Abstract
Background:
N,N-diethyl-m-toluamide (DEET) is a widely used insect repellent in the United States.
Objectives:
To assess exposure to DEET in a representative sample of persons 6 years and older in the U.S. general population from the 2007–2010 National Health and Nutrition Examination Survey.
Methods:
We analyzed 5348 urine samples by using online solid-phase extraction coupled to isotope dilution-high-performance liquid chromatography-tandem mass spectrometry. We used regression models to examine associations of various demographic parameters with urinary concentrations of DEET biomarkers.
Results:
We detected DEET in ~3% of samples and at concentration ranges (>0.08 μg/L–45.1 μg/L) much lower than those of 3-(diethylcarbamoyl)benzoic acid (DCBA) (>0.48 μg/L–30,400 μg/L) and N,N-diethyl-3-hydroxymethylbenzamide (DHMB) (>0.09 μg/L–332 μg/L). DCBA was the most frequently detected metabolite (~84%). Regardless of survey cycle and the person’s race/ethnicity or income, adjusted geometric mean concentrations of DCBA were higher in May–Sep than in Oct–Apr. Furthermore, non-Hispanic whites in the warm season were more likely than in the colder months [adjusted odds ratio (OR) = 10.83; 95% confidence interval (CI), 3.28–35.79] and more likely than non-Hispanic blacks (OR = 3.45; 95% CI, 1.51–7.87) to have DCBA concentrations above the 95th percentile.
Conclusions:
The general U.S. population, including school-age children, is exposed to DEET. However, reliance on DEET as the sole urinary biomarker would likely underestimate the prevalence of exposure. Instead, oxidative metabolites of DEET are the most adequate exposure biomarkers. Differences by season of the year based on demographic variables including race/ethnicity likely reflect different lifestyle uses of DEET-containing products.
Keywords: Biomonitoring, Exposure assessment, Human urine, Insect repellent, NHANES
1. Introduction
Since its development in 1946, N,N-diethyl-m-toluamide (DEET) has become a widely used insect repellent in the United States (USEPA, 2015). DEET has important public health applications because it repels potentially disease-carrying insects and ticks such as deer ticks associated with Lyme disease, and mosquitoes that can transmit malaria, encephalitis, Dengue fever, and the Zika and West Nile viruses (CDC, 2015b). DEET dermal toxicity in animals is relatively low, although at large enough doses, DEET displays neurotoxicity primarily with acute inhalation or oral exposure (ATSDR, 2015; California Environmental Protection Agency, 2000), and DEET poisoning may result in death (Wiles et al., 2014). The mechanism behind DEET neurotoxicity is unclear, but DEET has low potency for inhibiting human acetylcholinesterase (Swale et al., 2014). In 2014, the US Environmental Protection Agency completed an interim review of DEET under the Registration Review Program and did not identify risks of concern to human health, non-target species or the environment (USEPA, 2015).
DEET has been detected in aquatic ecosystems worldwide (Costanzo et al., 2007)—including streams, surface waters, ground-water systems, and sewage treatment plant effluents, albeit at trace levels, throughout the United States (ATSDR, 2015)—likely because of its widespread use. Furthermore, because currently 225 insect repellent products containing DEET at concentrations ranging from 4% to 100% are registered (CDC, 2009), and, at least once per year, about one third of the US population uses these products (USEPA, 2015), human exposure to DEET likely occurs (ATSDR, 2015). To evaluate such exposures, biomonitoring—measuring the parent chemical (or its metabolite or reaction product) in human samples—is a useful tool (CDC, 2009; National Research Council, 2012). Upon exposure, DEET undergoes phase I and phase II reactions to multiple oxidative metabolites including N,N-diethyl-3-hydroxymethylbenzamide (DHMB) and 3-(diethylcarbamoyl)benzoic acid (DCBA) (ATSDR, 2015; California Environmental Protection Agency, 2000; Selim et al., 1995; Usmani et al., 2002; Wu et al., 1979). Both DEET and its metabolites may serve as biomarkers to assess recent exposure to DEET (ATSDR, 2015; Selim et al., 1995).
