Abstract
Aims
Our objective was to determine the ventricular arrhythmia burden in implantable cardioverter-defibrillator (ICD) patients during COVID-19.
Methods and results
In this multicentre, observational, cohort study over a 100-day period during the COVID-19 pandemic in the USA, we assessed ventricular arrhythmias in ICD patients from 20 centres in 13 states, via remote monitoring. Comparison was via a 100-day control period (late 2019) and seasonal control period (early 2019). The primary outcome was the impact of COVID-19 on ventricular arrhythmia burden. The secondary outcome was correlation with COVID-19 incidence. During the COVID-19 period, 5963 ICD patients underwent remote monitoring, with 16 942 episodes of treated ventricular arrhythmias (2.8 events per 100 patient-days). Ventricular arrhythmia burden progressively declined during COVID-19 (P < 0.001). The proportion of patients with ventricular arrhythmias amongst the high COVID-19 incidence states was significantly reduced compared with those in low incidence states [odds ratio 0.61, 95% confidence interval (CI) 0.54–0.69, P < 0.001]. Comparing patients remotely monitored during both COVID-19 and control periods (n = 2458), significantly fewer ventricular arrhythmias occurred during COVID-19 [incident rate ratio (IRR) 0.68, 95% CI 0.58–0.79, P < 0.001]. This difference persisted when comparing the 1719 patients monitored during both the COVID-19 and seasonal control periods (IRR 0.69, 95% CI 0.56–0.85, P < 0.001).
Conclusions
During COVID-19, there was a 32% reduction in ventricular arrhythmias needing device therapies, coinciding with measures of social isolation. There was a 39% reduction in the proportion of patients with ventricular arrhythmias in states with higher COVID-19 incidence. These findings highlight the potential role of real-life stressors in ventricular arrhythmia burden in individuals with ICDs.
Trial registration
Australian New Zealand Clinical Trial Registry; URL: https://www.anzctr.org.au/; Unique Identifier: ACTRN12620000641998
Keywords: COVID-19, Implantable cardioverter-defibrillator, Ventricular arrhythmia, Ventricular tachycardia, Ventricular fibrillation, Remote monitoring
Graphical Abstract
Introduction
The coronavirus disease 2019 (COVID-19) was identified in Wuhan, China in December 2019,1 with the first case of COVID-19 in the USA confirmed on 21 January 2020.2 A surge in cases in the USA ensued, followed by implementation of various public health interventions designed to reduce viral transmission, including social distancing, closures of schools and non-essential business, stay-at-home orders, and—in some cases—quarantine.3 To date, the COVID-19 pandemic has infected >5 million people and claimed >160 000 lives in the USA as of mid-August 2020.4 In addition, the pandemic has affected the day-to-day lives of millions, through a combination of the effects of enforced social distancing and lockdown, a surge in unemployment, larger economic impacts, and the methods by which healthcare is accessed.
Ventricular arrhythmias (VAs) are more likely to occur in settings associated with increased sympathetic tone, including physical activity, illness, and emotional distress.5 Historically, we have seen significant world events coinciding with a substantial rise in myocardial infarctions (MIs) as well as VAs and the need for implantable cardioverter-defibrillator (ICD) therapies.6–11 The health consequences of such events may be long lasting, with documented increases in MIs up to 3 years following earthquakes and tsunamis.12 Interestingly, a decline in hospitalization for cardiovascular-related illness during COVID-19 has been documented in multiple countries, seemingly attributable to fewer acute coronary syndrome (ACS) presentations.13–17
Patients with an ICD are at greater risk of VAs and sudden cardiac death. We hypothesized that the COVID-19 period would be associated with a greater burden of VAs. We sought to apply remote monitoring technology to assess the impact of the coronavirus pandemic on VA burden in ICD patients.
Methods
Study cohort and data source
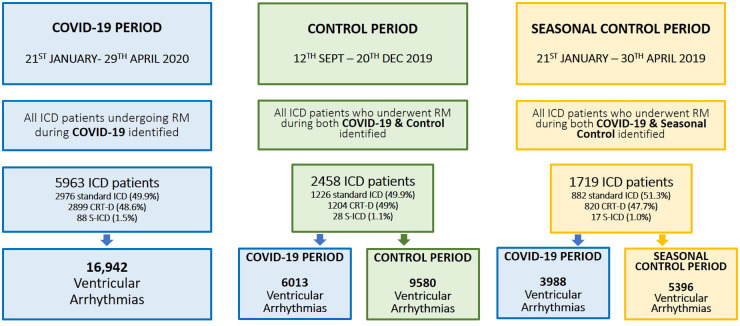
This is a multicentre cohort study. The study cohort included all patients with an ICD receiving remote monitoring via PaceMate™, a vendor-neutral service providing monitoring to a broad outpatient demographic, inclusive of hospital-based and community-based device clinics, across multiple states in the USA. This was an investigator-initiated study with data obtained from PaceMate LIVE™, a software system with automatic integration of all remote monitoring transmissions and alerts from multiple device vendor platforms, streamlined into a single user interface. The study evaluated all patients receiving remote monitoring during the 100 days following identification of the first COVID-19 case in the USA (COVID-19 period: 21 January to 29 April 2020 inclusive).
