Abstract
Background
Virtual consults have replaced in-person visits for many home-isolated patients with COVID-19 disease.
Objectives
To describe the natural history, clinical management and outcomes of community-dwelling patients with COVID-19, who received support from a family medicine-led, virtual CovidCare@Home program in Toronto, Ontario, Canada.
Methods
Observational, descriptive study conducted by retrospective chart review of 98 patients enrolled during the first 5 weeks of program implementation (8 April–11 May 2020); 73 patients with laboratory-confirmed COVID-19, with symptom onset ≤ 14 days before initial consult were included for analysis. Patients were classified as mild, moderate or severe based on WHO Criteria.
Results
All patients in the program experienced mild (88%) or moderate (12.3%) disease. No patients were hospitalized or died. Patients were mainly female (70%); with mean age of 43.3 years. Most patients (82.2%) worked in higher risk, healthcare settings. Almost 40% had no medical co-morbidities. Common symptoms were cough (65.8%), fatigue (60.3%), headache (42.5%) and myalgia (39.7%), followed by fever (32.9%), sore throat (21.9%), nasal congestion (21.9%) and rhinorrhea (20.5%). Headache (51%) and anosmia (45.1%) were common among females; fever and breathlessness among males (40.9%). Nine patients (12.3%) experienced worsening of symptoms (mainly respiratory) or exacerbation of co-morbidities, which required care outside the virtual service.
Conclusion
Patients with mild to moderate COVID-19 disease can be managed safely and effectively in a family medicine-led virtual program. Some sex differences in symptoms were observed. Future work should focus on long-term follow up in view of the existence of so-called ‘long-haulers’.
Keywords: COVID-19, family practice, multidisciplinary care, primary health care, SARS-CoV-2, telemedicine
Key Messages.
Case series describes the natural history of home-isolated, COVID-19 patients.
Our cohort experienced more milder, cold-like symptoms than previously described.
Sex differences in ever-presenting COVID-19 symptoms were observed.
Remote monitoring of patients with mild to moderate COVID-19 is safe and effective.
Assessing long-term effects of COVID-19 after program discharge is needed.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), first emerged from Wuhan City, Hubei Province, China in December 2019 (1). It rapidly spread internationally, and the World Health Organization (WHO) declared a global COVID-19 pandemic on 11 March 2020 (2). Since then more than 33.4 million people have been infected worldwide, with over one million deaths (3). These figures likely underestimate the true impact of the disease. The first report on the clinical characteristics and disease severity of 1099 COVID-19 patients emerged from China (4), with the predominant primary symptoms of dry cough, fever, myalgia, shortness of breath or diarrhea (4). Subsequent reports have described other presenting signs of COVID-19 infection, including: neurologic symptoms (e.g. headache, dizziness, confusion, ischemic and haemorrhagic stroke) (5–7); acute myocardial injury (e.g. chest pain, heart palpitations and severe tiredness) (8,9) and anosmia (10,11).
Presently, our understanding of the myriad of clinical presentations and complications of COVID-19 disease has come predominantly from patients seen in secondary and tertiary acute care settings, where the spectrum of disease is more severe. To our knowledge, the natural history of mild to moderate COVID-19 infection among community-dwelling, primary care patients who are in home isolation is not yet fully described. Elucidating the clinical course of patients with COVID-19 is important, as upwards of 80% of infected patients will have mild to moderate disease and can be managed at home (12).
In late March 2020, a virtual clinic, ‘CovidCare@Home’ (https://www.covidcareathome.ca/) (13) at Women’s College Hospital (WCH) in Toronto, Canada was created to provide virtual assessment and monitoring for community-dwelling people with COVID-19 experiencing mild to moderate illness symptoms. The program was established for people who either did not have their own family physician or who were unable to connect with them in the midst of the pandemic. The clinic was established using the principles and modified protocols described by Greenhalgh et al. (14) and was operational by 8 April 2020.
This study describes the natural history, clinical management and outcomes of patients who received care during the first 5 weeks of the CovidCare@Home virtual clinic.
