Abstract
Introduction
Preliminary reports indicated that smokers could be less susceptible to coronavirus SARS-CoV-2, which causes Covid-19. However, once infected an increased risk of severe disease is reported. We investigated the association between smoking and COVID-19 during an outbreak of the disease on a naval vessel.
Methods
We conducted a cross-sectional, observational study on the 1769 sailors of the same navy aircraft carrier at sea exposed at the same time to SARS-CoV2 to investigate the link between tobacco consumption and Covid-19.
Results
Among the 1688 crewmembers (87% men; median age = 28 [interquartile range 23–35]) included, 1279 (76%) developed Covid-19 (1038 [62%] reverse-transcriptase- polymerase chain reaction testing–positive and 241 [14%] with only clinical signs). One hundred and seven patients were hospitalized. The univariable analysis odds ratio (OR) for Covid-19 infection was 0.59 (95% confidence interval [CI], 0.45–0.78; p < .001) for current smokers versus former and nonsmokers; sex, body mass index or blood group had no significant impact. Crewmembers >50 years old had an increased risk of contracting Covid-19 (OR, 2.84 [95% CI, 1.30–7.5]; p = .01). Multivariable analysis retained the lower risk of current smokers becoming infected (OR, 0.64 [0.49–0.84]; p < .001) and age >50 years was significatively associated with Covid-19 (OR, 2.6 [1.17–6.9]; p = .03).
Conclusions
Current smoking status was associated with a lower risk of developing Covid-19 but cannot be considered as efficient protection against infection. The mechanism of the lower susceptibility of smokers to SARS-CoV-2 requires further research.
Trial Registration
IRB no.: 0011873-2020-09
Implications
(1) Recent epidemiologic data suggest a paradoxical link between smoking and COVID-19. (2) Among the 1688 crewmembers (with an attack rate of 76% and exposed at the same time in the same place to SARS-CoV2), we found a significantly lower risk for developing COVID-19 in current smokers (71%) versus former and nonsmokers (80%). This finding strongly supports the need for further research on nicotine physiological pathway and its impact on COVID-19 infection whilst emphasizing that tobacco smoking should not be considered as efficient protection against COVID-19.
Introduction
Recent epidemiologic data suggest a paradoxical link between smoking and becoming infected with SARS-CoV-2. Smoking seems to lower the susceptibility to infection1–11 but increases the risk of developing severe disease.1–3,7,8
Regarding Covid-19 severity, a recent systematic review of five studies on SARS-CoV-2–infected patients found smokers had relative risks of 1.4 (95% confidence interval [CI] 0.98–2.00) to develop severe Covid-19 and 2.4 (95% CI 1.43–4.04) for intensive care unit (ICU) admission.4 Among 8910 patients hospitalized for Covid-19 (low smoking rate, 6% smokers), Mehra et al. observed a greater risk of death for current smokers than nonsmokers or former smokers (adjusted odds ratio [aOR], 1.79 [95% CI, 1.29–2.47]).5 However, recent results indicated very low rates of smoking among hospitalized Covid-19 patients in China, the United States, and France.4,6–10 For example, the Centers for Disease Control summary of all confirmed Covid-19 cases until March 28, 2020 in the United States reported that only 1.3% were current smokers and 2.3% former smokers. This compares to 13.4% adult smoking prevalence in the United States which reaches 19.4% when including e-cigarette users.11 However, studies reporting a low rate of smoking have been criticized for the poor quality and retrospective collection of smoking status data, the comparison of smoking of subjects Covid-19 to smoking of the general population sometimes without taking into account sex and age1,3,5,7–9 and for having included only symptomatic or in-hospital subjects, creating possible selection bias.1,3,5,7–10 In this context, it is important to further explore the relationships between smoking and Covid-19, and to determine whether these relationships could reflect an effect of nicotine or smoke itself. It is also necessary to determine whether lower infection rates among smokers reflects infection prevention or an increase of asymptomatic or mild forms (not seeking medical assistance), which would affect collective immunity because a higher rate of asymptomatic patients could increase the virus spread.
A COVID-19 outbreak occurred in April 2020 on the French Navy nuclear aircraft carrier Charles de Gaulle, which was carrying 1769 crew members. During the mission, the crewmembers were contained onboard without mixing with other populations and, thus, were exposed at the same time and in the same place to SARS-CoV-2. When the ship returned to France, the entire crew was locked down at different military bases and benefited from daily medical monitoring for 14 days after landing. This study was undertaken with the main objective was to investigate the possible relationship between tobacco consumption and SARS-CoV-2 infection, in this particular and well-defined population and conditions of exposure.