The study was reviewed and approved by the Human Research Ethics Committees of the Royal Adelaide Hospital and the University of Adelaide, Adelaide, Australia. The study was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12620000641998).
Control groups
For comparison, two further 100-day periods in 2019 were included. The first was for determining the direct impact of the COVID-19 period and used a 100-day period in late 2019 (control period: 12 September to 20 December 2019 inclusive, after excluding the Christmas period). The second was to control for potential seasonal variations in VA burden and used the same period in 2019 (seasonal control period: 21 January to 30 April 2019 inclusive).
Study design
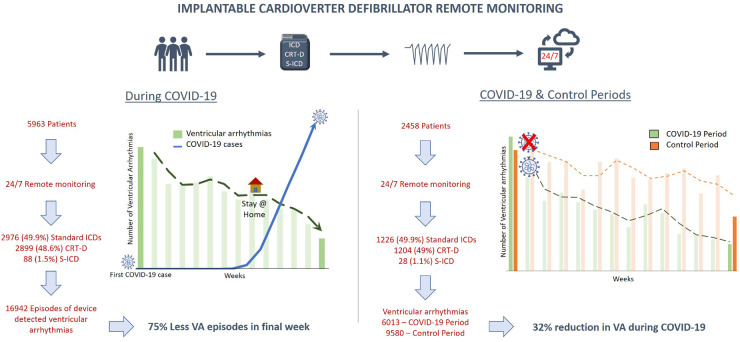
Analysis of the primary study cohort was designed to demonstrate the burden of VAs during the COVID-19 pandemic, and to compare VA burden between states according to COVID-19 incidence. All patients with an ICD [standard ICD, cardiac re-synchronization therapy with defibrillator (CRT-D), or subcutaneous defibrillator (S-ICD)] who received remote monitoring during the COVID-19 period, following the first confirmed case of COVID-19 in the USA, were included in the analysis (n = 5963; Figure 1). Patients who transmitted at least one arrhythmia episode for VA [ventricular fibrillation (VF) or ventricular tachycardia (VT)] needing device therapy were identified.
Figure 1.
CONSORT figure demonstrating the breakdown of the remote monitoring population and VA episodes in each period: COVID-19 period, control period, and seasonal control period.
Episode classification occurred according to the arrhythmia adjudication algorithms of each defibrillator model, combined with programmed arrhythmia detection thresholds at the treating physician’s discretion. All current ICDs monitored by PaceMate transmit automatically if alert thresholds are met, and thus, depending on alert programming, VA episodes were automatically transmitted, providing that the patient’s remote monitor was connected. Further, all ICDs were programmed for a scheduled download every 30–90 days (variable time frame as per physician preference), with a standard follow-up via phone call for any patient not transmitting according to their schedule, to ensure compliance. Upon receipt, all ICD alerts and transmissions were adjudicated by cardiac device specialists certified by the International Board of Heart Rhythm Examiners. The VA burden was determined across all monitoring sites, divided into their respective states, and correlated with per state COVID-19 incidence.
Analysis of the secondary study cohort was designed to determine the impact of the pandemic on VA occurrence, by assessment of VA burden during the COVID-19 period in comparison with a 100-day period in the months prior to the pandemic (control period, late 2019). The secondary cohort included all patients with an ICD who received remote monitoring during both the COVID-19 period and the control period (n = 2458; Figure 1), allowing for a paired analysis with direct comparison. Analysis of the tertiary cohort was designed to determine the presence of significant seasonal variations, by assessment of VA burden during the COVID-19 period in comparison with the identical 100-day period 1 year previously (seasonal control period, early 2019). Again, to allow direct comparison, only patients who underwent remote monitoring during both the COVID-19 period and the seasonal control period (n = 1719; Figure 1) were included in this analysis.