Methods
An observational, descriptive study was conducted by retrospective chart review of patients with laboratory-confirmed COVID-19 infection who were registered in the CovidCare@Home virtual program between 8 April and 11 May 2020, and received virtual consults and remote monitoring, as needed.
CovidCare@Home patients were referred from WCH’s COVID-19 Assessment Centre (CAC) after testing positive for COVID-19. The CAC, established to deliver COVID-19 testing to the public and health care workers, offers testing based on provincial public health algorithms that consider symptoms, exposure to COVID-19, travel history and involvement with vulnerable at-risk populations. Access to the Centre is by walk-in, or after an on-line self-assessment with telephone triage and a fast track visit for swabbing. Patients with symptom onset more than 14 days prior to their initial CovidCare@Home appointment were excluded from data analysis.
Setting
The CovidCare@Home clinic is led by a small group of family medicine residents and staff physicians, nurses, and with interprofessional support from two nurse practitioners, four social workers, a pharmacist, a pharmacist resident, and a clinic secretary and administrator.
CovidCare@Home initial virtual video assessments were conducted by a family medicine resident and staff family physician. Follow-up visits by a family medicine resident and staff family physician or nurse were conducted by video or by telephone every 1–2 days, based on the severity of symptoms, age and medical comorbidities. Patients’ symptoms were gathered and recorded by clinicians at each visit using a flowsheet developed for the program within the WCH electronic health record, provided by EPIC® (Epic Systems Corporation).
Management aligned with current guidelines, including reinforcing the need for self-isolation, adequate hydration, acetaminophen, inhaled corticosteroids for those with a history of asthma and antibiotics for presumed secondary infections (15). Pulse oximeters and thermometers were couriered to patients who were at increased risk for clinical deterioration based on clinicians’ assessment. Higher risk patients were followed more frequently (up to twice daily), and all patients were given an after-hours telephone number to reach an on-call family physician.
Medical back-up for the CovidCare@Home program is provided by two general internists, a respirologist and two psychiatrists. Patients seen in consultation and thought to be too ill to be managed remotely, but not necessarily requiring hospital admission, could be assessed in the WCH’s Acute Ambulatory Care Unit (AACU), a short stay medical unit that provides urgent assessment, investigation and management for patients with new medical problems and those with chronic medical illnesses.
Data collection
Clinical data from EPIC flowsheets were exported into a Microsoft Excel spreadsheet, which included: patient demographics (age, sex, rostered with a family physician), occupation (e.g. front-line, essential worker), medical risk factors (e.g. asthma, hypertension, etc.), COVID-19 diagnosis (i.e. swab positive, presumed) and symptoms (e.g. breathlessness, fever, cough, sputum production, fatigue, chest tightness, myalgias, etc.). Symptom data were collected during any visit with a clinician while a patient was enrolled in the Program (versus solely presenting symptoms at the initial visit). Data not available from flowsheets were extracted by chart review including: contextual risk factors (e.g. high risk occupations); other symptoms (i.e. anosmia, dysgeusia, headache, other gastrointestinal symptoms, dizziness, loss of appetite, sore throat, sweats/chills, nasal congestion, rhinorrhea, tachycardia, etc.); distribution of supportive monitoring tools (e.g. pulse oximeters, thermometers); and referral for additional medical services (virtual general internal medicine consultations, AACU and emergency department visits, hospitalizations). Data inconsistencies were resolved by chart review.
The WHO classification of COVID-19 severity was used to categorize patient’s symptoms as consistent with ‘mild’ or ‘moderate’ disease (16). Patients with ‘moderate’ disease were defined as those who experienced all of cough, fever and breathlessness, but without signs of severe pneumonia, including blood oxygen saturation levels (SpO2) ≥ 90% on room air. Since patients who were at high risk for deterioration based on clinicians’ impressions were couriered pulse oximeters, we assumed that patients without a pulse oximeter maintained SpO2 > 90% unless otherwise documented. All other patients were classed as ‘mild’.