Biomonitoring data on the US general population exposure to DEET, based on the measurement of DEET, suggest limited prevalence of exposure to DEET (CDC, 2009). In particular, the 90th percentile of DEET urinary concentrations from participants of the 2001–2002 National Health and Nutrition Examination Survey (NHANES) was 0.11–0.13 μg/L, close to the method limit of detection (LOD) of 0.1 μg/L (CDC, 2009). However, for some other short-lived chemicals such as certain phthalates, oxidative (i.e., phase I) metabolites rather than other metabolites or the parent compounds are better exposure biomarkers (Calafat et al., 2011; Kato et al., 2004; Koch et al., 2006; Koch and Angerer, 2007; Leng et al., 2014). Therefore, assessing exposure to DEET using additional urinary biomarkers is of interest. Here, we present the first nationally representative data on the concentrations of oxidative metabolites of DEET in urine among general population Americans 6 years of age and older, stratified by age group, sex, and race/ethnicity.
2. Materials and methods
2.1. Study population
Since 1999, the Centers for Disease Control and Prevention (CDC) conducts annually the National Health and Nutrition Examination Survey (NHANES) (CDC, 2011; CDC, 2015a). NHANES provides data, released in two year intervals, to evaluate the health and nutritional status of the civilian, noninstitutionalized U.S. population of all ages. NHANES includes household interviews, standardized physical examinations, and collection of medical histories and biologic specimens (CDC, 2015a). Some of these specimens are used to assess exposure to environmental chemicals.
For the present study, we analyzed a total of 5348 (2597 [2007–2008], 2751 [2009–2010]) spot urine specimens, collected from a one-third subset of 2007–2010 NHANES participants 6 years of age and older. The representative design of the survey was maintained because the subset was random. The National Centers for Health Statistics (NCHS) Research Ethics Review Board reviewed and approved the study protocol. All participants gave informed written consent; parents or guardians provided consent for participants younger than 18 years of age.
2.2. Urinary concentrations of DEET biomarkers
The urine samples were shipped on dry ice to CDC’s National Center for Environmental Health and stored at −20 °C or below until analyzed. At these temperatures, the integrity of the specimens is maintained for years. The analytical method for measuring DEET, DCBA and DHMB involved enzymatic hydrolysis of the conjugated species of the target analytes in 100 μL of urine, followed by on-line solid-phase extraction, separation with high-performance liquid chromatography, and detection by isotope-dilution positive ion atmospheric pressure chemical ionization tandem mass spectrometry (Kuklenyik et al., 2013). Calibration standards, quality control, and reagent blank samples were included in each analytical batch along with the study samples. The LODs were 0.93 μg/L (DCBA, NHANES 2007–2008), 0.48 μg/L (DCBA, NHANES 2009–2010), 0.09 μg/L (DHMB), and 0.08 μg/L (DEET). We prepared low-concentration (3 μg/L–20 μg/L) and high-concentration (40 μg/L–200 μg/L) quality control materials (QCLs and QCHs, respectively) with pooled human urine that was analyzed with standards, reagent blanks, and urine samples. The precision of measurements, expressed as the relative standard deviation of multiple measures of the QC materials, was 4%–8%, depending on the target analyte and concentration range (i.e., QCH and QCL). Details of the analytical procedures used are available on the NHANES website (http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/DEET_F_met.pdf).
2.3. Statistical analysis
We used SAS (version 9.3; SAS Institute Inc.; Cary, North Carolina) and SUDAAN (version 11; Research Triangle Institute; Research Triangle Park, North Carolina) to perform statistical analyses. SUDAAN calculates variance estimates that account for the complex, clustered design of NHANES. As recommended by NCHS, we used the environmental subsample population C weights to produce estimates that are representative of the U.S. population. For concentrations below the LOD, we imputed a value equal to the LOD divided by the square root of 2 (Hornung and Reed, 1990). Statistical significance was set at p < 0.05.
We stratified age, reported in years at the last birthday, in four groups (6–11 years, 12–19 years, 20–59 years, and 60 years and older). On the basis of self-reported data, we categorized race/ethnicity as non-Hispanic black, non-Hispanic white, and Mexican American. Participants not defined by these racial/ethnic categories were included only in the total population estimate. For each age, sex, and race/ethnic group, we calculated the geometric means (if the overall weighted frequency of detection was >60%) and distribution percentiles for both volume-based (μg/L) and creatinine-corrected concentrations (μg/g creatinine). We also determined weighted Pearson correlations among the concentrations (log10 transformed) of DCBA and DHMB in the 766 samples with detectable concentrations of both compounds.