COVID-19 infection incidence
The 100-day COVID-19 incidence per 10 000 people per state was obtained via COVID-19 case data from the United States Centers for Disease Control and Prevention website2 and population data from the United States Census Bureau.18 The number of infections per state as at 29 April (end of the 100-day COVID-19 study monitoring period) was recorded. For the purposes of our analysis, states were classified as either high COVID-19 incidence (≥15 COVID cases per 10 000) or low COVID-19 incidence (<15 COVID cases per 10 000).
Study outcomes
The primary outcomes of the study were the VA burden in ICD patients during the COVID-19 pandemic, and the impact of the pandemic on VA burden, as determined by comparison with two time periods in the preceding year. The secondary outcome was the association of VA burden with COVID-19 infection incidence per state.
Statistical analysis
The summary statistics are presented as frequencies and percentages, and continuous variables as mean [standard deviation (SD)] or median [interquartile range (IQR)]. Paired data regarding VAs from patients undergoing monitoring in both the COVID-19 period and control periods were analysed using McNemar’s χ2 test. We used univariable and multivariable negative binomial regression analyses to compare the incidence of VA episodes adjusted for patient age and geographical location (US state) to derive incident rate ratios (IRRs) with 95% confidence intervals (CIs). The state with the lowest COVID-19 incidence was considered as the reference group to estimate the IRR for primary events. We evaluated the association between incidence of VAs and COVID-19 incidence per state using logistic regression and reported odd ratios (ORs) with 95% CIs. A two-sided P-value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata 16 (StataCorp LLC, TX, USA).
Results
Study population
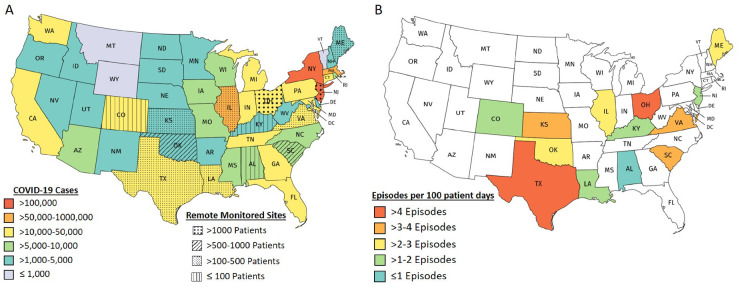
During the COVID-19 period, 5963 ICD patients, representing 20 centres from 13 states, were being remotely monitored via PaceMate™. The cohort included 2976 (49.9%) standard ICDs, 2899 (48.6%) CRT-Ds, and 88 (1.5%) S-ICDs (Figure 1). The average patient age was 69 ± 12 years. Details of the number of patients monitored per state and device types are provided in Figure 2 and in the Supplementary material online, Tables S1 and S2.
Figure 2.
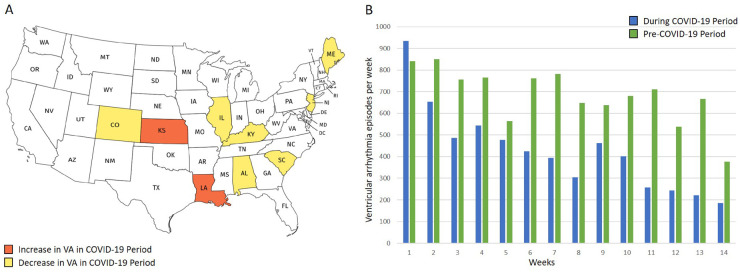
(A) The map of the USA colour coded for the prevalence of COVID-19 infection on 29 April 2020. Also shown are the states with centres undertaking remote monitoring services through PaceMate™. The figure is colour coded to show the number of patients being monitored with implantable cardioverter-defibrillators suitable for the study population. (B) The number of VAs per patient being monitored during the COVID-19 period in the various states. Note the relatively reduced VA burden in regions with a high prevalence of COVID-19 infection.
Of the 5963 patients receiving remote monitoring during the COVID-19 period, we identified all ICD patients who had also undergone remote monitoring during the control period (n = 2458), and included them in the analysis determining the impact of COVID-19 on VA burden. These 2458 patients represented 15 centres in nine states. The cohort consisted of 1226 (49.9%) standard ICDs, 1204 (49.0%) CRT-Ds, and 28 (1.1%) S-ICDs (Figure 1). The average patient age was 69 ± 12 years.
Of the 5963 patients monitored during the COVID-19 period, we identified all patients who had also undergone remote monitoring during the seasonal control period (n = 1719) and included them in the analysis to determine the presence of significant seasonal variation. These 1719 patients represented 14 centres in eight states. The cohort consisted of 882 (51.3%) standard ICDs, 820 (47.7%) CRT-Ds, and 17 (1.0%) S-ICDs (Figure 1). The average patient age was 68 ± 12 years.
