Abstract
Background
The agricultural food products industry in Bangladesh depends on utilizing antimicrobials indiscriminately as growth promoters and for controlling infectious diseases. Thus, there is always a risk of antimicrobial agent accumulation in food sources that originate from agricultural production.
Methods
In the present study, we collected data from published articles between January, 2013 and December, 2019 on antimicrobial residues in human food sources such as meat, milk, eggs, and fishes.
Results
Liver contained the highest percentage of antimicrobial residues (74%; 95% CI: 59.66–85.37) against the invitro enteric pathogen Escherichia coli in layer chickens. Similar results were demonstrated in liver (68%; 95% CI: 53.30–80.48) and kidney (66%, 95% CI: 51.23–78.79) of layer chickens against Bacillus cereus and Bacillus subtilis. Amongst all antibiotics, the highest concentrations of ciprofloxacin were detected in kidney (48.57%; 95% CI: 31.38–66.01), followed by liver (47.56; 95% CI: 40.88–54.30) of broiler chickens. Ciprofloxacin was also present in liver (46.15%; 95% CI: 33.70–58.96) of layer chickens. The percentage of ciprofloxacin in thigh and breast meat in broiler bird were 41.54% (95% CI: 34.54–48.79) and 37.95% (95% CI: 31.11–45.15) respectively. Enrofloxacin was the second most dominant antimicrobial agent and was present in the liver of both types of poultry (Broiler and Layer chickens: 41.54%; 95% CI: 29.44–54.4 and 437.33%; 95% CI: 30.99–44.01). The prevalence rates of enrofloxacin in thigh and breast meat of broiler chickens were 24.10% (95% CI: 18.28–30.73) and 20.51% (95% CI: 15.08–26.87), respectively. Tetracycline, a commonly used antibiotic in livestock, was present in the liver (49.23%; 95% CI: 36.60–61.93) of layer chickens. In case of aquaculture food products, the highest amount of amoxicillin (683.2 mg/kg) was detected in Tilapia fish (Oreochromis niloticus), followed by 584.4 mg/kg in climbing perch (Anabas testudineus) and 555.6 mg/kg in Rui fish (Labeo rohita). Among the five types of fishes, Rui fish (0.000515 mg/kg) contained the highest concentrations of chloramphenicol antibiotic residues.
Conclusions
The presence of antimicrobial residues in meat, milk, egg, and fish is a serious public health threat due to the potential induction of antimicrobial resistance. It can negatively impact the food supply chain, especially with the current strain that it is already facing with the current COVID-19 pandemic. The findings of the present study highlight the ongoing risk of residual antimicrobial agents in food of animal origin in Bangladesh and countries with similar practices. This can draw the attention of public health officials to propose plans to mitigate or stop this practice.
Keywords: Antimicrobial agents, Residual, Animals, Livestock, Growth promotors, Food chain, COVID-19 pandemic
1. Introduction
Antimicrobial resistance, emerging viruses such as (SARS-CoV-1, MERS-CoV, and SARS-CoV-2), avian influenza and several other zoonotic infectious diseases pose continuous threats and challenges to human public health. Antimicrobial resistance incurs increasing costs in lives and money, and is threatening modern medicine as we know it today. Antimicrobial agents have been used globally for many years in human and veterinary practices (Prescott, 2017). However, the indiscriminate use of antimicrobial agents may lead to the development of resistance through pathways that include the accumulation of antibiotic residues in the human food chain. Hence, maximum residue limits (MRLs) have been implemented by the Food and Agricultural Organization (FAO) for public health safety (Food and Agricultrual Organization of the United Nations, 2018). Antimicrobial resistance (AMR) has become a massive public health threat in many countries including Bangladesh, in which the practice of boosting agricultural production systems with overwhelming amounts of antibiotics is prevalent.
Bangladesh is a lower middle-income overpopulated country with increasing demands for protein-based food sources; which led to the establishment of extensive dairy, poultry, and aquaculture industries across the country. These farms play a significant role in the economy of Bangladesh through the production of poultry, meat, milk, and eggs (Department of Livestock Service, 2020). Moreover, indigenous or family-reared chicken/poultry supplied a significant amount of meat and eggs throughout the years to meet the protein demands of the rural communities. This created pressure on commercial farmers to produce more meat and eggs for the growing population and triggered the unregulated use of growth promoters and probiotics to meet the high production demands. More recently, milk and meat production have increased several folds by implementing specific and outcome-oriented initiatives by the government, non-government organizations (NGOs), and farmers (Ministry of Fisheries and Livestock, 2020). Farmers are, unfortunately, not very well aware about the antibiotic withdrawal period and they seem to be more concerned about the economic impacts of the products such as milk. This has led to commercial food products with varying amounts of heavy metals (Kundu et al., 2017) and antimicrobials used to prevent diseases and thus enhance farm production (Hasan et al., 2011). More precisely, farmers frequently treat the whole flock with antibiotics and growth promoters without consulting veterinarians, physicians, or public health experts (Chowdhury et al., 2009). They also do not typically maintain the prescribed antibiotic withdrawal period before marketing their food products such as meat, milk, egg and fish (Nonga et al., 2010). In broiler chickens, antimicrobial agents are used in feed and water to prevent diseases. Hence, the meat of broiler chickens, egg, and milk may contain antimicrobial residues which increase the possibility of developing resistant bacteria (Hasan et al., 2012; Hassan et al., 2014) which represents a serious threat to public health (Sachi et al., 2019). Aquaculture such as commercial fish and prawn farming is popular in Bangladesh as a source of low-cost protein to underprivileged groups of the society (Ahmed et al., 2008; Das et al., 2018). Antimicrobial agents are also used as feed additives in fish farming triggering antimicrobial resistant bacteria in water bodies and contaminating the aquaculture and the environment (Ahaduzzaman et al., 2014; Hassan et al., 2015).
Humans consuming livestock and aquaculture-derived food products might have the chance to develop resistance against specific antimicrobials due to antimicrobial residues being introduced into the human food chain (Roess et al., 2013). To investigate the situation of antimicrobial residues in animal-derived food, we consulted previous reports (Bakar et al., 2013; Barman et al., 2018; Bristy et al., 2019; Chowdhury et al., 2015; Ferdous et al., 2019; Hossain et al., 2018; Islam et al., 2016; Khan et al., 2018; Sarker et al., 2018; Sattar et al., 2014). Some studies proposed different techniques for antimicrobial residue detection in different types of animal-derived food products. In this review, we assessed the current reported data on antimicrobial residues in order to guide future public health measures in Bangladesh and other developing countries that have similar antibiotic-residue problems.
2. Materials and methods
2.1. Review protocol
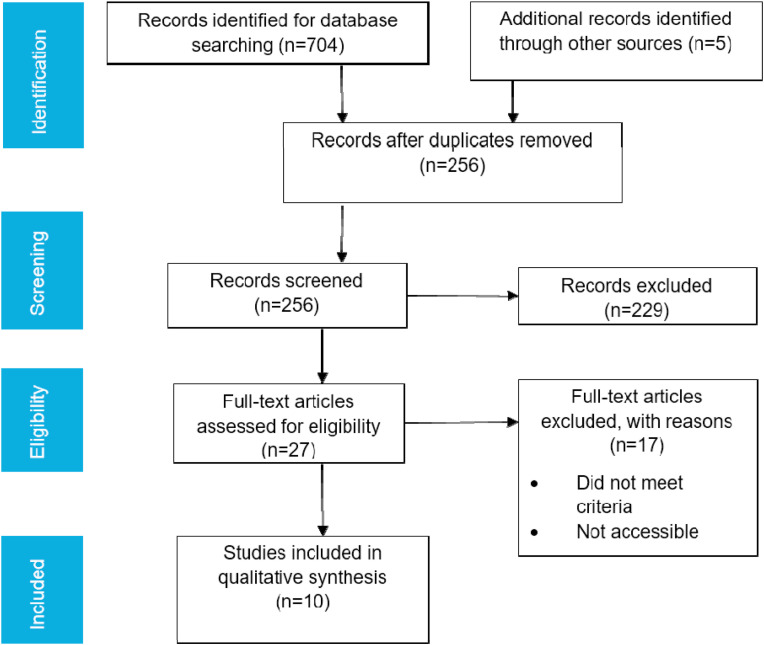
The review followed the standard systematic review procedures established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Liberati et al., 2009). The procedural guidelines shown in Fig. 1 were followed: (a) database search to categorize potentially relevant articles, (b) assessment of the relevance of the articles, (c) quality assessment and (d) extraction of data.