Methods
Study Oversight and Design
To investigate the effect of smoking on developing Covid-19, we conducted this observational study on a retrospective cohort comprised of the aircraft carrier’s crew. The local Institutional Ethics Committee approved the study (IRB no.: 0011873-2020-09). All data were collected in the context of care from completely anonymized files, in accordance with French and European laws, including the General Data Protection Regulation. All patients were informed and provided written consent to use their data.
Population Screening and Data Collection
The aircraft carrier was deployed at sea from January 22 to April 13, as part of an operational mission. A SARS-COV-2 epidemic broke out on-ship, requiring its early (2 weeks) return to Toulon (main harbor of the ship). All 1769 persons onboard underwent a physical examination, with reverse-transcriptase–polymerase chain reaction testing (RT-PCR) of nasopharyngeal samples obtained immediately upon arrival in Toulon. All crew members were individually housed during confinement (for 14 days and at least 2 days after symptoms disappeared), patients with clinical signs and/or PCR-positive were isolated in a given area and asymptomatic PCR-negative subjects in another. During this period vital signs (eg, fever, respiratory symptoms) were monitored daily by the medical teams of the French Army Health Service (Service de Santé des Armées). During medical monitoring, all crewmembers completed an information questionnaire on their smoking habits (Appendix) under the supervision of an investigator.
Definitions and Data Collected
SARS-CoV-2-infected subjects (cases) were defined as RT-PCR-positive (confirmed) or patients with clinical symptoms highly suggestive of Covid-19 (fever, myalgias, arthralgias, dyspnea, cough, headache, anosmia, ageusia, rhinitis, diarrhea, fatigue, cutaneous signs) (suspected). Non-Covid-19 participants had no clinical signs and were RT-PCR-negative. The questionnaire on smoking status specifically asked about current tobacco consumption and frequency and intensity of current smoking (daily or occasional smoking, number of daily cigarettes). Crewmembers were considered current smokers when they had smoked (any type of products including e-cigarette and shisha) during the last 3 months (daily or not). Former smokers included anyone who had smoked (any tobacco product corresponding to >100 cigarettes during their whole life) in the past and had abstained from smoking for at least the preceding 3 months. “Never smokers” had smoked <100 cigarettes in their whole lifetime. E-cigarette vaping was also recorded, as was the use of nicotinic replacement therapy or other smoking-cessation treatments (eg, varenicline).
In addition to smoking status, the following medical information was extracted from medical records: age, sex, height, weight, blood group, Covid-19–related symptoms and their duration, the indication of hospitalization, and whether or not a Chest Tomography scan (CT) was done.
RNA Extraction and Real-Time RT-PCR Assay
Total RNAs were extracted from the nasopharyngeal swab samples of all crewmembers, and 200 μL of the sample were subjected to thermal and chemical inactivation and RNA extraction with Magnapure (Roche Diagnostic, Basel, Switzerland) or Genoxtract (Biocentric, Bandol, France). The RT-PCR assay was run according to the French National Center protocol, targeting IP2 and IP4 regions of the SARS-CoV-2–specific RNA-dependent RNA polymerase (RdRp) gene on Light Cyler 480 (Lightcycler 480, Roche, Basel, Switzerland).
Statistical Analyses
The primary analysis investigated the impact of smoking status on the risk of developing Covid-19 (symptoms and/or positive RT-PCR). Secondary analyses were the risk evaluations for confirmed Covid-19 (RT-PCR–positive) being symptomatic, for the impact of age, sex, blood group or body mass index (BMI) on the risk of SARS-CoV-2 infection, for the impact of smoking habits (cigarette, e-cigarette, shisha, etc.) on developing Covid-19, and the initial clinical picture of Covid-19. A descriptive analysis was first conducted. Dichotomous variables are expressed as numbers (percentages) with 95% CI, and continuous variables as means or medians with range, 95% CI, and/or interquartile range (IQR). Age and BMI were grouped into categories (age <20, 20–30, 30–40, 40–50, and >50 years; BMI <20, 20–25, 25–30, and >30 kg/m2). Then, after verifying the conditions of use, categorical variables were compared in univariable analyses (chi2 test) and the means of continuous variables with the Student’s t test.