COVID-19 burden per state
The number of confirmed COVID-19 cases per monitored state,4 state population, and 100-day COVID-19 incidence per state as at 29 April 2020 is detailed in Figure 2 and Supplementary material online, Figure S1 and Table S3).
Ventricular arrhythmia burden during COVID-19
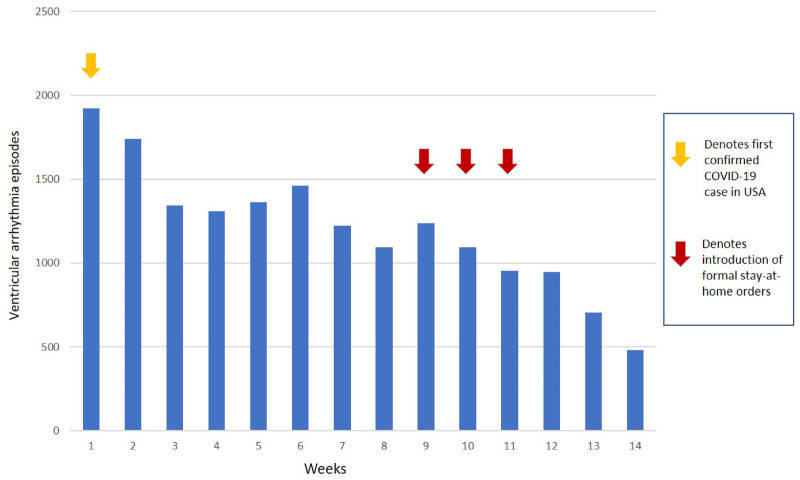
During the COVID-19 period, there were 16 942 VA episodes needing device therapies (Figure 1). This translated to 2.8 episodes per 100 patient-days. Figure 2B and Supplementary material online Figure S2 and Table S4 provide details regarding episodes per state. The COVID-19 period was divided into 14 distinct weeks and demonstrated a significant progressive reduction in events during the 100-day time frame (P < 0.001). During the first week, 1923 (11.4%) VA episodes were transmitted, reducing to 482 (2.8%) episodes in the final week (Figure 3). Implementation dates of governor-issued stay-at-home orders per state were noted. The lowest VA episode rates followed application of stay-at-home orders, which occurred in weeks 9–11, depending on the individual state19 (Figures 3 and 4B; Supplementary material online, Figures S3 and S4). There were no programming changes for VF or VT detection in 99.2% and 95%, respectively, during the COVID-19 period (Supplementary material online, Appendix).
Figure 3.
The weekly breakdown of VA burden requiring device therapies from the first detected case of COVID-19 infection in the USA. While various degrees of social lockdown were initiated, the red arrows highlight the weeks in which lockdown was mandated.
Figure 4.
(A) A map of the USA colour coded to denote which states saw an increase vs. a decrease in VAs during COVID-19, compared with the control period. (B) The number of VA episodes per week, before (control period: green) and during (COVID-19 period: blue) COVID-19. The figure demonstrates the natural incidental decline and incline in VA burden in the control period and highlights the markedly reducing burden of VAs during the COVID-19 period with social lockdown strategies.
Impact of COVID-19 on ventricular arrhythmia burden
VAs transmitted by the 2458 patients remotely monitored during both the COVID-19 period and the control period were compared. During the COVID-19 period, 572 (23.3%) patients experienced 6013 VA episodes, while during the control period, 549 (22.3%) patients were responsible for 9580 VA episodes (Figure 4; Supplementary material online, Figure S5 and Table S5). The number of patients who experienced an arrhythmia episode during the COVID-19 and control periods did not significantly differ (OR 0.88, 95% CI 0.71–1.09, P = 0.24). Multivariable negative binomial regression analysis, adjusted for patient age and state, demonstrated significantly fewer VA events during the COVID-19 period compared with the control period (IRR 0.68, 95% CI 0.58–0.79, P < 0.001; Supplementary material online, Table S6). There were no changes for VF or VT detection in 99.2% and 93.8%, respectively, that occurred in the secondary cohort during both the COVID-19 and the control period (Supplementary material online, Appendix).
Impact of 100-day COVID-19 incidence per state on ventricular arrhythmia burden
Of the 13 states with centres undergoing remote monitoring during both the COVID-19 period and the control period, 6 were classified as high COVID incidence (≥15 cases per 10 000 population: Colorado, Illinois, Louisiana, Maine, New Jersey, and Virginia) and 7 were classified as low COVID-19 incidence (<15 cases per 10 000 population: Alabama, Kansas, Kentucky, Ohio, Oklahoma, South Carolina, and Texas). States with high-COVID-19 incidence saw a 39% reduction in the proportion of patients with VAs during the COVID-19 period, compared with low-COVID-19 incidence states (OR 0.61, 95% CI 0.54–0.69, P < 0.001; Figure 4; Supplementary material online, Figure S5).