Fig. 1.
Flow chart of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) showing the search strategy and selection process for research articles published between 2013 and 2019. Based on the search criteria, a total of 704 English language published articles were identified and were further refined into 10 articles as described in the PRISMA flowchart.
2.2. Search strategy
A structured literature search approach was used to identify published studies reporting the presence of antimicrobial residues in foods of animal origin in Bangladesh. The scientific databases Google scholar, PubMed, and Science Direct were searched for relevant studies published between 2013 and 2019. Specific Boolean words were developed based on the objectives of the study. The search terms have been categorized into outcome, population, descriptive, and area categories. The articles were downloaded using the Chattogram Veterinary and Animal Sciences University (CVASU) library network. The Boolean words of each category were combined using “AND” whereas “OR” was used to join the term within a category. Some modifications have been made based on the requirements of the search engines, and advanced search criteria have been used to search Google scholar. Duplicate entries were identified and removed before the final selection of articles. Studies that did not meet the predetermined inclusion criteria were removed. Studies outside the scope were excluded. This included antimicrobial residues, poultry/livestock/fish, detection methods such as microbial inhibition test (MIT)/thin layer chromatography (TLC)/ultra-high-performance liquid chromatography (UHPLC)/liquid chromatography-mass spectrometry (LC-MS), conducted/published before 2013, studies published in languages other than English, reviews, abstracts, and conference proceedings.
2.3. Data screening
The full texts of retrieved published articles were screened for inclusion. Studies were selected for evaluation if they met the following inclusion criteria.
-
•
Any research article published between January 2013 and December 2019 that reported residual antimicrobial agents in poultry, livestock, and fish in Bangladesh.
-
•
Any research article that reported the prevalence, investigation, incidence, occurrence, survey, characterization and identification of antimicrobial residues from Bangladesh.
-
•
Data were extracted and recorded for study location, citation, first author, time of study, year of publication, type of specimen, sample size, number of positive specimens, presence or absence of antimicrobial residue, specific antibiotic sensitivity or resistance level percentages, methods used for detection, and antimicrobial residue level.
Eligible articles were retrieved in full text format and were assessed using the case definitions specified by the respective studies (Table 1 ).
Table 1.
Detection, sources, and prevalence of antimicrobial residues between 2013 and 2019 in Bangladesh.
| Area | Duration | Publication year | Sample source | Sample category | Sample | Method | Antibiotics | Total samples | Outcome | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Chattogram | 2009 | 2016 | Poultry farm | Layer chickens | Thigh Breast Liver Kidney Eggs |
MIT TLC | 4 | 100 | Ciprofloxacin Enrofloxacin Tetracycline Amoxicillin | Islam et al. (2016) |
| Chattogram | 2011–2012 | 2015 | Poultry farm Dairy farm |
Layer chickens Livestock | Eggs Milk |
MIT TLC UHPLC | 3 | 210 | Ciprofloxacin Tetracycline Amoxicillin |
Chowdhury et al. (2015) |
| Chattogram | 2012 | 2014 | Live bird market | Layer chickens Broiler chickens | Thigh Breast Liver Kidney |
TLC UHPLC | 4 | 50 | Ciprofloxacin Enrofloxacin Tetracycline Amoxicillin | Sattar et al. (2014) |
| Chattogram | 2013 | 2013 | Live bird market Fish market |
Broiler chickens Indigenous chicken Fish |
Meat Liver Tilapia Pungas Anabas Trout Rui fish |
LC-MS | 1 | 80 | Chloramphenicol | Bakar et al. (2013) |
| Chattogram | 2015 | 2019 | Live bird market Fish market |
Broiler chickens Indigenous chicken Fish Shrimp |
Thigh Breast Liver Climbing perch Rui fish Tilapia Bombay duck Shrimp |
TLC, UHPLC | 4 | 335 | Ciprofloxacin Enrofloxacin Amoxicillin Oxytetracycline | Ferdous et al. (2019) |
| Sylhet | 2016 | 2018 | Fish market | Fish | Tilapia | UHPLC | 1 | 24 | Oxytetracycline | Barman et al. (2018) |
| Sylhet | 2016 | 2018 | Fish market | Fish | Pungas | UHPLC | 1 | 24 | Oxytetracycline | Hossain et al. (2018) |
| Gazipur Mymensingh |
2017 | 2018 | Live bird market and farm | Broiler chickens | Thigh Breast Liver |
TLC | 5 | 160 | Ciprofloxacin Enrofloxacin Oxytetracycline Amoxicillin Doxycycline | Sarker et al. (2018) |
| Mymensingh | 2017 | 2019 | Poultry farm | Broiler chickens | Meat | TLC | 1 | 108 | Colistin | Bristy et al. (2019) |
| Mymensingh | 2017 | 2018 | Live bird market | Broiler chickens | Breast Liver |
TLC | 3 | 30 | Ciprofloxacin Enrofloxacin Amoxicillin |
Khan et al. (2018) |
Microbial Inhibition Test (MIT); Thin Layer Chromatography (TLC); Ultra-High-Performance Liquid Chromatography (UHPLC); Liquid Chromatography-Mass Spectrometry (LC-MS).
2.4. Data analysis
All data extracted from different publications were sorted in Microsoft excel for statistical analysis. Data were analyzed using STATA/IC version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX, USA) to get the prevalence and 95% confidence interval (CI). Descriptive statistics were done to identify the numbers of articles. The study results from those articles were classified according to sample category and method of detection and were arranged in tables with the identified antimicrobials.
3. Results
3.1. Data acquisition
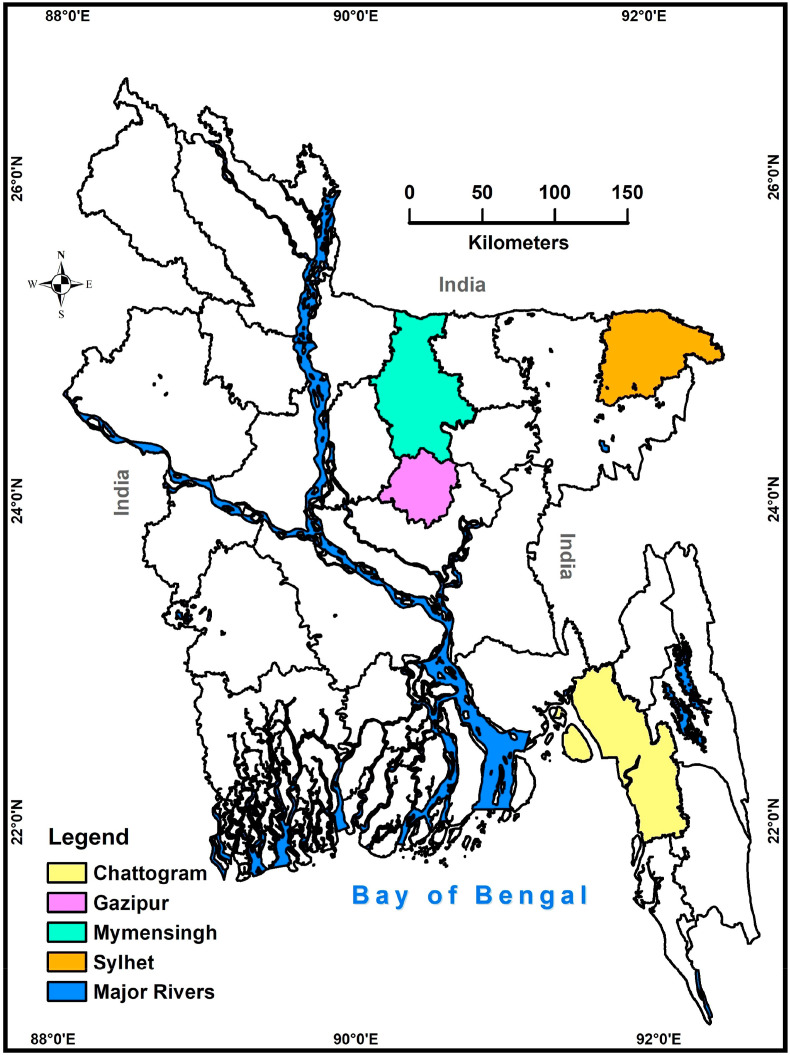
The preliminary search yielded 704 articles. Manual search identified five additional articles. Deduplication yielded 256 unique articles. Reports were considered duplicated if they had the same information of authors, year of publication, title of the article, volume, issue, and page number fields. After the exclusion of articles that did not meet the inclusion criteria, 10 articles were identified as eligible for data extraction and qualitative analysis (Table 1). Among the 64 districts of Bangladesh, research studies on residual antimicrobial agents were focused in only four districts (Fig. 2 ). Screening of residual antimicrobial agents in other districts is necessary to determine the overall situation in the country.