We used multiple regression to examine association between several variables (i.e., age group, sex, race/ethnicity, creatinine concentration, household income, and season of year) and the log-transformed urine concentrations of DCBA, the only DEET biomarker detected in >60% of the participants. Study participants reported annual household income in increments of $5000 (from <$5000 to ≥$75,000); to obtain a comparable number of participants per group, we categorized income as <$20,000, $20,000–$45,000, $45,000–$75,000, and >$75,000. Based on the month of the physical examination at the mobile examination center, we categorized season of the year as winter (Nov–Apr) or summer (May–Sep). For the multiple regression models, we used the above variables and all their possible two-way interactions to calculate the adjusted geometric mean (GM) concentrations of DCBA (in μg/L). We log transformed the concentrations of DEET, its metabolites and creatinine because their distributions were right-skewed.
To arrive at the final model for DCBA, we used backward elimination with SUDAAN to remove the nonsignificant interactions one at a time. Nonsignificant main effects were then removed one at a time, and the model was rerun to determine whether the beta coefficients for significant main effects or interactions changed by >10%. If any did, we retained the nonsignificant main effect in the model. Once the backward procedure was completed, we added main effects and interactions back into the model one at a time to determine whether any were significant. We retained all significant main effects and their interactions in the final model.
We also conducted weighted univariate and multiple logistic regressions to examine the association of the concentrations of DCBA above the 95th percentile (an arbitrary value selected as an example of higher than average concentrations) with sex, age group, race/ethnicity, household income, cycle of data collection, log10(creatinine), and season of year.
3. Results
3.1. Urinary concentrations of DEET biomarkers
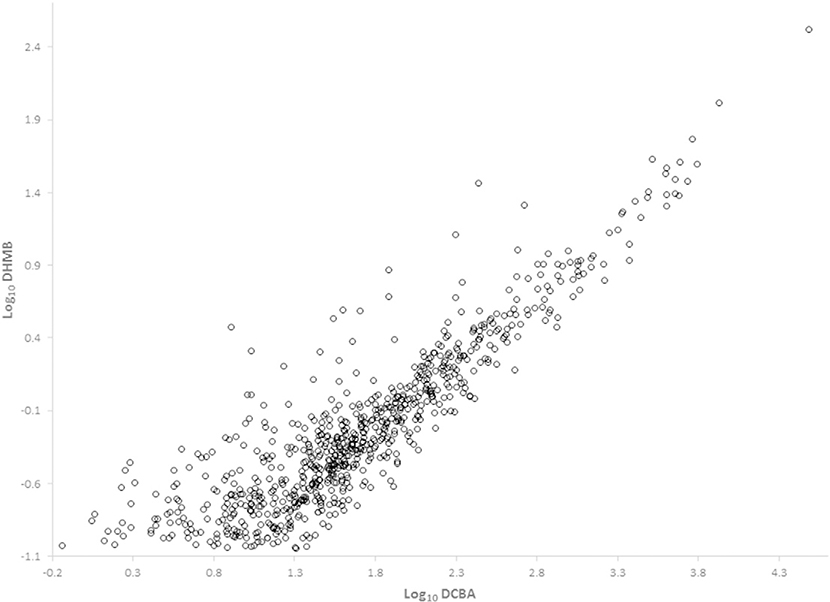
The geometric mean and selected percentile concentrations stratified by age, sex, and race/ethnicity are given in Tables 1–2 for DCBA and DHMB, respectively, two metabolites that have not been evaluated previously in NHANES, and in the supporting information for DEET (Supplemental Table S1). We detected DCBA at concentrations ranging from >0.48 μg/L to 30,400 μg/L in approximately 84% of persons examined. By contrast, DHMB was detected in approximately 15.5% of persons at concentrations of >0.09 μg/L–332 μg/L, while DEET, the parent compound, was only detected in ~3% of samples (range: 0.08 μg/L–45.1 μg/L) (Table 3). Of note, the person with the highest concentration of DCBA (30,400 μg/L) also had the highest concentrations of DHMB (332 μg/L) and DEET (45.1 μg/L). The correlation between the concentrations of DCBA and DHMB among the 766 persons with detectable concentrations of both compounds was excellent [Pearson correlation coefficient (R) = 0.90] (Fig. 1).
Table 1.
Geometric mean and selected percentiles of 3-(diethylcarbamoyl)-benzoic acid (DCBA) concentrations in urine (in μg/L first row and in μg/g creatinine second row) for the U.S. population 6 years of age and oldera.