Lastly, the impact of smoking on SARS-CoV-2 susceptibility was evaluated using logistic-regression univariable and multivariable analyses (risks assessed by ORs). The multivariable model included as covariates some parameters of clinical interest (age, sex, and BMI); the parameter “blood group” was only integrated when the univariate p-value was <.2. All analyses were computed with R software, version 3.6.3.12 p < .05 defined statistical significance.
Results
Patients
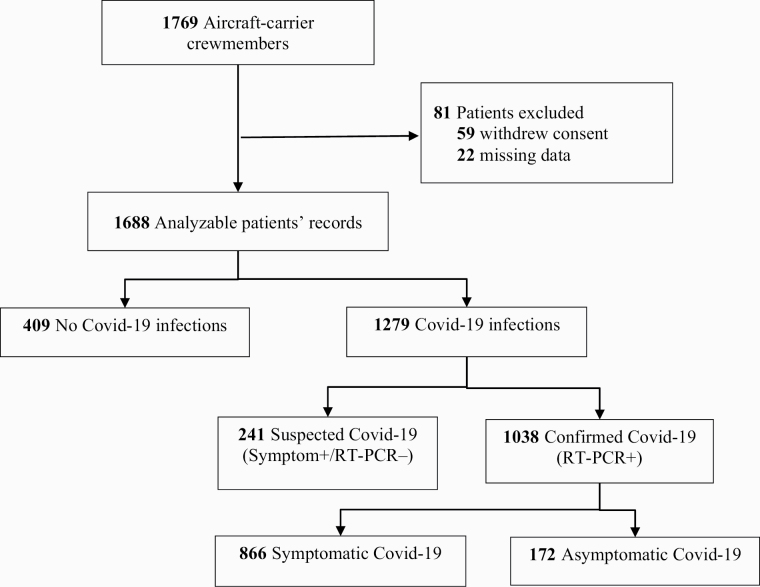
Of the 1769 crew members on the ship, 1688 (95%) were included in this study (59 withdrew consent and 22 had missing data: most of them had left the ship because of specific duty). The flow chart (Figure 1) shows the pathways and distribution of Covid-19 diagnoses. The demographic and clinical characteristics of all participants and Covid-19 status are reported in Table 1. Their median age was 28 years and 13% of the crew members were women. The population appeared to be healthy, with a median BMI of 24 and no significant co-morbidities. Notably, 48% were current smokers, with mean consumption of 9.7 cigarettes per day (95% CI, 9.7–10.15), 23% were former smokers (who had quit before January 2020) and 29% were never smokers. Among the 249 e-cigarettes users, two were exclusive users, 247 were dual users who simultaneously used e-cigarettes and smoked tobacco; 78 had smoked shisha between January and March 2020. The most frequent blood group was O (45%) followed by A (40%), with only 11% B and 4% AB.
Figure 1.
Flow-chart. RT-PCR denotes reverse-transcriptase–polymerase chain reaction. .
Table 1.
Characteristics of the Participants.
| Characteristic | All patients | Confirmed/suspected COVID-19 cases | No COVID-19 |
|---|---|---|---|
| Participants—no. (%) | 1688 (100) | 1279 (76) | 409 (24) |
| Median age (IQR)—yr | 28 (23–35) | 28 (23–36) | 27 (23–33) |
| Male—no. (%) | 1466 (87) | 1112 (87) | 354 (87) |
| Median BMI—kg/m2 (n = 1670)† | 24 (22–26) | 24 (22–26) | 24 (22–26) |
| <20—no. (%) | 85 (5) | 59 (5) | 26 (6) |
| 20–25—no. (%) | 945 (57) | 725 (57) | 220 (54) |
| 25–30—no. (%) | 556 (33) | 420 (33) | 136 (33) |
| >30—no. (%) | 84 (5) | 59 (5) | 25 (6) |
| Smoking status—no. (%) | |||
| Never smoker | 487 (29) | 391 (31) | 96 (23) |
| Former smoker | 386 (23) | 309 (24) | 77 (19) |
| Current smoker | 815 (48) | 579 (45) | 236 (58) |
| Current e-cigarette use—no. (%) (%)* | 249 (15) | 172 (13) | 77 (19) |
| Blood group—no. (%) (n=1669)‡ | |||
| A—no. (%)1669 | 674 (40) | 521 (41) | 153 (38) |
| B—no. (%) | 183 (11) | 135 (11) | 48 (12) |
| AB—no. (%) (%) | 70 (4) | 54 (4) | 16 (4) |
| O—no. (%) | 742 (45) | 553 (44) | 189 (46) |
| Number of hospitalizations—no. (%) | |||
| Medicine | 107 (6) | 107 (8) | — |
| ICU | 3 (0.2) | 3 (0.2) | — |
| Mortality | 0 (0) | 0 (0) | 0 (0) |
IQR denotes interquartile interval, BMI body mass index.