Fig. 2.
A map of Bangladesh showing the study areas and the spatial distribution of residual antimicrobial agents (4 districts in different colors; Chattogram, Sylhet, Gazipur and Mymensingh). The map was plotted using ArcMap, version 10.2, Environmental Systems Research Institute, Redlands, California, USA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Residual antimicrobial agents in poultry products
Using the microbial inhibition method, qualitative detection of antimicrobial residues (Table 2 ) showed the highest prevalence of 68% in liver (95% CI: 53.30–80.48) followed by 60% in kidney (95% CI: 45.18–73.59), 60% in breast meat (95% CI: 45.18–73.59), 50% in thigh meats of chicken (95% CI: 35.73–64.47), and 64% in egg (95% CI: 49.19–77.08) when tested with B. cereus. In case of B. subtilis, the highest prevalence of 66% was in kidney (95% CI: 51.23–78.79) followed by 50% in liver (95% CI: 35.73–64.47), 40% in breast meat (95% CI: 26.41–54.82), 44% in thigh meat of chicken (95% CI: 29.99–58.75), and 54% in eggs (95% CI: 39.32–68.19). In case of E. coli, the highest prevalence of 74% was in liver (95% CI: 59.66–85.37) followed by 72% in kidney (95% CI: 57.51–83.77), 70% in breast meat (95% CI: 55.39–82.14), 54% in thigh meat of chicken (95% CI: 39.32–68.19), and 60% in eggs (95% CI: 45.18–73.59). About 27% of ciprofloxacin (95% CI: 20.98–33.72) was detected in eggs. Amoxicillin and tetracycline have also been detected in eggs with the respective percentages of 11% (95% CI: 7.02–16.18) and 16% (95% CI: 11.21–21.83).
Table 2.
Qualitative detection of antimicrobial residues in foods of animal origin using the Microbial inhibition test (MIT).
| Sample category | Sample | Ciprofloxacin N, p, %, 95%CI |
Tetracycline N, p, %, 95%CI |
Amoxicillin N, p, %, 95%CI |
B. cereus N, p, %, 95%CI |
B. subtilis N, p, %, 95%CI |
E. coli N, p, %, 95%CI |
References |
|---|---|---|---|---|---|---|---|---|
| Layer chickens | Liver | 50, 34, 68, 53.30–80.48 | 50, 25, 50, 35.73–64.47 | 50, 37, 74, 59.66–85.37 | (Chowdhury et al., 2015; Islam et al., 2016) | |||
| Kidney | 50, 30, 60, 45.18–73.59 | 50, 33, 66, 51.23–78.79 | 50, 36, 72, 57.51–83.77 | |||||
| Breast | 50, 30, 60, 45.18–73.59 | 50, 20, 40, 26.41–54.82 | 50, 35, 70, 55.39–82.14 | |||||
| Thigh | 50, 25, 50, 35.73–64.47 | 50, 22, 44, 29.99–58.75 | 50, 27, 54, 39.32–68.19 | |||||
| Eggs | 200, 54, 27, 20.98–33.72 | 200, 32, 16, 11.21–21.83 | 200, 22, 11, 7.02–16.18 | 50, 32, 64, 49.19–77.08 | 50, 27, 54, 39.32–68.19 | 50, 30, 60, 45.18–73.59 | ||
| Livestock | Milk | 200, 25, 12.5, 8.26–17.90 | 200, 35, 17.5, 12.50–23.49 | 200, 52, 26, 20.07–32.66 |
The published articles that used the TLC method for the qualitative detection of antimicrobial residues (Table 3 ) were assessed. The highest prevalence of ciprofloxacin was detected in kidney and liver of broiler chickens and were 48.57% (95% CI: 31.38–66.01) and 47.56% (95% CI: 40.88–54.30), respectively. The lowest prevalence was detected in thigh and breast meat of layer chickens and were 30.77% (95% CI: 19.91–43.44) and 29.23% (95% CI: 18.60–41.82) respectively. The highest prevalence of enrofloxacin was detected in liver of layer chickens and was 41.54% (95% CI: 29.44–54.44) while the lowest prevalence was detected in breast meat of broiler chickens and was 20.51% (95% CI: 15.08–26.87). The highest prevalence of tetracycline was detected in the liver of layer chickens and was 49.23% (95% CI: 36.60–61.93) whereas the lowest prevalence was detected in thigh meat of layer chickens and was 18.46% (95% CI: 9.92–30.03). The highest prevalence of amoxicillin was detected in indigenous chicken and was 88.24% (95% CI: 72.55–96.70) whereas the lowest prevalence was detected in eggs of indigenous chicken and was 5% (95% CI: 1.64–11.28). The highest prevalence of doxycycline was detected in liver and was 43.13% (95% CI: 35.33–51.18) whereas the lowest prevalence was detected in breast meat and was 25.63% (95% CI: 20.38–35.20). In broiler chickens, the highest prevalence of oxytetracycline was detected in the liver and was 46.25% (95% CI: 38.35–54.29) whereas the lowest prevalence was detected in breast meat and was 22.5% (95% CI: 16.28–29.76). The prevalence of colistin sulfate residue was 55.55% (95% CI: 45.68–65.12) in broiler meat. As detected by the UHPLC method, broiler meat (522.9 mg/kg) contained more amoxicillin residues than the meat of indigenous chicken (444.3 mg/kg) followed by eggs which had 29.64 mg/kg. Using the LC-MS method, meat (0.405 μg/kg) and liver (0.438 μg/kg) of indigenous chicken contained more chloramphenicol residues than meat (0.275 μg/kg) and liver (0.403 μg/kg) of broiler chickens (Table 4 ).
Table 3.