Data analysis
De-identified data from the Microsoft Excel spreadsheet was exported into SPSS statistics version 26.0 (IBM Corp, Armonk, NY) for descriptive statistical analysis (counts, percentage, means, standard deviation), and Fisher exact test was for discrete variables. Symptom prevalence was expressed as the percentage of total patients, and 95% confidence intervals were calculated using the Clopper–Pearson method. P values <0.05 were considered statistically significant. The prevalence of ever-presenting COVID-19 symptoms were compared with estimates gathered from a portal that provides clinical decision support tools to clinicians worldwide.
Results
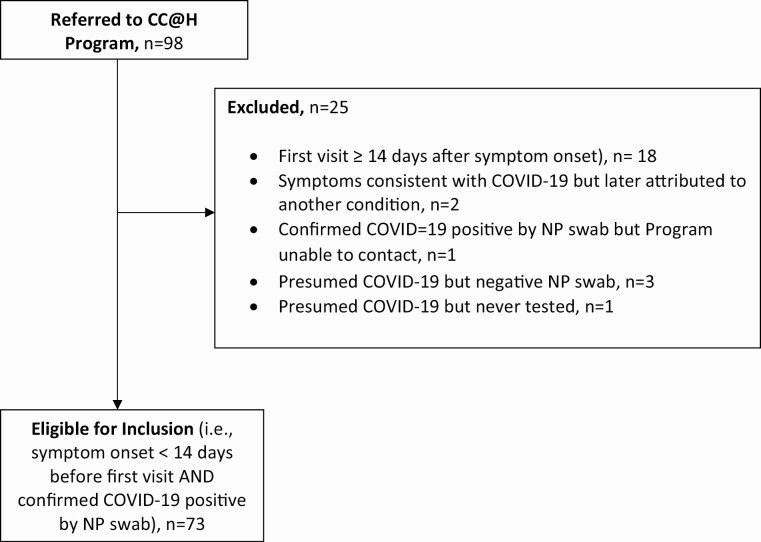
A total of 98 patients were seen in the CovidCare@Home in the first 5 weeks of operation (8 April–11 May 2020). Of these, 25 were ineligible and were excluded from analysis (Fig. 1). Eighteen patients had their first virtual care visit 14 or more days after symptom onset; six were presumed to have COVID-19 (based on symptoms) and one had laboratory-confirmed COVID-19, but the Program was unable to contact the patient.
Figure 1.
Eligibility flow diagram. NP, nasopharyngeal
Patient characteristics
A total of 73 patients were included in the analysis. Their demographic and clinical characteristics are summarized in Table 1. About 70% of patients (51/73) were female. The mean age was 43.3 years (SD 13.2).
Table 1.
Characteristics of 73 patients with nasopharyngeal-swab confirmed COVID-19
| Characteristics | Female | Male | Total |
|---|---|---|---|
| Individuals, no. (% of total) | 51 (69.9) | 22 (30.1) | 73 (100) |
| Age, mean (SD), min/max, years | 43.3 (14.3), 19/68 | 43.8 (10.7), 28/63 | 43.5 (13.2), 19/68 |
| Age range, no. (%a), years | |||
| ≤19 | 1 (2.0) | 0 | 1 (1.4) |
| 20–29 | 11 (21.6) | 1 (4.5) | 12 (16.4) |
| 30–39 | 12 (23.5) | 9 (40.9) | 21 (28.8) |
| 40–49 | 8 (15.7) | 5 (22.7) | 13 (17.8) |
| 50–59 | 9 (17.6) | 5 (22.7) | 14 (19.2) |
| 60–69 | 10 (19.6) | 2 (9.1) | 12 (16.4) |
| Pregnant, no. (%a) | 4 (7.8) | - | 4 (5.5) |
| Current cigarette smoker, no. (%a) | 3 (5.9) | 1 (4.5) | 4 (5.5) |
| Medical co-morbidities, no. (%a) | |||
| Anxiety | 7 (13.7) | 2 (9.1) | 9 (12.3) |
| Hypertension | 6 (11.8) | 3 (13.6) | 9 (12.3) |
| Dyslipidemia | 4 (7.8) | 3 (13.6) | 7 (9.6) |
| Asthma | 3 (5.9) | 3 (13.6) | 6 (8.2) |
| Diabetes | 3 (5.9) | 2 (9.1) | 5 (6.8) |
| Autoimmune disorder or otherwise immunocompromised | 1 (2.0) | 2 (9.1) | 3 (4.1) |
| Depression | 2 (3.9) | 1 (4.5) | 3 (4.1) |
| Heart conditions (e.g. heart failure/cardiovascular disease) | 1 (2.0) | 1 (4.5) | 1 (2.8) |
| Other | 13 (25.5) | 5 (22.7) | 18 (24.7) |