Data from National Health and Nutrition Examination Survey 2007–2010.
| Survey years | Geometric mean |
Selected percentile (95% confidence interval) |
Sample size | ||||
|---|---|---|---|---|---|---|---|
| (95% confidence limit) | 50th | 75th | 90th | 95th | |||
| Total | 07–08 | 3.50 (2.64–4.64) | 2.37 (1.88–3.10) | 9.14 (5.61–14.5) | 33.9 (20.5–53.1) | 79.2 (37.9–145) | 2538 |
| 3.60 (2.79–4.65) | 2.79 (2.14–3.55) | 8.55 (5.49–13.2) | 27.3 (17.8–47.9) | 70.8 (34.1–170) | 2537 | ||
| 09–10 | 4.54 (3.35–6.15) | 3.40 (2.31–4.95) | 13.8 (8.63–20.6) | 51.9 (31.1–108) | 165 (57.8–464) | 2735 | |
| 4.74 (3.48–6.46) | 3.35 (2.30–5.26) | 12.9 (8.53–20.6) | 44.6 (28.3–86.3) | 131 (47.0–405) | 2735 | ||
| Age group | |||||||
| 6–11 years | 07–08 | 4.44 (3.73–5.29) | 3.44 (2.70–5.87) | 12.7 (9.54–15.9) | 42.0 (24.2–70.4) | 79.7 (44.9–114) | 378 |
| 5.64 (4.72–6.75) | 4.84 (3.65–5.78) | 14.2 (10.7–19.8) | 47.7 (34.2–55.1) | 88.6 (47.9–182) | 378 | ||
| 09–10 | 6.44 (3.72–11.1) | 5.35 (2.58–8.86) | 18.5 (8.15–37.9) | 83.8 (28.4–439) | 316 (41.2–3970) | 385 | |
| 8.72 (5.03–15.l) | 6.42 (4.02–11.6) | 23.5 (13.2–36.7) | 75.4 (28.6–673) | 365 (46.2–4980) | 385 | ||
| 12–19 years | 07–08 | 5.26 (3.47–7.98) | 4.37 (2.68–5.98) | 13.1 (6.81–25.8) | 35.4 (20.4–71.2) | 71.2 (30.7–700) | 380 |
| 4.08 (2.81–5.93) | 2.96(2.14–5.36) | 11.0 (6.38–16.0) | 24.3 (14.8–53.4) | 53.4 (19.3–345) | 379 | ||
| 09–10 | 6.58 (4.49–9.66) | 4.63 (2.82–8.64) | 18.9 (10.7–33.6) | 87.8 (32.9–186) | 186 (31.1–1130) | 398 | |
| 5.65 (3.76–8.50) | 3.76 (2.56–6.22) | 16.5 (7.29–31.7) | 68.6 (25.3–182) | 154 (25.3–1270) | 398 | ||
| 20–59 years | 07–08 | 3.33 (2.56–4.35) | 2.23 (1.83–2.90) | 7.95 (5.05–14.5) | 30.8 (17.4–53.1) | 75.6 (39.3–131) | 1157 |
| 3.34 (2.61–4.27) | 2.73 (1.99–3.46) | 7.57 (4.92–12.1) | 24.8 (14.9–44.7) | 57.8 (30.9–117) | 1157 | ||
| 09–10 | 4.39 (3.29–5.86) | 3.33 (2.23–4.95) | 14.0 (8.36–20.9) | 51.4 (32.6–95.8) | 138 (52.9–280) | 1300 | |
| 4.41 (3.25–5.97) | 2.98 (2.11–5.32) | 11.7 (7.71–19.6) | 39.1(24.7–82.6) | 112 (51.3–228) | 1300 | ||
| 60 years and older | 07–08 | 2.78 (1.75–4.42) | 1.64 (.936–3.06) | 6.15 (3.08–16.9) | 34.7 (16.3–75.4) | 103 (32.4–200) | 623 |
| 3.42 (2.33–5.02) | 2.47 (1.45–3.64) | 7.33 (4.25–16.8) | 33.8 (15.9–86.0) | 93.3 (34.2–244) | 623 | ||
| 09–10 | 3.42 (2.39–4.91) | 2.13 (1.45–4.00) | 9.63 (5.33–17.1) | 35.4 (19.7–63.8) | 103 (43.2–346) | 652 | |
| 4.06 (2.95–5.59) | 2.68 (2.10–3.96) | 10.8 (6.79–15.9) | 37.7 (22.2–51.1) | 108 (42.7–393) | 652 | ||