Qualitative detection of antimicrobials residues in foods of animal origin using the thin-layer chromatography (TLC).
| Sample category | Sample | Ciprofloxacin (N, p, %, 95%CI) |
Enrofloxacin (N, p, %, 95%CI) |
Tetracycline (N, p, %, 95%CI) |
Amoxicillin (N, p, %, 95%CI) |
Doxycycline (N, p, %, 95%CI) |
Oxytetracycline (N, p, %, 95%CI) |
Colistin (N, p, %, 95%CI) |
References |
|---|---|---|---|---|---|---|---|---|---|
| Layer chickens | Thigh | 65, 20, 30.77, 19.91–43.44 | 65, 15, 23.08, 13.53–35.19 | 65, 12, 18.46, 09.92–30.03 | 65, 17, 26.15, 16.03–38.54 | (Chowdhury et al., 2015; Islam et al., 2016; Sattar et al., 2014) | |||
| Breast | 65, 19, 29.23, 18.60–41.82 | 65, 12, 18.46, 09.91–30.03 | 65, 16, 24.62, 14.77–36.87 | 65, 14, 21.54, 12.31–33.49 | |||||
| Liver | 65, 30, 46.15, 33.70–58.96 | 65, 27, 41.54, 29.44–54.44 | 65, 32, 49.23, 36.60–61.93 | 65, 31, 47.69, 11.10–31.77 | |||||
| Kidney | 65, 25, 38.46, 26.65–51.36 | 65, 24, 36.92, 25.28–49.80 | 65, 15, 23.07, 13.53–35.19 | 65, 25, 38.46, 26.53–51.36 | |||||
| Eggs | 150, 60, 40.00, 32.09–48.31 | 50, 13, 26.00, 14.63–40.34 | 150, 43, 28.67, 21.59–36.61 | 150, 29, 19.33, 13.35–26.57 | |||||
| Broiler chickens | Thigh | 195, 81, 41.54, 34.54–48.79 | 195, 47, 24.10, 18.28–30.73 | 35, 8, 22.86, 10.42–40.13 | 195, 52, 26.67, 20.60–33.45 | 160, 45, 28.13, 21.31–35.77 | 160, 47, 29.38, 22.45–37.08 | (Bristy et al., 2019; Chowdhury et al., 2015; Ferdous et al., 2019; Khan et al., 2018; Sarker et al., 2018; Sattar et al., 2014) | |
| Breast | 195, 74, 37.95, 31.11–45.15 | 195, 40, 20.51, 15.08–26.87 | 35, 9, 25.71, 12.49–43.26 | 195, 47, 24.10, 18.28–30.73 | 150, 41, 25.63, 20.38–35.20 | 160, 36, 22.50, 16.28–29.76 | |||
| Liver | 225, 107, 47.56, 40.88–54.30 | 225, 84, 37.33, 30.99–44.01 | 65, 23, 35.38, 23.92–48.23 | 195, 81, 41.54, 34.54–48.79 | 160, 69, 43.13, 35.33–51.18 | 160, 74, 46.25, 38.35–54.29 | |||
| Kidney | 35, 17, 48.57, 31.38–66.01 | 35, 11, 31.43, 16.85–49.29 | 35, 9, 25.71, 12.49–43.26 | 35, 25, 71.43, 53.69–85.36 | |||||
| Meat | 148, 129, 87.16, 80.68–92.09 | 108, 60, 55.55, 45.68–65.12 | |||||||
| Indigenous chicken | Meat | 34, 30, 88.24, 72.55–96.70 | (Chowdhury et al., 2015; Ferdous et al., 2019) | ||||||
| Egg | 100, 9, 09.00, 04.20–16.40 | 100, 7, 07.00, 02.86–13.89 | 100, 5, 05.00, 01.64–11.28 | ||||||
| Livestock | Milk | 200, 22, 11.00, 07.02–16.18 | 200, 33, 16.50, 11.64–22.38 | 200, 48, 24.00, 18.26–30.53 | Chowdhury et al. (2015) | ||||
| Fish | Climbing perch | 24, 16, 66.67, 44.67–84.37 | 24, 2, 08.33, 01.02–27.00 | Ferdous et al. (2019) | |||||
| Rui fish | 33, 21, 63.63, 45.12–79.60 | 33, 10, 30.30, 15.59–48.71 | |||||||
| Tilapia | 33, 18, 54.54, 36.35–71.89 | 33, 1, 03.03, 00.07–15.76 | |||||||
| Bombay duck | 33, 4, 12.12, 03.40–28.20 | 33, 0, | |||||||
| Shrimp | 30, 15, 50.00, 31.30–68.70 | 30, 10, 33.33, 17.29–52.82 |
Table 4.
Quantitative detection of antimicrobial residues in foods of animal origin using Ultra-High-Performance Liquid Chromatography (UHPLC) and Liquid Chromatography-Mass Spectrometry (LC-MS).
| Sample category | Sample (n) | Amoxicillin (mg/kg) | Oxytetracycline (mg/kg) | Chloramphenicol (mg/kg) | Test methods |
|---|---|---|---|---|---|
| Broiler chickens | Breast (10) | 0.479 | UHPLC | ||
| Liver (10) | 0.0847 | ||||
| Liver (10) | 0.000403 | LC-MS | |||
| Meat (145) | 522.90 | UHPLC | |||
| Meat (10) | 0.000275 | LC-MS | |||
| Indigenous chickens | Meat (33) | 444.30 | UHPLC | ||
| Meat (10) | 0.000405 | LC-MS | |||
| Liver (10) | 0.000438 | ||||
| Eggs (10) | 29.64 | UHPLC | |||
| Livestock | Milk (10) | 33.00 | |||
| Fish | Climbing perch (5) | 584.40 | |||
| Climbing perch (10) | 0.000188 | LC-MS | |||
| Rui fish (8) | 555.60 | UHPLC | |||
| Rui fish (10) | 0.000515 | LC-MS | |||
| Trout (10) | 0.000328 | ||||
| Bombay duck (2) | 14.80 | UHPLC | |||
| Shrimp (7) | 419.50 | ||||
| Tilapia (6) | 683.20 | ||||
| Tilapia (24) | 0.0388 | ||||
| Tilapia (10) | 0.0003535 | LC-MS | |||
| Pungas (10) | 0.000133 | ||||
| Pungas (24) | 0.0351 | UHPLC |
Antibiotic concentrations are based on the following references (Bakar et al., 2013; Barman et al., 2018; Chowdhury et al., 2015; Ferdous et al., 2019; Hossain et al., 2018; Sattar et al., 2014).
3.3. Residual antimicrobial agents in livestock products
Using MIT, the prevalence rates of amoxicillin, ciprofloxacin, and tetracycline in milk were 26% (95% CI: 20.07–32.66), 12.5% (95% CI: 8.26–17.90), and 17.5% (95% CI: 12.50–23.49), respectively. Using TLC, the prevalence rates of ciprofloxacin and tetracycline residues in cow's milk were 11% (95% CI: 7.02–16.18) and 16.5% (95% CI: 11.64–22.38), respectively. From the published literature, the UHPLC method for the quantitative detection of amoxicillin residues in milk detected 33 mg/L (Table 4).
3.4. Residual antimicrobial agents in aquaculture products
Using the TLC method, the highest prevalence was detected in shrimp (Macrobrachium rosenbergii) and was 33.33% (95% CI: 17.29–52.82) whereas the lowest prevalence was detected in Tilapia fish and was 3.03% (95% CI: 0.07–15.76). Using the UHPLC method, the highest concentration of amoxicillin was detected in Tilapia fish (683.2 mg/kg). However, Climbing perch, Rui fish, Shrimp, and Bombay duck fish (Harpadon nehereus) contained amoxicillin residues of 584.4 mg/kg, 555.6 mg/kg, 419.5 mg/kg, and 14.8 mg/kg, respectively. Oxytetracycline residues were detected in Tilapia (0.0388 mg/kg) and Pungas fish (Pangasius pangasius) (0.0351 mg/kg). Using the LC-MS method, the quantitative detection of chloramphenicol residues (Table 4) indicated the highest concentration in Rui fish (0.000515 mg/kg) followed by Tilapia (0.0003535 mg/kg), Trout (0.000328 mg/kg), Climbing perch (0.000188 mg/kg) and Pungas (0.000133 mg/kg).