| Number of medical co-morbidities, no. (%a) | |||
| 0 | 22 (43.1) | 8 (36.4) | 30 (41.1) |
| 1 | 15 (29.4) | 8 (36.4) | 23 (31.5) |
| 2 | 9 (17.6) | 2 (9.1) | 11 (15.1) |
| 3 | 3 (5.9) | 3 (13.6) | 6 (8.2) |
| 4 | 1 (2.0) | 0 | 1 (1.4) |
| 5 | 1 (2.0) | 1 (4.5) | 2 (2.7) |
| High risk for occupational exposure, no. (%a) | 43 (84.3) | 17 (77.3) | 60 (82.2) |
| Health care setting | 28 (54.9) | 7 (31.8) | 35 (24.2) |
| Long term care | 16 (31.4) | - | 16 (21.9) |
| Acute care | 3 (5.9) | 5 (22.7) | 8 (11.0) |
| Complex continuing care or rehabilitation | 9 (17.6) | 2 (9.1) | 11 (15.1) |
| Shelter | 5 (9.8) | 3 (13.6) | 8 (11.0) |
| Grocery store | 3 (5.9) | 2 (9.1) | 5 (6.8) |
| Other essential workerb | 7 (13.7) | 5 (22.7) | 12 (16.4) |
| Exposure status no. (%a) | |||
| Known close contact | 36 (70.6) | 17 (77.3) | 53 (72.6) |
| Other exposure risk | 10 (19.6) | 3 (13.6) | 13 (17.8) |
| Unknown | 5 (9.8) | 2 (9.1) | 7 (9.6) |
a% within sex.
bSome other essential worker included: police, Canada Border Services agent, pharmacist, early childhood educator, security guard, mechanic, scientist in COVID lab
Over three-quarters worked in high-risk occupational settings (82.2%, 60/73). Among those females in the study, 55% (28/51) worked in healthcare settings, with 31% in long-term care (LTC; 16/51), whereas more males (45%; 10/22) were employed as essential workers (e.g. security guard, police, peer support worker in home care, scientist in a COVID lab) outside the health sector. Almost three-quarters (72.9%; 54/73) reported known COVID-19 exposure either through a close contact (53%; 39/73) or from working in high-risk settings or as essential workers. Some examples of other risk exposures included living with a spouse who worked in LTC or another healthcare setting, living in high-rise buildings or in a shelter. Almost 10% of patients (7/73) did not know how they got exposed to COVID-19.
Among all patients, 43% of females (22/51) and 36% of males (8/22) had no medical co-morbidities. The most common co-morbidities among females were anxiety (13.7%; 7/51) and hypertension (11.8%; 6/51); most common among males were hypertension, dyslipidemia and asthma (13.6% each; 3/22). No patients reported chronic obstructive pulmonary disease, chronic kidney disease or liver disease.
Symptoms
A majority of patients had mild disease (88%; 64/73), while 12% (9/73) had moderate disease. The most common symptoms among all patients were cough (65.8%; 48/73), fatigue (60.3%; 44/73) and headache (42.5%), followed by myalgia (39.7%), anosmia (37.0%), fever (32.9%) and breathlessness (31.5%; Table 2).
Table 2.