| Sex | |||||||
| Males | 07–08 | 4.15 (2.88–6.00) | 2.90 (2.13–4.34) | 11.3 (6.63–19.7) | 37.7 (20.7–82.0) | 112 (34.7–556) | 1269 |
| 3.46 (2.46–4.87) | 2.72 (1.76–3.73) | 8.68 (5.34–14.4) | 27.8 (16.9–69.4) | 87.0 (27.8–403) | 1269 | ||
| 09–10 | 5.58 (3.94–7.90) | 4.39 (2.67–6.24) | 18.7 (10.8–30.6) | 78.3 (37.3–174) | 199 (96.2–525) | 1340 | |
| 4.97 (3.49–7.08) | 3.29 (2.14–5.94) | 14.7 (9.13–24.0) | 60.0 (28.5–134) | 185 (74.8–433) | 1340 | ||
| Females | 07–08 | 2.97 (2.32–3.80) | 2.06 (1.64–2.59) | 6.84 (4.41–10.8) | 30.8 (15.0–40.8) | 52.6 (36.4–103) | 1269 |
| 3.74 (2.99–4.68) | 2.88 (2.27–3.63) | 8.55 (5.36–13.2) | 27.2 (16.2–46.3) | 54.8 (34.2–117) | 1268 | ||
| 09–10 | 3.73 (2.79–4.98) | 2.76 (1.87–4.24) | 9.91 (6.35–15.9) | 36.2 (22.4–70.4) | 94.9 (40.2–278) | 1395 | |
| 4.54 (3.40–6.05) | 3.35 (2.39–4.67) | 11.8 (7.55–18.4) | 35.3 (24.2–53.4) | 77.6 (36.7–252) | 1395 | ||
| Race/ethnicity | |||||||
| Non-Hispanic whites | 07–08 | 3.47 (2.35–5.14) | 2.22 (1.65–3.19) | 9.12 (4.82–17.0) | 36.5 (17.7–82.0) | 86.9 (32.9–356) | 1064 |
| 3.78 (2.69–5.32) | 2.82 (2.05–4.02) | 8.70 (5.23–14.9) | 30.7 (16.7–57.2) | 76.9 (26.7–432) | 1064 | ||
| 09–10 | 5.48 (3.83–7.84) | 4.31 (2.64–6.25) | 17.7 (10.5–28.4) | 67.9 (32.6–195) | 200 (63.8–832) | 1199 | |
| 5.97 (4.13–8.64) | 4.41 (2.61–7.43) | 17.4 (10.7–26.7) | 61.1 (29.0–189) | 189 (56.4–849) | 1199 | ||
| Mexican Americans | 07–08 | 3.70 (2.57–5.33) | 3.26 (1.87–5.17) | 9.63 (5.93–17.6) | 28.0 (14.7–69.1) | 69.1 (27.5–133) | 490 |
| 3.79 (2.57–5.58) | 3.60 (1.99–5.96) | 9.91 (6.55–16.9) | 27.8 (15.0–60.6) | 60.6 (24.4–107) | 490 | ||
| 09–10 | 2.63 (1.61–4.28) | 2.03 (.932–4.71) | 7.35 (4.22–14.5) | 23.1 (12.5–48.2) | 48.9 (26.0–94.3) | 599 | |
| 2.74 (1.75–4.28) | 2.03 (1.12–5.18) | 7.57 (3.92–14.0) | 23.3 (13.6–31.8) | 37.4 (23.6–90.5) | 599 | ||
| Non-Hispanic blacks | 07–08 | 4.36 (3.18–5.96) | 3.54 (2.24–6.04) | 10.3 (6.78–17.4) | 31.9 (19.3–51.6) | 62.4 (40.4–103) | 562 |
| 3.33 (2.46–4.49) | 2.74 (1.91–3.76) | 7.07 (4.88–11.9) | 22.5 (12.6–45.1) | 53.9 (28.7–103) | 561 | ||
| 09–10 | 3.91 (2.85–5.35) | 3.22 (2.24–4.75) | 9.53 (5.88–14.4) | 23.4 (18.0–33.4) | 38.4 (29.1–60.5) | 497 | |
| 2.98 (2.26v3.94) | 2.41 (1.70–3.37) | 6.73 (4.34–9.46) | 16.5 (13.0–20.4) | 30.6 (19.4–51.1) | 497 | ||
The 95% confidence intervals are shown in parentheses. Participants not defined by the three racial/ethnic groups shown were included only in the total population estimate.