4. Discussion
4.1. Agricultural production systems heavily depend on antimicrobial as growth promoter and probiotics
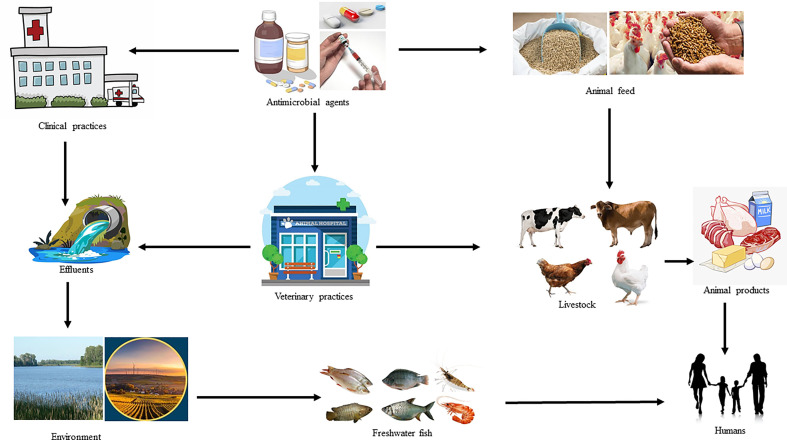
Antibiotics have been used in agricultural production systems, especially in animals since the mid-1940s, shortly after they became available for the treatment of human diseases (Gustafson & Bowen, 1997; McEwen, 2006). Antibiotics are now widely used for therapeutic, prophylactic, and growth promoting purposes within agricultural production systems including livestock and fish industries. A significant number of antibiotics used in human medicine either belongs to the same class or have the same mode of action as those used in agricultural systems. Several antibiotics used to treat bacterial infections in humans also have veterinary applications (Landers et al., 2012). In fact, the use of animal antibiotics for the treatment or prevention of disease closely followed their use in humans. Each year, a huge number of drugs, including antibiotics are administered to livestock to produce increased amounts of animal-derived food (meat, eggs, and dairy products) for human consumption around the globe (Vázquez‐Moreno et al., 1990; Roura et al., 1992; Rassow & Schaper, 1996). In 2010, China, the United States, Brazil, India, and Germany were the five countries with the largest amount of antibiotics consumed by food-producing animals (van Bijnen et al., 2011). In that same year, the estimated global amount of antibiotics used for food-producing animals was 63,151 (±1560) tons (Boeckel et al., 2015). Antimicrobials are prescription-only medications and their indiscriminate use can lead to the development of bacterial resistance, which is a major public health concern (Ferech et al., 2006; Liu et al., 1999; van Bijnen et al., 2011; Buke et al., 2003; Ahiabu et al., 2016; Al-Tawfiq et al., 2010; Costelloe et al., 2010; Vaananen et al., 2006; You et al., 2008; Mather et al., 2012). Using antibiotics in agricultural systems is a major contributing factor for resistance development in bacteria that may cause infections in humans (Molbak, 2004; Wassenaar, 2005). In Bangladesh, antimicrobial agents are used regularly for the treatment and control of diseases in the agricultural system, especially of food-producing animals, and are usually used as a feed additive for growth promotion. Antibiotics are used at suboptimal concentrations for the treatment of infections; a potentially dangerous recipe for developing resistant bacteria. In addition, this enhances potential allergic reactions and technological problems of fermented meat products. Interestingly, 80% of all food-producing animals receive medication in their entire productive period (Center for Veterinary Medicine, US Food and Drug Adminstration et al., 2017). In case of poultry, antibiotic usage has facilitated their efficient production, and also improved their health and well-being by reducing the incidence of infections (Tollefson & Miller, 2000). In Bangladesh, farmers frequently use antibiotics for therapeutic purposes and regularly add them in the feed and water at sub-therapeutic concentrations for prophylaxis and growth promotion. This unregulated administration of antimicrobial agents in livestock and aquaculture industry for treatment, prophylaxis, and growth promotion resulted in a huge risk of their deposition to the human food chain at levels exceeding the MRL (Food and Agricultrual Organization of the United Nations, 2018). The presence of antimicrobial residues in food of animal origin as a possible point of entry into the human food chain is shown in Fig. 3 . The presence of antimicrobial residues in meat, meat products, fish, and aquaculture products has been negatively impacting their international trade for the last few years (Mathews et al., 2003). The antimicrobials used as feed additive can suppress gut bacteria (Parker & Armstrong, 1987). Generally, the use of growth promoters in poultry promotes faster growth and plays a beneficial role in controlling some chronic disease conditions. Withdrawal of growth promoters can lead to reduced profitability of farming enterprises through increased capital and operational costs. However, studies from Nordic countries suggested that negative effects of removing growth promoters can be offset by improved animal husbandry (Krauβ et al., 1996). Antibiotics commonly used for treatment, prophylaxis, and growth promotion of food animals include doxycycline, colistin sulfate, neomycin, tetracycline, enrofloxacin, ciprofloxacin, and amikacin (Apata, 2009; Roess et al., 2013). There is always a possibility for these antibiotic residues to gain access to the consumers’ food products and the human food chain. Poor record keeping can exacerbate the problem, when farmers sell their animals and products of animal origin without having records indicating the completion of the specified withdrawal periods.
Fig. 3.
Flowchart showing the presence of antimicrobial residues arising from the use of antimicrobial agents in Bangladesh.
4.2. Non-judicial use of antibiotics in the poultry, livestock, and fish production systems can exacerbate the antibiotic residue accumulation problem
The unregulated accessibility of farmers to antimicrobial agents poses a serious risk for the development of antimicrobial resistance. This is especially problematic in areas where there is scarcity of authorized veterinarians, such as in remote and rural areas. In addition, antibiotics are readily available to the farmers in retail drug stores in Bangladesh without prescription by registered veterinarians or authorized personnel. Such practice is prevalent throughout the country and has led to the misuse of antibiotics with the associated high prevalence of antibiotic resistance in bacteria from animal and food sources. Commercial poultry farmers in Bangladesh use antibiotics indiscriminately without any prescription or veterinary consultation. Several factors including the lack of knowledge among local farmers, lack of proper veterinary services in rural and remote areas, and the lure of higher profits collectively contribute to the problem (Redding et al., 2013). Antibiotics in the growth promoting category are, in some cases, the same antibiotics used in the therapeutic category. The decision to use growth-promoting antibiotics in commercial poultry is mainly an economic decision that is made by the farmers (Al Masud et al., 2020). Farmers depend on antibiotics and believe that using different types of antibiotics will keep their farm animals healthier and more productive. Farmers are not well-informed about the risks associated with the abuse of antibiotics and the potential deleterious impacts of this practice on public health. Medical representatives of pharmaceutical companies also share some responsibility for this problem as they have to fulfill their monthly target of selling a certain amount of their products (Guha, 2004; Masood et al., 2009), and it was observed that they try to convince farmers to directly use antimicrobial agents in regular schedules in order to prevent diseases, especially in poultry farms (personal communications).
Medical representatives of human medicines frequently do the same with physicians in the country, trying to convince them to prescribe more antibiotics (Habib & Alam, 2011; D'Arcy & Moynihan, 2009).
In Bangladesh, only a few companies indicate the withdrawal period of their product on their drug labels and inserts, which can increase the residue accumulation risk. Finally, there is a lack of serious enforcement of rules that prevent the abuse of antibiotics and the consumption of foods that have antimicrobial residues (Apata, 2009). Altogether, these factors all contribute to the problem in Bangladesh and other countries.
4.3. The antimicrobial residues in food sources (milk, meat, eggs, fish) can lead to the evolution of resistant pathogens
The possible effects of widespread use of antibiotics in poultry, livestock, and aquaculture production have been reported elsewhere (Marshall & Levy, 2011). The use of antibiotics can lead to the entry of such drugs into the human food chain in the form of drug residues in animal source foods including meat, milk, eggs and fishes. This can occur when withdrawal periods are not maintained before selling animal products for human consumption. This practice may lead to lowering the efficacy of antibiotics when used for treatment of human diseases. Moreover, extensive antibiotic usage can also lead to the emergence of antibiotic-resistant bacterial strains with the possibility of transfer of resistant genes to other pathogenic and nonpathogenic bacteria (Peterson & Kaur, 2018) or to the human food chain (Smith, 1969). Humans can thus acquire antibiotic resistant bacteria following consumption of poultry, livestock, and fish products. Antibiotic resistance against human pathogens is now a major global public health issue (Martínez-Martínez & Calvo, 2010). There is an evident association between antibiotic use in animals and the development of resistance in human pathogens as was previously reported (Hoelzer et al., 2017; Landers et al., 2012; Wegener, 2012). Evidently, the use of antibiotics in livestock production has been associated with the development of antibiotic resistance in humans. The development of resistance in several microorganisms due to the use of sub-therapeutic concentrations of penicillin, tetracyclines, and sulfa drugs in the agricultural industry has been highlighted as a problem by the World Health Organization (Landers et al., 2012; Prajwal et al., 2017). The ability of tetracycline resistance to persist for a long period of time after the withdrawal period was previously reported (Grossman, 2016).