*E-cigarette users during the mission, exclusively or concurrently with cigarettes.
†Eighteen patients did not remember their exact weight or height: 16 COVID-19 cases and 2 not infected.
‡Nineteen patients had no blood group recorded in their medical records or the French blood-bank database: 16 COVID-19 cases and 3 not infected.
Covid-19
While onboard, 1279 patients developed Covid-19, representing a 76% (1279/1688) infection-attack rate. RT-PCR confirmed SARS-CoV-2 infection among 1038 (62%) patients, and 241 (14%) cases were clinically diagnosed. After returning to France, 107 crewmembers were hospitalized, including three requiring ICU admission. Covid-19 was asymptomatic in 172 (14%) cases. Among symptomatic cases, the mean duration of symptoms was 9 days (range, 1–40; 95% CI, 9.08–9.88). Table 2 gives the frequencies of patient-reported symptoms. The most common were headache (52%), ageusia, anosmia (48%), and fatigue (45%), with cough or dyspnea representing 22% and 34%, respectively; only 25% had a fever.
Table 2.
Clinical Picture of Covid-19 at Onset
| Covid-19 cases | p-value | |||
|---|---|---|---|---|
| All (N = 1279) | Current smokers (N = 472) | Former/never smokers (N = 807) | ||
| Mean symptom duration (days, 95% CI)* | 9.5 (9.08–9.88) | 9.10 (8.63–9.56) | 9.70 (9.20–10.20) | .15 |
| Asymptomatic infection—no. (%) | 172 (13) | 68 (14) | 104(13) | .22 |
| Symptom†—no. (%) | ||||
| Headache | 666 (52) | 226 (48) | 440 (55) | <.001 |
| Anosmia | 614 (48) | 212 (45) | 383 (47) | .20 |
| Asthenia | 575 (45) | 192 (41) | 383 (47) | <.001 |
| Myalgias | 523 (41) | 172 (36) | 351 (43) | <.001 |
| Ageusia | 499 (39) | 186 (39) | 313 (39) | .19 |
| Dyspnea | 437 (34) | 120 (25) | 317 (39) | <.001 |
| Rhinitis | 322 (25) | 119 (25) | 203 (25) | .29 |
| Fever | 322 (25) | 88 (19) | 234 (29) | <.001 |
| Cough | 286 (22) | 93 (20) | 193 (24) | <.001 |
*Patients with persistent clinical signs were censored on the date of the second RT-PCR.
†Symptoms listed were reported by at least 10% of the patients.
95% CI, 95% confidence interval.
Main Outcome
The rate of Covid-19 infection in the groups of current smokers, former smokers, and never smokers was respectively 71% (579/815), 80% (309/386), and 80% (391/487). Among Covid-19 patients, 45% (579/1279) were current smokers versus 58% (236/409) in the non-Covid-19 group (Table 1).
According to univariable analyses, current smoking was significantly associated with Covid-19 compared to former and nonsmokers and subjects >50 years old were at significantly higher risk of catching Covid-19 (Table 3). No significant relationship was found between SARS-CoV-2 infection and sex, BMI, or blood group. Multivariable analysis retained the significantly lower risk of SARS-CoV-2 infection for current smokers and age >50 years remained significantly associated with Covid-19. A similar result was found when cases were restricted to PCR positive patients (OR 0.57 [0.44–0.72]).
Table 3.