Ventricular arrhythmia burden during COVID-19 compared with seasonal controls
VAs transmitted by the 1719 patients remotely monitored during both the COVID-19 period and the seasonal control period were compared. During the COVID-19 period, 349 (20.3%) patients experienced 3988 VA episodes, while during the seasonal control period, 331 (19.3%) patients were responsible for 5396 VA episodes (Supplementary material online, Table S7). The number of patients who experienced an arrhythmia episode in each of the COVID-19 and seasonal control periods did not significantly differ (OR 0.87, 95% CI 0.67–1.12, P = 0.28). Multivariable negative binomial regression analysis, adjusted for patient age and state, demonstrated significantly fewer VA events during the COVID-19 period compared with the seasonal control period (IRR 0.69, 95% CI 0.56–0.85, P < 0.001; Supplementary material online, Table S7).
Discussion
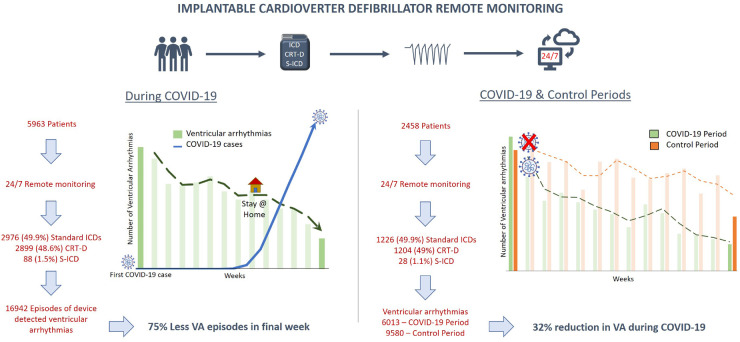
This study identifies a 32% reduction in the burden of VA needing device therapies in ICD patients during the COVID-19 pandemic. In states with a high COVID-19 incidence, 39% fewer patients were affected by VAs. In addition, there was a progressive decline in VAs following implementation of lockdown measures, suggestive of an association between VAs and societal adaptations in response to the pandemic (Take home figure).
Take home figure.
The left image demonstrates the dramatic reduction in the VA burden with stricter lockdown measures for COVID-19. The right image demonstrates the results of the cohort monitored during both COVID-19 and the control periods. This demonstrates the markedly reduced episodes of VA during COVID-19
Not for over a century have we experienced a pandemic of such magnitude as the novel coronavirus.20 In the past, a correlation between momentous world events, including natural disasters across multiple continents, and sudden death/cardiac arrhythmias has been identified,6,9–11,21,22 most probably as a result of increased sympathetic output during such crises. We hypothesized that COVID-19 would similarly be associated with a rise in VAs, in the context of stress related to economic circumstances, social isolation, and fear of COVID-19 infection. Though we may expect to see a similar phenomenon during such a pandemic, the nature of our current predicament is distinct. Unlike a natural disaster, which is finite in duration, the pandemic has swept the globe and continued to spread for >7 months. In the USA, it is associated with extensive lockdown measures, social distancing, and record job losses, all of which have resulted in many people largely remaining in their homes. The daily routines of millions of Americans have been drastically altered since COVID-19 first surfaced in Washington state in late January 2020.2 Although the challenges faced during this time have been rather uncharted in terms of VAs, there are various potential explanations for the decline in VAs during COVID-19.
The COVID-19 pandemic is associated with record unemployment figures and temporary closure of many businesses. A rise in working from home arrangements for non-essential workers who remain employed has been observed. A resultant reduction in work/occupational stressors may impact VA incidence. In one previous study assessing septadian trends in VT and VF in patients with an ICD in situ, VT events were most likely to occur on a Monday, suggestive of a potential relationship between VAs and the working week.23
The link between physical stress and VAs is well established. Various studies have established a correlation between physical stress 5,24,25 and risk of VAs. A trend of increased sedentary behaviour has emerged during the pandemic, as people are largely confined to their homes and in many cases prohibited from attending exercise venues such as gyms and sports centres. Two studies observed a reduction in physical activity during lockdown in almost half (48.6% and 47.9%, respectively) of participants.26,27 Another study of >1000 survey respondents documented a 28% increase in sitting time during the pandemic.28 A reduction in physical activity may partially contribute to fewer VAs in our ICD cohort.