4.4. Practices that promote the spread of resistant bacteria to different components of the agricultural production system
Herds and flocks that have been treated with tetracycline and aminoglycosides might show widespread transmission of resistance genes or resistant pathogens. In case of the aquaculture industry, resistant pathogens have evolved via antibiotic use in feed additives, poultry offal, and litter which had an impact on fish in different water bodies. The contamination of water with antibiotic-contaminated waste and effluents from both veterinary practices and human hospitals may lead to antimicrobial residue deposition in water, which can subsequently transmit resistant bacteria to different components of the agricultural production system (Manyi-Loh et al., 2018). Vancomycin-resistant enterococci readily detected in healthy human fecal material is the ultimate result of the use of avoparcin in pig industry (Van den Bogaard et al., 2002). Avoparcin withdrawal has been successful in reducing vancomycin-resistant enterococci contamination in pork (Nilsson, 2012). Antibiotic resistance genes may be transferred from animal pathogens or commensals to human pathogens (Argudín et al., 2017, Blake et al., 2003, von Wintersdorff et al., 2016). Resistant bacteria with similar resistance genes and patterns in both animals and of humans were previously reported (Ahmed et al., 2019; Marshall & Levy, 2011; Wegener, 2012). It is evident that resistant bacteria with the same resistance patterns and/or of the same genotype in food are also increasing. There are possible One Health components that might contribute to the spread of resistant genes or bacteria through the human food chain (Ashour, 2014;Asante et al., 2019 Khan et al., 2020). Interestingly, the antimicrobial residues deposited into the fish bodies can also be transferred to the human food chain, an observation that was previously made for ampicillin and olaquindox (Smith, 1969). The MRLs of oxytetracycline for poultry liver, eggs, and milk were 0.06 mg/kg, 0.04 mg/kg, and 0.01 mg/L, respectively, whereas the MRLs for colistin sulfate were 0.015 mg/kg and 0.03 mg/kg for poultry liver and eggs, respectively (Food and Agricultrual Organization of the United Nations, 2018; Health Canada Government of Canada, 2017). The current study identified antimicrobial residue concentrations that are higher than the MRL for most of the antimicrobial agents. Existence of chloramphenicol residues in food of animal origin is an indication of a public health problem (Davies & Wales, 2019). The dynamics of the flow of antimicrobial residues to humans is summarized in Fig. 3.
Food-producing animals may receive antimicrobial agents directly as a treatment or in the feed as growth promoters. Some antimicrobial residues can remain in the food products if the withdrawal period of a specific antimicrobial agent was not maintained during human consumption of the animal-derived food products.
On the other hand, the effluents from medical hospitals and veterinary practices can indirectly contaminate water bodies in which fish is raised leading to the presence of antimicrobial residues in fish, which can then be consumed by humans.
5. Interventions and suggestions
Based on this study, we recommend the judicial use of antimicrobials in food-producing animals and to only administer antimicrobials to the food-producing animals when prescribed by registered health professionals. In addition, it is important to maintain withdrawal periods after antibiotic treatments and to discard the products for human consumption before the end of the withdrawal period. Furthermore, the use of antimicrobials as food additives in food-producing animals including fish should be restricted. We recommend against the use of poultry offal, litters, and livestock waste in aquacultures. Human and veterinary hospital effluents should be stopped from reaching aquatic environments without proper sewage treatment. Finally, there should be investment in improved animal husbandry to reduce the antimicrobial growth promoter use without losing revenue.
It is noteworthy that there is limited data on antimicrobial residue concentrations in foods originating from livestock and aquaculture. Some studies tested only a few antimicrobial agents or included a few animal species such as domestic poultry, broiler and layer chickens, fish, and shrimp.
6. Conclusions
In summary, residual antimicrobial agents in food products from animal sources including but not limited to meat, milk, egg, fish and shrimp pose the risk of inducing the development of antimicrobial resistance. The high residue concentrations might be the result of the indiscriminate use of antimicrobials in the agricultural production systems or an inappropriate withdrawal period. The residual antimicrobial agents can then be transferred to the human food chain causing further enhancement of resistance genes in human pathogens, and the potential development of antimicrobial resistance. Farmers need to adhere to the guidelines from health professionals, including guidelines on the prudent use of antimicrobials and observing the withdrawal periods. We recommend the removal (banning) of poultry offal and other livestock waste from the aquaculture industry. Following the appropriate guidelines and protocols will eventually limit the presence of antimicrobial residues in animal-derived food products and the development of antimicrobial resistance which will reduce the risk to the public. This will also support the food supply chain, which has been severely affected by the ongoing COVID-19 pandemic.
Declaration of competing interest
No conflict of interest.
Acknowledgment
We would like to thank the Bangladesh Bureau of Educational Information and Statistics (BANBEIS; #SD-2019967) and the Swedish Research Council (2016-02606) for support.
Disclaimer
Any opinions, findings, conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the funding agencies.
References
- Ahaduzzaman M., Hassan M.M., Alam M., Islam S., Uddin I. Antimicrobial resistance pattern against Staphylococcus aureus in environmental effluents. Research Journal for Veterinary Practitioners. 2014;2:13–16. [Google Scholar]
- Ahiabu M.A., Tersbol B.P., Biritwum R., Bygbjerg I.C., Magnussen P. A retrospective audit of antibiotic prescriptions in primary health-care facilities in Eastern Region, Ghana. Health Policy and Planning. 2016;31:250–258. doi: 10.1093/heapol/czv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N., Demaine H., Muir J.F. Freshwater prawn farming in Bangladesh: History, present status and future prospects. Aquaculture Research. 2008;39:806–819. [Google Scholar]
- Ahmed I., Rabbi M.B., Sultana S. Antibiotic resistance in Bangladesh: A systematic review. International Journal of Infectious Diseases. 2019;80:54–61. doi: 10.1016/j.ijid.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Al Masud A., Rousham E.K., Islam M.A., Alam M.-U., Rahman M., Al Mamun A., Sarker S., Asaduzzaman M., Unicomb L. Drivers of antibiotic use in poultry production in Bangladesh: Dependencies and dynamics of a patron-client relationship. Frontiers in Veterinary Science. 2020;7 doi: 10.3389/fvets.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Stephens G., Memish Z.A. Inappropriate antimicrobial use and potential solutions: A middle eastern perspective. Expert Rev Anti Infect Ther. 2010;8:765–774. doi: 10.1586/eri.10.56. [DOI] [PubMed] [Google Scholar]
- Apata D. Antibiotic resistance in poultry. International Journal of Poultry Science. 2009;8:404–408. [Google Scholar]