Table 2.
Geometric mean and selected percentiles of N,N-diethyl-3-(hydroxymethyl)benzamide (DHMB) concentrations in urine (in μg/L first row and in μg/g creatinine second row) for the U.S. population 6 years of age and oldera.
Data from National Health and Nutrition Examination Survey 2007–2010
| Survey years | Geometric mean |
Selected percentile (95% confidence interval) |
Sample size | ||||
|---|---|---|---|---|---|---|---|
| (95% confidence limit) | 50th | 75th | 90th | 95th | |||
| Total | 07–08 | * | < LOD | < LOD | .229 (<LOD–.525) | .780 (.326–1.51) | 2562 |
| * | < LOD | < LOD | .331 (<LOD–.452) | .628 (.393–1.32) | 2560 | ||
| 09–10 | * | < LOD | < LOD | .455 (.162–.956) | 1.34 (.644–3.10) | 2736 | |
| * | < LOD | < LOD | .449 (.300–.720) | 1.13 (.548–2.41) | 2736 | ||
| Age group | |||||||
| 6–11 years | 07–08 | * | < LOD | < LOD | .275 (.168–.433) | .640 (.264–2.64) | 380 |
| * | < LOD | < LOD | .370 (.289–.524) | .831 (.347–1.37) | 380 | ||
| 09–10 | * | < LOD | < LOD | .655 (<LOD–2.93) | 2.82 (.205–24.6) | 385 | |
| * | < LOD | < LOD | .572 (<LOD–3.40) | 3.12 (.370–18.4) | 385 | ||
| 12–19 years | 07–08 | * | < LOD | < LOD | .356 (<LOD–.879) | .665 (.165–8.14) | 386 |
| * | < LOD | < LOD | .253 (<LOD–.555) | .544 (.191–1.76) | 384 | ||
| 09–10 | * | < LOD | < LOD | .472 (<LOD–1.59) | 1.20 (.201–4.11) | 398 | |
| * | < LOD | < LOD | .436 (<LOD–.869) | .869 (.246–8.42) | 398 | ||
| 20–59 years | 07–08 | * | < LOD | < LOD | .188 (<LOD–.413) | .767 (.335–1.30) | 1167 |
| * | < LOD | < LOD | .331 (<LOD–.44l) | .582 (.441–.866) | 1167 | ||
| 09–10 | * | < LOD | < LOD | .498 (.172–.956) | 1.34 (.729–2.29) | 1304 | |
| * | < LOD | < LOD | .468 (.300–.702) | 1.10 (.572–1.79) | 1304 | ||
| 60 years and older | 07–08 | * | < LOD | < LOD | .256 (<LOD–.787) | .787 (.194–1.81) | 629 |
| * | < LOD | < LOD | .394 (<LOD–.70l) | 1.01 (.389–2.48) | 629 | ||
| 09–10 | * | < LOD | < LOD | .257 (.106–.512) | .840 (.521–2.46) | 649 | |
| * | < LOD | < LOD | .395 (.315–.489) | .875 (.548–2.41) | 649 | ||
| Sex | |||||||
| Males | 07–08 | * | < LOD | < LOD | .325 (.091–.909) | 1.05 (.249–4.86) | 1283 |
| * | < LOD | < LOD | .300 (.176–.826) | .866 (.304–3.33) | 1282 | ||
| 09–10 | * | < LOD | < LOD | .744 (.323–1.43) | 1.81 (.946–3.94) | 1339 | |
| * | < LOD | < LOD | .524 (.280–1.39) | 1.45 (.718–3.16) | 1339 | ||
| Females | 07–08 | * | < LOD | < LOD | .165 (<LOD–.326) | .512 (.256–.968) | 1279 |
| * | < LOD | < LOD | .341 (<LOD–.393) | .572 (.419–.734) | 1278 | ||
| 09–10 | * | < LOD | < LOD | .220 (<LOD–.52l) | .796 (.329–2.05) | 1397 | |
| * | < LOD | < LOD | .419 (<LOD–.488) | .723 (.458–1.85) | 1397 | ||
| Race/ethnicity | |||||||
| Non-Hispanic whites | 07–08 | * | < LOD | < LOD | .255 (<LOD–.861) | .884 (.225–4.84) | 1071 |
| * | < LOD | < LOD | .343 (<LOD–.583) | .826 (.349–2.99) | 1071 | ||
| 09–10 | * | < LOD | < LOD | .644 (.182–1.34) | 1.89 (.770–5.34) | 1195 | |
| * | < LOD | < LOD | .531 (.305–1.36) | 1.59 (.524–5.83) | 1195 | ||
| Mexican Americans | 07–08 | * | < LOD | < LOD | .216 (.092–.509) | .509 (.207–.989) | 499 |
| * | < LOD | < LOD | .315 (.203–.446) | .467 (.337–.720) | 498 | ||