With the COVID-19 pandemic has come cancellation of most sporting events across the country, which may have some impact on VA incidence. Watching a stressful sporting match has been correlated with increased arrhythmia burden in multiple studies. Katz et al. noted an increase in sudden cardiac death rates in Swiss men during both the 2002 and 2008 football World Cups.30,31 Wilbert et al. described a three-fold increase in serious arrhythmia presentations in Munich while Germany was hosting the 2006 football World Cup; on the days on which the German team played a match, cardiovascular hospital presentations were especially high.32 A similar effect was seen in patients with an ICD or pacemaker during the 2011 Baseball World Series in Missouri. Arrhythmia rates were significantly higher compared with the period prior to the playoffs; this difference was driven by a greater number of VAs.33 Fewer sporting matches of consequence may be a contributor to the results in our cohort.
ICD patients, many with an associated cardiomyopathy, often have strict regimens consisting of specific pharmacotherapy, fluid restrictions, and salt restrictions. One study of heart failure patients with an ICD in situ demonstrated a positive association between a rise in intrathoracic impedance reflecting fluid overload, and risk of VAs.34 A combination of time spent at home during lockdown without the usual daily distractions of social and work commitments, as well as restaurant closures reducing opportunity for dietary indiscretion, may translate to improved adherence to prescribed medication, dietary and lifestyle treatments in such patients, reducing the risk of cardiac decompensation and resultant arrhythmias.
As approaches to healthcare delivery are tailored to involve less in-person patient–physician contact during COVID-19, the rise in telehealth-based medicine may, for some patients, enhance outpatient care accessibility and improve attendance for clinical review. The postponement of elective procedures during the pandemic may allow electrophysiologists more time to dedicate to the outpatient and remote monitoring transmission review processes, allowing for timely intervention to prevent imminent VAs in patients exhibiting warning signs of cardiac decompensation.
Data from various European countries and US states have identified a reduction in the burden of acute coronary syndrome (ACS)-related hospital presentations. A French percutaneous coronary intervention registry identified an 18% reduction in admissions for ST-elevation myocardial infarctions (STEMIs) immediately following implementation of national lockdown restrictions in France.17 Similarly, in Northern Italy, De Filippo et al. described a significant fall of 21% in rates of ACS-related hospitalization following the first confirmed case of COVID-19 in Italy. Comparison with the same time period 1 year earlier confirmed the effect to be independent of seasonal variation.16 In Northern California, a dramatic reduction in weekly acute MI hospitalizations, of up to 48%, was identified after the first COVID-19 death in the region.15 Similarly, Garcia et al. detected a 38% reduction in monthly STEMI activations across nine USA centres during COVID-19, compared with the previous 14 months.14 Whether these trends reflect an actual decrease in ACS incidence, or patient reluctance to present to hospital in the COVID-19 climate, is unclear. If ACS incidence has indeed fallen, this could have potential links to VA incidence, as coronary ischaemia can precipitate such arrhythmias.
Limitations
The data for this study have been obtained from a real-world remote monitoring registry. While this provides details of the VA and device parameters, details of clinical characteristics, pharmacological therapies and other treatments, and healthcare utilization are not available. In addition, we do not have information regarding individual behavioural changes around physical activity, eating habits, and working from home arrangements, that were adopted by individuals included in this analysis, or their exposure to COVID-19 infection. The infection rates may not represent the true infection rates of each state; however, we have used the data available from the United States Centers for Disease Control and Prevention website at the date to ensure the best representation of known infection rates within states.
Conclusions
The COVID-19 pandemic has been associated with a marked reduction in VAs requiring device therapies. The VA burden was most dramatically reduced in states with a greater prevalence of COVID-19 infection, suggesting a potential relationship to the degree of social lockdown. This was further supported by the progressive reduction in VA burden with duration of social lockdown. These data implicate a potential role for real-life stressors in VA burden in individuals with an ICD.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
Study concept and design: C.J.O., C.H., K.C., and P.S. Statistical analysis: G.T. Drafting of the manuscript: C.J.O., M.E.M., D.H.L., and P.S. Critical revision of the manuscript for important intellectual content: C.J.O., G.T., M.E.M., C.H., A.D.E., D.H.L., N.R., K.C., and P.S.
Funding
This study was supported by the Centre for Heart Rhythm Disorders at the University of Adelaide, Adelaide, Australia. Several of the authors are employees or students of the University of Adelaide. The sponsor has had no direct involvement in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. C.J.O. and M.E.M. are supported by a Postgraduate Scholarship from the University of Adelaide. D.H.L. is supported by a mid-career Fellowship from The Hospital Research Fund. P.S. is supported by a Practitioner Fellowship from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia.