Univariable and Multivariable Analyses of Factors Associated with COVID-19
| Factor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age—years | <.0001 | |||
| <20 | 0.98 (0.61–1.63) | .94 | 0.99 | NS |
| 20–30 | 1.00 | — | 1.00 | — |
| 30–40 | 1.01 (0.78–1.31) | .93 | 1.00 | NS |
| 0–50 | 1.42 (0.77–2.12) | .07 | 1.30 | NS |
| >50 | 2.84 (1.30–7.5) | .01 | 2.6 (1.17–6.9) | .03 |
| Sex | .83 | NS | ||
| Male | 1.00 | — | 1.00 | — |
| Female | 0.96 (0.70–1.35) | — | 1.00 | — |
| Body mass index | ||||
| <20 | 0.68 (0.42–1.13) | .13 | 0.70 | NS |
| 20–25 | 1.00 | — | 1.00 | — |
| 25–30 | 0.93 (0.73–1.20) | .60 | 0.90 | NS |
| >30 | 0.71 (0.44–1.18) | .18 | 0.69 | NS |
| Tobacco | ||||
| Never smoker | 1.00 | — | 1.00 | — |
| Former smoker | 0.98 (0.70–1.38) | .93 | 0.98 | NS |
| Active smoker | 0.59 (0.45–0.78) | <0.001 | 0.64 (0.49–0.84) | .001 |
| Blood group | ||||
| O | 0.86 (0.67–1.10) | .22 | NA | — |
| A | 1.00 | — | NA | — |
| B | 0.82 (0.57–1.21) | .31 | NA | — |
| AB | 0.99 (0.56–1.83) | .97 | NA | — |
OR, odds ratio; aOR, adjusted OR; NS, nonsignificant; NA, not applicable.
Secondary Outcomes
Smoking status did not delay Covid-19 onset, as new symptomatic infections peaked between April 6 and 8 for smokers and nonsmokers. The Covid-19-case rate was not significantly different for e-cig users (OR, 0.75 [95% CI 0.54–1.05]; p = .1) or shisha smokers (OR, 0.92 [95% CI 0.54–1.55]; p = .79).
Among current smokers (807 patients), compared to the group of patients who didn’t smoke everyday (166 patients), we found an OR for Covid-19 0.62 [95% CI 0.39–0.94]; p = .003 for smokers <10 cigarettes/day (373 patients) and 0.33 [95% CI 0.19–0.55]; p <.0001 for smokers > 10 cigarettes/day (123 patients).
Among 107 hospitalized Covid-19 cases, 31 were current smokers and 76 former or nonsmokers (p = .09). Among the 20 cases requiring oxygen therapy, two were current smokers (OR, 0.16 [95% CI 0.02–0.69]; p = .005). Smoking was not associated with a higher number of clinical signs (OR, 0.80 [95% CI 0.55–1.12]; p = .20) in the RT-PCR positive subgroup. Among Covid-19 patients, current smoking was associated with less frequent fever >38°C, dyspnea, coughing, myalgias, diarrheas, and headache. Other symptoms were unaffected by smoking status (ie, rhinitis, ageusia, anosmia, and cutaneous signs), with comparable mean symptom duration (Table 2).
Discussion
We found evidence of a lower occurrence of Covid-19 infection among smokers, with the greatest reduction in risk, seem among heavier smokers. The outbreak affected 76% of the crew. Most cases were mild forms, with only 107 (6%) patients hospitalized, including three in ICU; none died. The SARS-CoV-2-infection rate observed herein was much higher than that on the Diamond Princess ocean liner (700/3700, 18.9%) or the Roosevelt aircraft carrier (1100/4800, 22.3%),13,14 but those investigations only took into account, RT-PCR-positive patients. Our RT-PCR positive Covid-19-case rate was 61.5% (1038/1688 patients). The proximity of crew is far greater on an aircraft carrier than for passengers on a cruise ship and could partly explain the rapid progression of the outbreak. A more detailed epidemiologic investigation is underway to try to understand these differences. Aboard ship, smoking is only allowed in two limited areas and crewmembers are not allowed to smoke at their workstations. The organization of the day requires them to go for several hours every day without smoking. There are many interactions on-board: at workplaces, relaxation areas, or mess halls. Because sleeping berths were assigned by the command and not by affinity, we thought it unnecessary to look for a “subgroup effect.” Smoking did not change the date of symptom onset. Further studies are needed to describe and compare the degree of social interactions among smokers and nonsmokers.