| 09–10 | * | < LOD | < LOD | .228 (<LOD–.504) | .507 (.223–.866) | 598 | |
| * | < LOD | < LOD | .297 (<LOD–.401) | .415 (.299–.718) | 598 | ||
| Non-Hispanic blacks | 07–08 | * | < LOD | < LOD | .310 (.091–.470) | .640 (.378–1.29) | 567 |
| * | < LOD | < LOD | .234 (.175–.411) | .487 (.315–1.05) | 566 | ||
| 09–10 | * | < LOD | < LOD | .135 (<LOD–.292) | .449 (.212–.884) | 503 | |
| * | < LOD | < LOD | .221 (<LOD–.262) | .362 (.246–.648) | 503 | ||
The 95% confidence intervals are shown in parentheses. Participants not defined by the three racial/ethnic groups shown were included only in the total population estimate. Limit of detection (LOD)=0.09 μg/L.
Not calculated: proportion of results below limit of detection was too high to provide a valid result.
Table 3.
2 × 2 table for DEET, DCBA and DMHB [weighted percent of participants]a.
| DCBA urinary concentration |
DMHB urinary concentration |
|||||
|---|---|---|---|---|---|---|
| Detectable | Non-detectable | Total | Detectable | Non-detectable | Total | |
| DEET urinary concentration | ||||||
| Detectable | 2.92 | 0.06 | 2.98 | 2.69 | 0.28 | 2.97 |
| Non-detectable | 81.00 | 16.02 | 97.02 | 12.76 | 84.27 | 97.03 |
| Total | 83.92 | 16.08 | 100 | 15.45 | 84.55 | 100 |
The LODs were 0.93 μg/L (DCBA, NHANES 2007–2008), 0.48 μg/L (DCBA, NHANES 2009–2010), 0.09 μg/L (DHMB), and 0.08 μg/L (DEET).
Fig. 1.
Correlation analysis of the log-transformed urinary concentrations of DCBA and DHMB.
3.2. Determinants of DEET exposure
The final DCBA model included significant interactions among the following variables (selected on the basis of statistical, demographic, and biologic considerations): season of year and race/ethnicity, season of year and survey cycle, season of year and household income, and age group and race/ethnicity (Table 4, Supplemental Table S2). Adjusted GM DCBA concentrations in May–Sep were higher than in Nov–Apr, but differences only reached statistical significance (p = 0.017) during the 2009–2010 survey cycle (Table 4, Supplemental Table S2). Similarly, regardless of income, adjusted GM concentrations were higher in May–Sep than in Nov–Apr (Table 4, Supplemental Table S2), although the differences did not reach statistical significance (p = 0.294) for persons in the lowest income category and were of borderline statistical significance (p = 0.059) for persons in the next to lowest income category. Persons in the three race/ethnicity categories examined had higher adjusted GM concentrations in the summer than in the winter months (Supplemental Table S2). Non-Hispanic white children and adolescents had significantly higher adjusted GM concentrations than adults and 60+ year old seniors, but the differences between children and adolescents did not reach statistical significance (p = 0.286). Similarly, non-Hispanic black children and adolescents had higher adjusted GM concentrations than adults and 60+ year old seniors, although concentrations between children and adolescents (p = 0.451) and between adolescents and seniors (p = 0.242) did not differ statistically. Among Mexican Americans, children had statistically significantly higher concentrations than all other age groups (p = <0.001–0.042).
Table 4.