Symptoms experienced by 73 patients with nasopharyngeal-swab confirmed COVID-19
| Characteristic | Female (No., %) |
Male (No., %) |
P-valuesa | Total (No., %; 95% CIb) |
Published Estimates (ref: 19,20) (%) |
|---|---|---|---|---|---|
| Symptoms | |||||
| Cough | 34 (66.7) | 14 (63.6) | 0.80 | 48 (65.8; 53.7–76.5) | 58.3 |
| Fatigue | 28 (54.9) | 16 (72.7) | 0.20 | 44 (60.3; 48.1–71.5) | 34.0 |
| Headache | 26 (51.0) | 5 (22.7) | 0.04 | 31 (42.5; 31.0–54.6) | 11.3 |
| Myalgia | 21 (41.2) | 8 (36.4) | 0.80 | 29 (39.7; 28.5–51.9) | 21.9 |
| Anosmia | 23 (45.1) | 4 (18.2) | 0.04 | 27 (37.0; 26.0–49.1) | 52.7 |
| Fever | 15 (29.4) | 9 (40.9) | 0.42 | 24 (32.9; 22.3–44.9) | 78.4 |
| Breathlessness | 14 (27.5) | 9 (40.9) | 0.28 | 23 (31.5; 21.1–43.4) | 20.6 |
| Anxiety/depression | 17 (33.3) | 7 (31.8) | 1.00 | 24 (32.9; 22.3–44.9) | Not reported |
| Dysgeusia | 13 (25.5) | 5 (22.7) | 1.00 | 18 (24.7; 15.3–36.0) | 43.9 |
| Sputum production | 12 (23.5) | 5 (22.7) | 1.00 | 17 (23.3; 14.2–34.6) | 22.7 |
| Sweats/chills | 14 (27.5) | 3 (13.6) | 0.24 | 17 (23.3; 14.2–34.7) | Not reported |
| Nasal congestion | 12 (23.5) | 4 (18.2) | 0.76 | 16 (21.9; 13.1–33.1) | 4 |
| Sore throat | 11 (21.6) | 5 (22.7) | 1.00 | 16 (21.9; 13.1–33.1) | 11.6 |
| Loss of appetite | 12 (23.5) | 3 (13.6) | 0.53 | 15 (20.5; 12.0–31.6) | 22.7 |
| Rhinorrhea | 12 (23.5) | 3 (13.6) | 0.53 | 15 (20.5; 12.0–31.6) | 7.3 |
| Diarrhea | 9 (17.6) | 2 (9.1) | 0.49 | 11 (15.1; 7.8–25.4) | 7.7 |
| Dizzy/light-headed | 9 (17.6) | 5 (22.7) | 0.75 | 14 (19.2; 10.9–30.1) | 12.1 |
| Nausea/vomiting | 10 (19.6) | 3 (13.6) | 0.71 | 9 (12.3; 5.8–22.1) | 7.8 |
| Chest tightness | 5 (9.8) | 3 (13.6) | 0.69 | 8 (11.0; 4.9–20.5) | 22.9 |
| Tachycardia | 3 (5.9) | 2 (9.1) | 0.63 | 5 (6.8; 2.3–15.3) | Not reported |
| Cold clammy skin | 1 (2.0) | 0 | 1.00 | 1 (1.4; 0.0–7.4) | Not listed |
| Decrease urine Output | 0 | 1 (4.5) | 0.30 | 1 (1.4; 0.0–7.4) | Not listed |
| Hemoptysis | 0 | 1 (4.5) | 0.30 | 1 (1.4; 0.0–7.4) | 0.9 |
| Confusion | 0 | 0 | - | 0 | 9 |
| Cutaneous symptoms | 0 | 0 | - | 0 | 7.8 |
| Additional Symptoms | 20 (39.2) | 2 (9.1) | 0.02 | 22 (30.1; 19.9–42.0) | - |
| Number of symptoms | |||||
| 0 | 2 (3.9) | 3 (13.6) | 5 (6.8; 2.3–15.3) | ||
| 1–3 | 12 (23.5) | 4 (18.2) | 16 (21.9; 13.1–33.1) | ||
| 4–6 | 11 (21.6) | 7 (31.8) | 0.83 | 18 (24.7; 15.3–36.0) | |
| 7–9 | 20 (39.2) | 5 (22.7) | 25 (34.2; 23.5–46.3) | ||
| 10–12 | 4 (7.8) | 3 (13.6) | 7 (9.6; 3.9–18.8) | ||
| ≥ 13 | 2 (4.0) | 0 | 2 (2.7; 0.3–9.6) | ||
| COVID-19 severityc | |||||
| Mild | 48 (94.1) | 16 (72.7) | 0.02 | 64 (87.7; 77.9–94.2 | |
| Moderate | 3 (5.9) | 6 (27.3) | 9 (12.3; 5.8–22.1) |
aFisher exact test.
b95% confidence intervals calculated by Clopper–Pearson method.
cBased on World Health Organization Classification of COVID-19 severity.