Conflict of interest: C.H., N.R., and K.C. are employees of PaceMate. D.H.L. reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Abbott Medical, Bayer, Biotronik, Boehringer Ingelheim, Medtronic, Microport, and Pfizer/BMS. P.S. reports having served on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx, and PaceMate; that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott Medical, and Boston Scientific; and that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott Medical, Boston Scientific, and Microport. All other authors report no conflicts.
Supplementary Material
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. CDC, Washington State Report First COVID-19 Death. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 3.Hartley DM, Perencevich EN.. Public health interventions for COVID-19: emerging evidence and implications for an evolving public health crisis. JAMA 2020;323:1908–1909. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Coronavirus Disease 2019 (COVID-19): Cases, Data, & Surveillance. Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 5.Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D.. Emotional and physical precipitants of ventricular arrhythmia. Circulation 2002;106:1800–1805. [DOI] [PubMed] [Google Scholar]
- 6.Shedd OL, Sears SF Jr, Harvill JL, Arshad A, Conti JB, Steinberg JS, Curtis AB.. The World Trade Center attack: increased frequency of defibrillator shocks for ventricular arrhythmias in patients living remotely from New York City. J Am Coll Cardiol 2004;44:1265–1267. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Yamada S, Kamiyama Y, Takeishi Y.. Efficacy of intrathoracic impedance and remote monitoring in patients with an implantable device after the 2011 great East Japan earthquake. Int Heart J 2014;55:53–57. [DOI] [PubMed] [Google Scholar]
- 8.Ranjbar F, Akbarzadeh F, Kazemi B, Ranjbar A, Sharifi Namin S, Sadeghi-Bazargani H.. Increased likelihood of arrhythmic events associated with increased anxiety in patients with implanted cardiac defibrillators after the Ahar-Varzegan Earthquake in East Azarbaijan, 2012. Bull Emerg Trauma 2016;4:202–210. [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, David D.. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet 1991;338:660–661. [DOI] [PubMed] [Google Scholar]
- 10.Leor J, Poole WK, Kloner RA.. Sudden cardiac death triggered by an earthquake. N Engl J Med 1996;334:413–419. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg JS, Arshad A, Kowalski M, Kukar A, Suma V, Vloka M, Ehlert F, Herweg B, Donnelly J, Philip J, Reed G, Rozanski A.. Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. J Am Coll Cardiol 2004;44:1261–1264. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Tanaka K, Tanaka F, Matsuura Y, Komi R, Niiyama M, Kawakami M, Koeda Y, Sakai T, Onoda T, Itoh T, Northern Iwate Heart Registry Consortium. Long-term effects of the 2011 Japan earthquake and tsunami on incidence of fatal and nonfatal myocardial infarction. Am J Cardiol 2017;120:352–358. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa S, Spaccarotella C, Basso C, Calabro MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C, Società Italiana di Cardiologia and the CCU Academy investigators group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD.. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020;75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung SH, Ambrosy AP, Sidney S, Go AS.. The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med 2020;383:691–693. [DOI] [PubMed] [Google Scholar]
- 16.De Filippo O, D’Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A, Secco GG, Campo G, Gallone G, Verardi R, Gaido L, Iannaccone M, Galvani M, Ugo F, Barbero U, Infantino V, Olivotti L, Mennuni M, Gili S, Infusino F, Vercellino M, Zucchetti O, Casella G, Giammaria M, Boccuzzi G, Tolomeo P, Doronzo B, Senatore G, Grosso Marra W, Rognoni A, Trabattoni D, Franchin L, Borin A, Bruno F, Galluzzo A, Gambino A, Nicolino A, Truffa Giachet A, Sardella G, Fedele F, Monticone S, Montefusco A, Omede P, Pennone M, Patti G, Mancone M, De Ferrari GM.. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in Northern Italy. N Engl J Med 2020;383:88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Range G, Hakim R, Motreff P.. Where have the STEMIs gone during COVID-19 lockdown? Eur Heart J Qual Care Clin Outcomes 2020;6:223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Census Bureau. US Census Data; 2020.