Our findings suggest that current smoking appears to significantly lower the odds of Covid-19 occurrence by –36% compared to nonsmokers and former smokers. However, the odds ratio will over-estimate the strength of the relative risk reduction (particularly as the prevalence of the outcome was high). Univariable and multivariable analyses identified and retained, respectively, that significant independent association (Table 3), as well as age >50 years. We found no significant association between Covid-19 and BMI, sex, or blood group. The Covid-19 clinical picture also appeared to differ according to smoking status, with fewer general symptoms (fever, asthenia, and headache) and respiratory signs (cough and dyspnea) among current smokers and some evidence of less severe infections—for example, among 20 patients requiring oxygen therapy, only two were current smokers, Previous studies showed conflicting results with most studies finding higher levels of severe Covid-19 in smokers (usually older and chronic obstructive pulmonary disease [COPD] patients).15 As the numbers with severe disease in our study were low, these results need to be treated with caution
The mechanism of interaction between smoking and SARS-CoV-2 infection remains unknown. Nicotine could be responsible for the effect,16–24 as well as tobacco smoke by for example its fine particles and numerous chemical agents which could have a nonspecific effect of worsening of all viral related lung diseases.25 The ACE2 receptor is the site of specific binding to the cell of SARS-CoV-1 and SARS-CoV-2,16–19 but not of Middle-East respiratory syndrome (MERS)-CoV (dipeptidyl peptidase IV [DPP4] is involved).22 In MERS-CoV-related disease, nonspecific tobacco-related worsening of viral diseases had been reported, but no clinical protective effect. The ACE2 receptor hypothesis has been strongly advocated, but recent studies,18,19 in contradiction with older data,24 suggest a greater expression of these receptors in smokers. This would be expected to result in an increased risk of contracting SARS-CoV2 infection in smokers, as well as in women who have more ACE2 receptors than men. Neither of these is observed. Two other hypotheses have been proposed, but actually, none of them give a global explanation for the lower rate of Covid-19 in smokers: the first hypothesis supposes a global effect on the renin-angiotensin system of which ACE2 is only part of, by up-regulating the ACE1 receptor and down-regulating the ACE2 receptor.24 The second hypothesis involves an interaction between ACE2 and the nicotinic receptors (in particular α7 nicotine receptors), which is very close on the cell membrane to the ACE2 receptors and whose dysregulation could trigger a Th1 immune response.16 Nicotin and SARS-Cov2 could also compete for binding to nAchR.16,17
The key strength of this study is that virtually the whole population was included, so smoking type did not affect the likelihood of being tested for Covid-19 (no selection bias). We were also able to investigate dose-response in relation to current intensity of smoking.
The generalizability of these findings of this study is uncertain. For example, results from this young and healthy population may not apply in a more to the general (older and with more co-morbidities) population. However, our findings broadly align with previously identified risk factors for SARS-CoV-2 infection or protection against Covid-19 (age and smoking status). The clinical presentation of cases was also consistent with current literature.
We were unable to account for the impact of onboard lifestyle or the degree of social interactions on the spread of the infection because this is the subject of a more extensive epidemiologic investigation still in progress. This could potentially be a confounding factor, if, for example, smokers have a lower degree of interaction with other crew members. However, we found that all areas of the ship were affected, which is consistent with the very confined space on board. It is therefore unlikely that taking this factor into account would modify the results on the impact of smoking.
The retrospective nature of the study is also a limitation, although the study was conducted when the outbreak was still in progress when all the crewmembers were still gathered. We paid particular attention to the reliability of the information collected by assigning medical staff to data collection. A tobacco consumption rate of 50% in the French Navy was reported previously, thus our data are consistent.26
The quantification of smoking was difficult because most very young patients had been smoking for a short time and very irregularly, with notable differences between onboard and on-shore periods. So we were unable to assess dose-response in relation to pack years of smoking. Given the fact that only two patients were exclusive e-cigarette smokers, no conclusion can be drawn concerning the association between vaping and Covid-19 infection.
Conclusions
The results of this study support, in accordance with some previous findings, that smoking is associated with a lower susceptibility to SARS-CoV-2 infection. However, 71% of smokers developed Covid-19, so it seems unreasonable to say that smoking strongly protects against Covid-19 infection in this population. The finding of a lower odds of infection among smokers and the trend towards a lower risk among e-cigarettes users, although not significant, encourages further research on nicotine’s physiologic pathway. Further studies also have to be conducted to specify mechanisms that could underlie this relationship, particularly socializing habits and smoker’s lifestyles, which could affect interhuman SARS-CoV-2 transmission. Moreover, any hypothetical “protective” effect of smoking has to be put in context with the high tobacco burden worldwide. Smoking certainly should not be recommended as a public health measure.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Appendix
Study group members’ full names: Damien Pascaud, M.D, Jean-Baptiste Morvan, M.D., Olivier Cathelinaud, M.D., Loraine Vatin, M.D., François Le Saos, M.D., Lucie Fabry, Emma Neny, Jean-Baptiste Quilly, Lucie Quevedo, Camille Louis, Cyrille Brochard, Tanguy Chapeaux, Amaury Jouvencel, Valentin Raud, Chiara Aicardi, Justine Defrance, Camille Charruau, Corinne Dachary, Céline Fernandez, Bernard Valero, M.D., Ludivine Gan, M.D., Marie Desjardins, M.D., Aude Valois, M.D., Christelle Lavagna, Ph.D.