- Argudín M.A., Deplano A., Meghraoui A., Dodémont M., Heinrichs A., Denis O., Nonhoff C., Roisin S. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics. 2017;6:12. doi: 10.3390/antibiotics6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante J., Noreddin A., El Zowalaty M.E. Systematic review of important bacterial zoonoses in Africa in the last decade in light of the “One Health” concept. Pathogens. 2019;8(2) doi: 10.3390/pathogens8020050. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour H.M. One health—people, animals, and the environment. Clinical Infectious Diseases. 2014;59(10) 1510-1510. [Google Scholar]
- Bakar M., Morshed A., Islam F., Karim R. Screening of chloramphenicol residues in chickens and fish in Chittagong city of Bangladesh. Bangladesh Journal of Veterinary Medicine. 2013;11:173–175. [Google Scholar]
- Barman A.K.A., Hossain M.M., Rahim M.M., Hassan M.T., Begum M. Oxytetracycline residue in Tilapia. Bangladesh Journal of Scientific & Industrial Research. 2018;53:41–46. doi: 10.3329/bjsir.v53i1.35909. [DOI] [Google Scholar]
- van Bijnen E.M., den Heijer C.D., Paget W.J., Stobberingh E.E., Verheij R.A., Bruggeman C.A., Pringle M., Goossens H., Schellevis F.G. The appropriateness of prescribing antibiotics in the community in europe: Study design. BMC Infectious Diseases. 2011;11:293. doi: 10.1186/1471-2334-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D., Hillman K., Fenlon D., Low J. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. Journal of Applied Microbiology. 2003;95:428–436. doi: 10.1046/j.1365-2672.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- Boeckel T.P.V., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P.…Laxminarayan R. Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristy N.I., Das S., Noman Z.a., Ferdous J., Sachi S., Kabir S.M.L., Sikder M.H. Colistin residue in broiler: Detection in different growth stages. Asian-Australasian Journal of Food Safety and Security. 2019;3:43–47. [Google Scholar]
- Buke A.C., Ermertcan S., Hosgor-Limoncu M., Ciceklioglu M., Eren S. Rational antibiotic use and academic staff. International Journal of Antimicrobial Agents. 2003;21:63–66. doi: 10.1016/s0924-8579(02)00272-8. [DOI] [PubMed] [Google Scholar]
- Center for Veterinary Medicine, US Food and Drug Adminstration, MD, USA Summary report on antimicrobials sold or distributed for use in food-producing animals. 2017. https://www.fda.gov/media/119332/download
- Chowdhury M.H., Islam K., Khaleduzzaman A. A review on antibiotics in an animal feed. Bangladesh Journal of Animal Science. 2009;38:22–32. [Google Scholar]
- Chowdhury S., Hassan M.M., Alam M., Sattar S., Bari M.S., Saifuddin A.K.M., Hoque M.A. Antibiotic residues in milk and eggs of commercial and local farms at Chittagong, Bangladesh. Veterinary World. 2015;8:467–471. doi: 10.14202/vetworld.2015.467-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costelloe C., Metcalfe C., Lovering A., Mant D., Hay A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- D'Arcy E., Moynihan R. Can the relationship between doctors and drug companies ever be a healthy one? The International Journal of Risk and Safety in Medicine. 2009;21:185–191. doi: 10.1371/journal.pmed.1000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Islam M., Akter T., Kawser A., Mondal M. Present status, problems and prospect of fish farming at Gazipur Sadar upazila in Bangladesh. Progressive Agriculture. 2018;29:53–63. [Google Scholar]
- Davies R., Wales A. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Comprehensive Reviews in Food Science and Food Safety. 2019;18:753–774. doi: 10.1111/1541-4337.12438. [DOI] [PubMed] [Google Scholar]
- Department of Livestock Service Bangladesh. Livestock economy at a glance. http://dls.portal.gov.bd/sites/default/files/files/dls.portal.gov.bd/page/ee5f4621_fa3a_40ac_8bd9_898fb8ee4700/Livestock%20Economy%20at%20a%20glance%20%20%282017-2018%29.pdf
- Ferdous J., Bradshaw A., Islam S.A., Zamil S., Islam A., Ahad A., Fournie G., Anwer M.S., Hoque M.A. Antimicrobial residues in chicken and fish, chittagong, Bangladesh. EcoHealth. 2019;16:429–440. doi: 10.1007/s10393-019-01430-6. [DOI] [PubMed] [Google Scholar]
- Ferech M., Coenen S., Malhotra-Kumar S., Dvorakova K., Hendrickx E., Suetens C., Goossens H., Group E.P. European surveillance of antimicrobial consumption (ESAC): Outpatient antibiotic use in europe. Journal of Antimicrobial Chemotherapy. 2006;58:401–407. doi: 10.1093/jac/dkl188. [DOI] [PubMed] [Google Scholar]
- Food and Agricultrual Organization of the United Nations Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. 2018. http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXM%2B2%252FMRL2e.pdf 8.
- Grossman T.H. Tetracycline antibiotics and resistance. Cold Spring Harbor perspectives in medicine. 2016;6(4):a025387. doi: 10.1101/cshperspect.a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A. A comparison of codes of pharmaceutical marketing practices. Indian journal of medical ethics. 2004;1:19–21. doi: 10.20529/IJME.2004.009. [DOI] [PubMed] [Google Scholar]
- Gustafson R., Bowen R. Antibiotic use in animal agriculture. Journal of Applied Microbiology. 1997;83:531–541. doi: 10.1046/j.1365-2672.1997.00280.x. [DOI] [PubMed] [Google Scholar]
- Habib M.A., Alam M.Z. Business analysis of pharmaceutical firms in Bangladesh: Problems and prospects. Journal of Business and Technology (Dhaka) 2011;6:61–77. [Google Scholar]
- Hasan B., Faruque R., Drobni M., Waldenström J., Sadique A., Ahmed K.U.…Alam M. High prevalence of antibiotic resistance in pathogenic Escherichia coli from large-and small-scale poultry farms in Bangladesh. Avian Diseases. 2011;55:689–692. doi: 10.1637/9686-021411-Reg.1. [DOI] [PubMed] [Google Scholar]
- Hasan B., Sandegren L., Melhus Å., Drobni M., Hernandez J., Waldenström J.…Olsen B. Antimicrobial drug–resistant Escherichia coli in wild birds and free-range poultry, Bangladesh. Emerging Infectious Diseases. 2012;18:2055. doi: 10.3201/eid1812.120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M., Ahaduzzaman M., Alam M., Bari M., Amin K., Faruq A. Antimicrobial resistance pattern against E. coli and Salmonella spp. in environmental effluents. International Journal of Natural Sciences. 2015;5:52–58. [Google Scholar]
- Hassan M., Amin K.B., Ahaduzzaman M., Alam M., Faruk M.S., Uddin I. Antimicrobial resistance pattern against E. coli and Salmonella in layer poultry. Research Journal for Veterinary Practitioners. 2014;2:30–35. [Google Scholar]
- Health Canada Government of Canada . Health Canada Ottawa; Ontario, Canada: 2017. List of maximum residue limits (MRLs) for veterinary drugs in foods.https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/vet/mrl-lmr/mrl-lmr_versus_new-nouveau-20170802-eng.pdf [Google Scholar]
- Hoelzer K., Wong N., Thomas J., Talkington K., Jungman E., Coukell A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Veterinary Research. 2017;13:211. doi: 10.1186/s12917-017-1131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.M., Barman A.A., Rahim M.M., Hassan M.T., Begum M., Bhattacharjee D. Oxytetracycline residues in Thai pangas Pangasianodon hypophthalmus sampled from Sylhet sadar upazila, Bangladesh. Bangladesh Journal of Zoology. 2018;46:81–90. [Google Scholar]
- Islam A., Saifuddin A., Al Faruq A., Islam S., Shano S., Alam M., Hassan M.M. Antimicrobial residues in tissues and eggs of laying hens at Chittagong, Bangladesh. International Journal One Health. 2016;2:75–80. [Google Scholar]
- Khan M., Ferdous J., Ferdous M., Islam M., Rafiq K., Rima U. Study on indiscriminate use of antibiotics in poultry feed and residues in broilers of Mymensingh city in Bangladesh. Progressive Agriculture. 2018;29:345–352. [Google Scholar]
- Khan S.A., Shawn A.I., Syeed A., Shaikat A.H., Hassan M.M. Antimicrobial Resistance Pattern in Domestic Animal- Wildlife- Environmental niche via the food chain to humans with a Bangladesh perspective; A systematic review. BMC Veterinary Research. 2020;16(1):302. doi: 10.1186/s12917-020-02519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauß J., van der Linden M., Grebe T., Hakenbeck R. Penicillin-binding proteins 2x and 2b as primary PBP targets in Streptococcus pneumoniae. Microbial Drug Resistance. 1996;2:183–186. doi: 10.1089/mdr.1996.2.183. [DOI] [PubMed] [Google Scholar]