Sex differences in symptoms were observed and included greater prevalence of headache (51% versus 22.7%; P < 0.04), anosmia (45.1% versus 18.2%; P < 0.04), sweats and chills (27.5% versus 13.6%), rhinorrhea (23.5% versus 13.6%) and anorexia (23.5% versus 13.6%) amongst females (Table 2). Fatigue (72.7% versus 54.9%), shortness of breath (40.9% versus 27.5%) and fever (40.9% versus 29.4%) were more prevalent amongst males, although not statistically significant (Table 2).
The next most common symptoms among both sexes included myalgia and anxiety/depression. From chart review, 40% (20/51) of women reported additional symptoms that were not captured from the clinical flowsheets. Most symptoms were non-specific, such as restlessness, burning sensations of the eyes, nose, back, trunk and neck.
Clinical course and monitoring
Patients reported a mean of 3.5 days (SD 4.2) between symptom onset and their COVID-19 tests, and patients had their first virtual care clinic visit a mean of 3.2 (SD 1.6) days after undergoing COVID-19 testing. Overall, most patients in the Program were seen for approximately five virtual care visits over a mean of 8.1 (SD 6.0 days). Nearly one-third of patients were sent an oximeter (32.9%; 24/73) and 6.8% (5/73) received a thermometer to provide additional monitoring (Table 3). Of the six males with moderate disease, 66.7% (4/6) were monitored with oximetry and two had asthma. All three females with moderate disease received a pulse oximeter, with one reporting asthma.
Table 3.
Clinical course and monitoring of 73 patients with nasopharyngeal-swab confirmed COVID-19
| Characteristic | Female | Male | Total |
|---|---|---|---|
| Pulse oximeter, no. (% within sex) | 18 (35.3) | 6 (27.3) | 24 (32.9) |
| Thermometer, no. (% within sex) | 4 (7.8) | 1 (4.5) | 5 (6.8) |
| Clinical course, no. (% within sex) | |||
| Worsening Symptoms Attributed to COVID-19 | |||
| Hospital admission | 0 | 0 | 0 |
| Emergency department | 3 (5.9) | 2 (9.1) | 5 (6.8) |
| AACU | 1 (2.0) | 0 | 1 (1.4) |
| General internal medicine virtual consultation | 1 (2.0) | 0 | 1 (1.4) |
| Co-morbidity management | |||
| Hospital admission | 0 | 0 | 0 |
| Emergency department | 0 | 0 | 0 |
| AACU | 0 | 0 | 0 |
| General internal medicine virtual consultation | 1 (2.0) | 1 (4.5) | 2 (2.7) |
AACU, acute ambulatory care unit.
None of the patients were admitted to hospital or died. Nine patients (12.3%) experienced worsening of COVID-related symptoms (mainly respiratory) or exacerbation of co-morbidities; five patients (6.8%) were referred to the emergency department, one to the AACU (1.4%) and three (4.1%) received a virtual general internal medicine (GIM) consultation (Table 3).
Discussion
To our knowledge, this is the first case series describing the natural history of COVID-19 in community-dwelling people well enough at presentation to be followed by a virtual family medicine program. There are several key findings relevant to a primary care audience where most care for people with COVID-19 occurs.
A major finding is that remote monitoring of COVID-19 patients in a family medicine setting as adapted from that described by Greenhalgh et al. (14) appears to be both safe and effective in spite of a lack of validated clinical predictive tools to identify those most likely to progress to pneumonia and require hospital admission (17). In this case series, all patients experienced mild to moderate illness with resolution of symptoms at the time of discharge.
Amongst those cared for in the first 5 weeks of the program, there were no hospital admissions and no deaths. This suggests that those with mild to moderate disease may do very well at home with virtual support from an interprofessional primary care team.