From the Head and Neck Unit, HIA Sainte-Anne, Toulon, (D.P., J.-B.M., O.C.); Aeronaval Medical Center, Landivisiau (L.V., F.L.S.); French Military Medical School (ESA) students, Lyon (F.L., E.N., J.-B.Q., L.Q., Ca.L., C.B., T.C., A.J., V.R., C.A.); Clinical Research Unit Registered Nurses, HIA Sainte-Anne, Toulon (J.D., C.C., C.D., C.F.,); Ophthalmology Unit, HIA Sainte-Anne, Toulon (B.V.); Hepato-Gastro-Enterology Unit, HIA Sainte-Anne, Toulon (L.G., M.D.); Dermatology Unit, HIA Sainte-Anne, Toulon (A.V.); French Military Center for Epidemiology and Public Health (CESPA), Marseille, (Ch.L.)—all in France.
Declaration of Interests
None declared.
Authors Contributions
Conceptualization: NP, AM, OB, BD; Data curation: NP, VM, AP, HB, FXB, FJ, OB; writing original draft: NP, OB, BD; statistical analysis: NP, OB. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. The main author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that there was no discrepancy from the study as originally.
Data Availability
We commit to making the relevant anonymized patient-level data available on reasonable request, especially concerning RT-PCR results, smoking, and demographic status.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in china. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontanet A, Tondeur L, Madec Y, et al. Cluster of COVID-19 in northern France: A retrospective closed cohort study (submitted). https://www.medrxiv.org/content/10.1101/2020.04.18.20071134v1.
- 3. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 15(5):e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Inf Dis. 2020. doi: 10.1016/s1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID-19. New Engl J Med. 2020. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Miyara M, Tubach F, Pourcher V, et al. Low incidence of daily current tobacco smoking in patients with symptomatic COVID-19. (submitted) https://www.qeios.com/read/WPP19W.3.
- 7. Farsalinos K, Barbouni À, Niaura R. Smoking, vaping and hospitalization for COVID-19. (submitted) https://www.qeios.com/read/Z69O8A.8.
- 8. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: A systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 11. Preliminary estimates of the prevalence of selected underlying health condition among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 13. Rocklöv J, Sjödin H, Wilder-Smith A. COVID-19 outbreak on the diamond princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. J Travel Med. 2020. doi: 10.1093/jtm/taaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://www.nbcsandiego.com/news/local/uss-theodore-roosevelt-COVID-19-cases-exceed-1100-navy-to-decrease-reporting/2316749/.
- 15. Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of Covid-19: a systematic review and meta analysis. J Med Virol. 2020. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Changeux JP, Amoura Z, Rey FA, Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Académie des sciences-Comptes Rendus. Biologies####-2020;343(preprint)DOI::33105802/crbiol8 10.5802/crbiol.8 [DOI] [PubMed] [Google Scholar]
- 17. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. [DOI] [PubMed] [Google Scholar]
- 21. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking. Nicotine Tobacco Res. 2020. (in press). doi: 10.1093/ntr/ntaa059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai G. Tobacco-use disparity in gene expression of ACE2, the receptor of 2019-nCov. Preprints 2020:202002005. doi: 10.20944/preprints202002.0051.v1. [DOI] [Google Scholar]
- 23. Wang J, Luo Q, Chen R, Chen T, Li J. Susceptibility analysis of COVID-19 in smokers based on ACE2. Preprints 2020:2020030078. doi: 10.20944/preprints202003.0078.v1. [DOI] [Google Scholar]
- 24. Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R895–R906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. [DOI] [PubMed] [Google Scholar]
- 26. Mayet A, Marimoutou C, Haus-Cheymol R, et al. Etat des lieux des conduites addictives dans les armées françaises: Une méta-analyse des enquêtes de prévalence conduites entre 2005 et 2009. Médecine et Armées 2014;42:113–122. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We commit to making the relevant anonymized patient-level data available on reasonable request, especially concerning RT-PCR results, smoking, and demographic status.