- Kundu G.K., Alauddin M., Akter M.S., Khan M.S., Islam M.M., Mondal G., Islam D., Mohanta L.C., Huque A. Metal contamination of commercial fish feed and quality aspects of farmed tilapia (Oreochromis niloticus) in Bangladesh. Bioresearch Communications. 2017;3:345–353. [Google Scholar]
- Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Reports. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A.…Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine. 2009;6:W49–W65. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.C., Huang W.K., Huang T.S., Kunin C.M. Extent of antibiotic use in Taiwan shown by antimicrobial activity in urine. Lancet. 1999;354:1360. doi: 10.1016/S0140-6736(99)07446-2. [DOI] [PubMed] [Google Scholar]
- Manyi-Loh C., Mamphweli S., Meyer E., Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules. 2018;23:795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: Impacts on human health. Clinical Microbiology Reviews. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Martínez L., Calvo J. The growing problem of antibiotic resistance in clinically relevant gram-negative bacteria: Current situation. Enfermedades Infecciosas Y Microbiologia Clinica. 2010;28:25–31. doi: 10.1016/S0213-005X(10)70027-6. [DOI] [PubMed] [Google Scholar]
- Masood I., Ibrahim M., Hassali M., Ahmed M. Evolution of marketing techniques, adoption in pharmaceutical industry and related issues: A review. Journal of Clinical and Diagnostic Research. 2009;3:1942–1952. [Google Scholar]
- Mather A.E., Matthews L., Mellor D.J., Reeve R., Denwood M.J., Boerlin P.…Reid S.W.J. An ecological approach to assessing the epidemiology of antimicrobial resistance in animal and human populations. Proceedings of the Royal Society B: Biological Sciences. 2012;279:1630–1639. doi: 10.1098/rspb.2011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J., Kenneth H., Bernstein J., Buzby J.C. International trade of meat/poultry products and food safety issues. International trade and food safety: Economic Theory and Case Studies. 2003:48–73. [Google Scholar]
- McEwen S.A. Antibiotic use in animal agriculture: What have we learned and where are we going? Animal Biotechnology. 2006;17:239–250. doi: 10.1080/10495390600957233. [DOI] [PubMed] [Google Scholar]
- Ministry of Fisheries and Livestock Chapter-10: Ministry of fisheries and livestock. https://mof.gov.bd/sites/default/files/files/mof.portal.gov.bd/budget_mof/d9f53c16_c377_420a_9ba4_271ef1396986/G-1_09_144_Fisheries_English.pdf accessed on 03-03-2020.
- Molbak K. Spread of resistant bacteria and resistance genes from animals to humans--the public health consequences. Journal Vet Medicine B Infect Dis Vet Public Health. 2004;51:364–369. doi: 10.1111/j.1439-0450.2004.00788.x. [DOI] [PubMed] [Google Scholar]
- Nilsson O. Vancomycin resistant Enterococci in farm animals–occurrence and importance. Infection Ecology & Epidemiology. 2012;2:16959. doi: 10.3402/iee.v2i0.16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonga H.E., Simon C., Karimuribo E.D., Mdegela R.H. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro municipality, Tanzania. Zoonoses Public Health. 2010;57:339–344. doi: 10.1111/j.1863-2378.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Parker D., Armstrong D. Antibiotic feed additives and livestock production. Proceedings of the Nutrition Society. 1987;46:415–421. doi: 10.1079/pns19870056. [DOI] [PubMed] [Google Scholar]
- Peterson E., Kaur P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Frontiers in Microbiology. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajwal S., Vasudevan V., Sathu T., Irshad A., Nayankumar S., Pame K. Antibiotic residues in food animals: Causes and health effects. The Pharma Innovation J. 2017;6:1–4. [Google Scholar]
- Prescott J.F. History and current use of antimicrobial drugs in veterinary medicine. Microbiology Spectrum. 2017;5 doi: 10.1128/microbiolspec.ARBA-0002-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassow D., Schaper H. The use of feed medications in swine and poultry facilities in the Weser-Ems region. DTW. Deutsche tierarztliche Wochenschrift. 1996;103:244–249. [PubMed] [Google Scholar]
- Redding L., Barg F., Smith G., Galligan D., Levy M., Hennessy S. The role of veterinarians and feed-store vendors in the prescription and use of antibiotics on small dairy farms in rural Peru. Journal of Dairy Science. 2013;96:7349–7354. doi: 10.3168/jds.2013-7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roess A.A., Winch P.J., Ali N.A., Akhter A., Afroz D., El Arifeen S., Darmstadt G.L., Baqui A.H., Group B.P.S. Animal husbandry practices in rural Bangladesh: Potential risk factors for antimicrobial drug resistance and emerging diseases. The American Journal of Tropical Medicine and Hygiene. 2013;89:965–970. doi: 10.4269/ajtmh.12-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura E., Homedes J., Klasing K.C. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. Journal of Nutrition. 1992;122:2383–2390. doi: 10.1093/jn/122.12.2383. [DOI] [PubMed] [Google Scholar]
- Sachi S., Ferdous J., Sikder M.H., Hussani S.A.K. Antibiotic residues in milk: Past, present, and future. Journal of advanced veterinary and animal research. 2019;6:315. doi: 10.5455/javar.2019.f350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker Y.A., Hasan M.M., Paul T.K., Rashid S.Z., Alam M.N., Sikder M.H. Screening of antibiotic residues in chicken meat in Bangladesh by thin layer chromatography. Journal of Advanced Veterinary and Animal Research. 2018;5:140–145. [Google Scholar]
- Sattar S., Hassan M.M., Islam S., Alam M., Al Faruk M.S., Chowdhury S., Saifuddin A. Antibiotic residues in broiler and layer meat in Chittagong district of Bangladesh. Veterinary World. 2014;7 [Google Scholar]
- Smith H.W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. The Lancet. 1969;293:1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Tollefson L., Miller M.A. Antibiotic use in food animals: Controlling the human health impact. Journal of AOAC International. 2000;83:245–254. [PubMed] [Google Scholar]
- Vaananen M.H., Pietila K., Airaksinen M. Self-medication with antibiotics--does it really happen in Europe? Health Policy. 2006;77:166–171. doi: 10.1016/j.healthpol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Van den Bogaard A., Willems R., London N., Top J., Stobberingh E. Antibiotic resistance of faecal Enterococci in poultry, poultry farmers and poultry slaughterers. Journal of Antimicrobial Chemotherapy. 2002;49:497–505. doi: 10.1093/jac/49.3.497. [DOI] [PubMed] [Google Scholar]
- Vázquez‐Moreno L., Bermúdez A M., Langure A., Higuera‐Ciapara I., Aguayo M., Flores E. Antibiotic residues and drug resistant bacteria in beef, and chicken tissues. Journal of Food Science. 1990;55:632–634. [Google Scholar]
- Wassenaar T.M. Use of antimicrobial agents in veterinary medicine and implications for human health. Critical Reviews in Microbiology. 2005;31:155–169. doi: 10.1080/10408410591005110. [DOI] [PubMed] [Google Scholar]
- Wegener H.C. Institute of medicine (US). Improving food safety through a one health approach: Workshop summary. A15. National Academies Press (US); Washington (DC): 2012. Antibiotic resistance—linking human and animal health; pp. 331–349. [PubMed] [Google Scholar]
- von Wintersdorff C.J., Penders J., van Niekerk J.M., Mills N.D., Majumder S., van Alphen L.B., Savelkoul P.H., Wolffs P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J.H., Yau B., Choi K.C., Chau C.T., Huang Q.R., Lee S.S. Public knowledge, attitudes and behavior on antibiotic use: A telephone survey in Hong Kong. Infection. 2008;36:153–157. doi: 10.1007/s15010-007-7214-5. [DOI] [PubMed] [Google Scholar]