The positive outcomes might be explained by an overall younger and healthier cohort of people than those described elsewhere, as well as a predominantly female cohort of patients who have been shown to have better outcomes with COVID-19 (18). Daily monitoring for people during the ‘riskiest’ period of their illness (days 5–10) and for those who experienced an increased number or severity of symptoms, or co-morbid conditions, may also explain why patients did well. Finally, those deemed at higher risk clinically were provided with a pulse oximeter and thermometer, which provided objective data to complement their regular clinical assessment and guide decision-making.
The second key finding is that we observed a heterogeneity of symptoms, as well as differences in symptom prevalence between females and males. Sex differences in symptoms observed included greater prevalence of headache and anosmia amongst females. Fatigue, shortness of breath and fever were more prevalent amongst males, but the differences did not achieve statistical significance. As far as we are aware, such sex differences in symptom prevalence have not been described elsewhere.
Our approach reflects symptoms gathered during clinicians’ assessments on multiple days which may more accurately reflect common symptomatology of the disease. In contrast, other studies have captured and described symptoms only at time of presentation to the hospital or emergency department (2,4).
In this study, only 6.8% of patients were asymptomatic as compared with the higher rates described in the literature (19). This may be because when the Program started, Ontario limited COVID-19 testing to those in occupations with a high risk of exposure (health care workers of various kinds), who made up 82% of our patient population (Table 1).
Finally, we compared symptom prevalence in our cohort versus what has been described and summarized to date in the literature (20,21). Our cohort had a higher prevalence of head and neck cold-like symptoms, including headache, sore throat, rhinorrhea and nasal congestion. Our cohort also had a lower prevalence of certain symptoms, such as anosmia and dysgeusia. There was a marked difference in the prevalence of fever in our cohort versus that described in the literature. Only 32.9% of our patients reported fever versus 78.4% of patients described in the literature (20,21). We speculate that fever itself may possibly be an early warning sign of patients who may be at risk of progressing to more severe disease based on the pathophysiology of SARS-Cov-2 (22) and fever (23).
Strength of this study
There are several strengths to this study. First, these are community-dwelling patients in a virtual family medicine clinic with mild to moderate COVID-19 illness. As far as we are aware, this is the first description of a cohort of such patients and their clinical course in the literature. Second, we captured ‘ever-presenting’ symptoms (clinicians probing over time) versus initial presenting symptoms in the emergency department or hospital setting previously reported (2,3). Last, a key strength of our program and the study is its family medicine orientation with an inter-professional team led by family medicine residents and staff family physicians who were able to address other acute, non-COVID-19 health problems that arose.
Limitations of this study
This study has limitations. It represents a small cohort of 73 patients and is likely not representative of the general population since patients were younger, predominantly female with fewer co-morbid health conditions. Furthermore, patients included many healthcare workers or people who were high risk for COVID-19 exposure. Patients were only followed for a 5-week time period.
Future research should focus on capturing larger case series and include longer term follow-up to determine the clinical course beyond the initial acute illness period. Although patients with persisting symptoms (so-called ‘long-haulers’) have been described in the media (24), as far as we are aware, to date there are no published studies describing such patients in detail. The role of clinical symptoms as well as remote monitoring tools, such as pulse oximetry to identify patients at higher risk of progression to more severe disease requires more robust evaluation. Future research should also assess the role of the routine use of acetaminophen and inhaled corticosteroids in mild to moderate COVID-19 management overall.
Conclusions
This case series from a family medicine setting reveals that people with mild to moderate COVID-19 disease can present with a wide range of symptoms, but may have a greater prevalence of milder, cold-like symptoms including headache, sore throat, rhinorrhea and nasal congestion than previously described. It is likely feasible, safe and effective to care for people with mild to moderate COVID-19 virtually in a primary care setting. Primary care/family medicine teams are ideally suited to manage people with mild to moderate COVID-19 disease in the community.
Acknowledgements
The authors would like to acknowledge the invaluable contributions of the CovidCare@Home clinical team, administrative team, our patient representative, WCH inter-professional and specialist colleagues, as well as all members of the COVIDCare@Home Steering Committee.