- 19.Mervosh S, Lu D, Swales V.. See which states and cities have told residents to stay at home. The New York Times 2020. [Google Scholar]
- 20.Dicke T. Waiting for the flu: cognitive inertia and the Spanish influenza pandemic of 1918–19. J Hist Med Allied Sci 2015;70:195–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kario K, Matsuo T, Kobayashi H, Yamamoto K, Shimada K.. Earthquake-induced potentiation of acute risk factors in hypertensive elderly patients: possible triggering of cardiovascular events after a major earthquake. J Am Coll Cardiol 1997;29:926–933. [DOI] [PubMed] [Google Scholar]
- 22.Trichopoulos D, Katsouyanni K, Zavitsanos X, Tzonou A, Dalla-Vorgia P.. Psychological stress and fatal heart attack: the Athens (1981) earthquake natural experiment. Lancet 1983;1(8322):441–444. [DOI] [PubMed] [Google Scholar]
- 23.Peters RW, McQuillan S, Resnick SK, Gold MR.. Increased Monday incidence of life-threatening ventricular arrhythmias. Experience with a third-generation implantable defibrillator. Circulation 1996;94:1346–1349. [DOI] [PubMed] [Google Scholar]
- 24.Fries R, Konig J, Schafers HJ, Bohm M.. Triggering effect of physical and mental stress on spontaneous ventricular tachyarrhythmias in patients with implantable cardioverter-defibrillators. Clin Cardiol 2002;25:474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siscovick DS, Weiss NS, Fletcher RH, Lasky T.. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med 1984;311:874–877. [DOI] [PubMed] [Google Scholar]
- 26.Galle F, Sabella EA, Da Molin G, De Giglio O, Caggiano G, Di Onofrio V, Ferracuti S, Montagna MT, Liguori G, Orsi GB, Napoli C.. Understanding knowledge and behaviors related to CoViD-19 epidemic in Italian undergraduate students: the EPICO Study. Int J Environ Res Public Health 2020;17:3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almandoz JP, Xie L, Schellinger JN, Mathew MS, Gazda C, Ofori A, Kukreja S, Messiah SE.. Impact of COVID-19 stay-at-home orders on weight-related behaviours among patients with obesity. Clin Obes 2020;10:e12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, Bouaziz B, Bentlage E, How D, Ahmed M, Muller P, Muller N, Aloui A, Hammouda O, Paineiras-Domingos LL, Braakman-Jansen A, Wrede C, Bastoni S, Pernambuco CS, Mataruna L, Taheri M, Irandoust K, Khacharem A, Bragazzi NL, Chamari K, Glenn JM, Bott NT, Gargouri F, Chaari L, Batatia H, Ali GM, Abdelkarim O, Jarraya M, Abed KE, Souissi N, Van Gemert-Pijnen L, Riemann BL, Riemann L, Moalla W, Gomez-Raja J, Epstein M, Sanderman R, Schulz SV, Jerg A, Al-Horani R, Mansi T, Jmail M, Barbosa F, Ferreira-Santos F, Simunic B, Pisot R, Gaggioli A, Bailey SJ, Steinacker JM, Driss T, Hoekelmann A.. Effects of COVID-19 home confinement on eating behaviour and physical activity: results of the ECLB-COVID19 International Online Survey. Nutrients 2020;12:1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasso S. China’s divorce spike is a warning to rest of locked-down world. Bloomberg Businessweek 2020. [Google Scholar]
- 30.Katz E, Metzger JT, Schlaepfer J, Fromer M, Fishman D, Mayer L, Niquille M, Kappenberger L.. Increase of out-of-hospital cardiac arrests in the male population of the French speaking provinces of Switzerland during the 1998 FIFA World Cup. Heart 2005;91:1096–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz E, Metzger JT, Marazzi A, Kappenberger L.. Increase of sudden cardiac deaths in Switzerland during the 2002 FIFA World Cup. Int J Cardiol 2006;107:132–133. [DOI] [PubMed] [Google Scholar]
- 32.Wilbert-Lampen U, Leistner D, Greven S, Pohl T, Sper S, Volker C, Guthlin D, Plasse A, Knez A, Kuchenhoff H, Steinbeck G.. Cardiovascular events during World Cup soccer. N Engl J Med 2008;358:475–483. [DOI] [PubMed] [Google Scholar]
- 33.Stolker JM, Aziz Z, Kennedy KF, Hauptman PJ.. Electrical triggers of adverse events during the Baseball World Series. Am J Cardiol 2018;122:1805–1806. [DOI] [PubMed] [Google Scholar]
- 34.Osman M, Ahmed A, Alzubi H, Kheiri B, Osman K, Barbarawi M, Rios-Bedoya CF, Bachuwa G, Hassan M.. Association between changes in the intrathoracic impedance and ventricular arrhythmias in patients with heart failure. Pacing Clin Electrophysiol 2018;41:1577–1582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.