Declarations
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. This study was undertaken with in-kind resources provided by Women’s College Hospital.
Ethical approval: This study was reviewed and approved by the Women’s College Hospital Research Ethics Board (2020-0058-E).
Conflict of interest: none.
Author contributions
NP conceived and designed the study with PA and RH. NP wrote the initial draft with contributions from SH, LM and ML. LM, SH and ML conducted the analyses. All authors contributed to data interpretation and edited and approved the final version of the manuscript.
Data availability
Data sharing is not applicable because we did not receive participant’s informed.
References
- 1.Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579(7798): 270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus/ (accessed 25 September 2020).
- 4.Guan W, Ni Z, Liang W, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J, Kremer S, Merdji H, et al. . Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382: 2268–70. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao, H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-Cov-2 infection: causality or coincidence? Lancet Neurol 2020; 19(5): 383–4. doi: 10.1016/51474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toscano G, Palmerini F, Ravaglia S, et al. . Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med 2020; 382: 2574–6. doi:10/1056?NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (Covid-19) with myocardial injury and mortality. JAMA Cardiol 2020; 5(7): 751–3. [DOI] [PubMed] [Google Scholar]
- 9.Inciardi RM, Lupi L, Zaccone G, et al. . Cardiac involvement in a patient with coronavirus disease 2019 (covid-19). JAMA Cardiol 2020; 5(7): 819–24. doi: 10.1001/jamacardio.2020.1096pmid:32219357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomelli A, Pezzati L, Conti F, et al. . Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020;ciaa330. 10.1093/cid/ciaa33032215618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinato G, Fabbris C, Polosel J, et al. . Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020; 323(20): 2089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. New Engl J Med 2020; 383: 1757–66. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal P, et al. . COVIDCare@Home: lessons from a Family Medicine Led Remote Monitoring Program. Submitted to CMAJ Open. [DOI] [PMC free article] [PubMed]
- 14.Greenhalgh T, Choon Huat Koh G, Car J. Covid-19: a remote assessment in primary care. BMJ 2020; 368: m1182. https://www.bmj.com/content/368/bmj.m1182 (accessed 30 April 2020). [DOI] [PubMed] [Google Scholar]
- 15.Bobrovitz N, Lee J, Mahtani KR. Preventing non-COVID-19 hospital admissions during a pandemic: a rapid overview of the evidence for high-value medications. The Centre for Evidence-Based Medicine. 2020. https://www.cebm.net/covid-19/preventing-non-covid-19-hospital-admissions-during-a-pandemic-a-rapid-overview-of-the-evidence-for-high-value-medications/ (accessed 23 June 2020). [Google Scholar]
- 16.Clinical management of COVID-19. WHO. 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Page 13 (accessed 22 June 2020). [Google Scholar]
- 17.McIsaac WJ, Upshur R, Kukan S. Challenges in the virtual assessment of COVID-19 infections in the community. 2020. https://www.cfp.ca/news/2020/06/02/06-02 (accessed 16 June 2020). [DOI] [PubMed] [Google Scholar]
- 18.Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Diff 2020; 11:29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19: What proportion are asymptomatic? The Centre for Evidence-Based Medicine. 2020. https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/ (accessed 20 June 2020).
- 20.Coronavirus disease 2019 (COVID-19). BMJ Best Practice. 2020. https://bestpractice.bmj.com/topics/en-gb/3000201 (accessed 20 June 2020). [Google Scholar]
- 21.Grant MC, Geoghegan L, Arbyn M, et al. . The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARSCoV-2; COVID-19): A systematic review and metaanalysis of 148 studies from 9 countries. PLoS ONE 2020; 15(6): e0234765. 10.1371/journal.pone.0234765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zirui Tay M, Poh CK, Renia L, MacAry PA,Ng LFP. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020; 20(6): 363–74. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Critical Care 2016; 20:200 doi 10.1186/s13054-016-1375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogaert D. The coronavirus ‘long-haulers’ show how little we still know. The Guardian. 2020. https://www.theguardian.com/commentisfree/2020/jun/28/coronavirus-long-haulers-infectious-disease-testing (accessed 23 July 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable because we did not receive participant’s informed.