Adjusted geometric mean (GM) concentrations (95% confidence intervals [CI]) in μg/L of 3-(diethylcarbamoyl)-benzoic acid (DCBA) in various demographic groups.
| Variable | GM (95% CI) |
|---|---|
| Winter, Mexican American | 2.83 (1.95–4.12) |
| Winter, non-Hispanic White | 2.58 (1.99–3.36) |
| Winter, non-Hispanic Black | 2.81 (2.21–3.58) |
| Summer, Mexican American | 3.6 (2.27–5.71) |
| Summer, non-Hispanic White | 6.07 (4.45–8.29) |
| Summer, non-Hispanic Black | 3.48 (2.66–4.55) |
| Winter, 2007–2008 | 2.87 (1.98–4.17) |
| Winter, 2009–2010 | 2.39 (1.93–2.95) |
| Summer, 2007–2008 | 3.76 (2.58–5.47) |
| Summer, 2009–2010 | 7.28 (4.88–10.85) |
| 6–11 years, Mexican American | 5.27 (3.85–7.22) |
| 6–11 years, non-Hispanic White | 7.67 (5.07–11.62) |
| 6–11 years, non-Hispanic Black | 4.46 (3.33–5.98) |
| 12–19 years, Mexican American | 3.1 (2.32–4.14) |
| 12–19 years, non-Hispanic White | 6.4 (4.59–8.93) |
| 12–19 years, non-Hispanic Black | 3.93 (2.95–5.23) |
| 20–59 years, Mexican American | 2.98 (2.13–4.15) |
| 20–59 years, non-Hispanic White | 4.04 (3.3–4.95) |
| 20–59 years, non-Hispanic Black | 2.93 (2.41–3.56) |
| 60 + years, Mexican American | 3.74 (2.71–5.16) |
| 60 + years, non-Hispanic White | 3.51 (2.65–4.66) |
| 60 + years, non-Hispanic Black | 3.24 (2.36–4.45) |
| Winter, <$20K | 2.87 (2.2–3.74) |
| Winter, $20–$45K | 2.97 (2.11–4.16) |
| Winter, $45K–$75K | 2.2 (1.7–2.84) |
| Winter, >$75K | 2.53 (1.98–3.22) |
| Summer, <$20K | 4.3 (3.15–5.88) |
| Summer, $20–$45K | 5.44 (4.06–7.29) |
| Summer, $45–$75K | 5.16 (4.01–6.64) |
| Summer, >$75K | 5.84 (4.05–8.43) |
In weighted univariate analysis, season of year (p = 0.001), sex (p = 0.003), race/ethnicity (p = 0.013), household income (p = 0.016), and the log10(creatinine) (p < 0.001), but not cycle of data collection (p = 0.23) or age group (p = 0.44) were significantly associated with the likelihood of DCBA exceeding the 95th percentile (Supplemental Table S3).
In the final multiple logistics regression, the log10(creatinine) (p < 0.001) and the interaction term season * race (p = 0.005) were significantly associated with the likelihood of DCBA exceeding the 95th percentile (Supplemental Table S4). People with higher urinary creatinine were more likely than others to have DCBA concentrations above the 95th percentile [adjusted odds ratio (OR) = 4.59; 95% confidence interval (CI), 2.31–9.13]. Non-Hispanic whites were 10.83 times more likely to have DCBA concentrations above the 95th percentile (OR = 10.83; 95% CI, 3.28–35.79) in the summer than in the winter season (p < 0.001). For Mexican Americans and Non-Hispanic blacks, although concentrations were higher in the summer than in the winter season, the differences were not statistically significant. Furthermore, in the summer season, compared to non-Hispanic blacks, non-Hispanic whites were 3.45 times more likely to have DCBA concentrations above the 95th percentile [OR = 3.45; 95% CI, 1.51–7.87] (p = 0.005). We observed no statistically significant differences between non-Hispanic blacks and Mexican Americans regardless of the season, and between non-Hispanic whites and non-Hispanic blacks in the winter season (Supplemental Table S4).
4. Discussion
4.1. Urinary concentrations of DEET biomarkers
We detected DCBA in more than three quarters of the samples analyzed (~84%), and much less frequently DHMB (~15%) and DEET, the parent compound (~3%). DEET concentration ranges were also considerably lower than those of DCBA and DHMB. Interestingly, these data are in agreement with the concentrations among a group of Puerto Rican pregnant women in the only study published to date that has evaluated these three compounds (Lewis et al., 2014). Consistent with expectations, urinary concentrations of DCBA and DHMB—metabolites of the same parent compound—correlated well with each other. For the first time, we report concentrations of DEET metabolites among school-age children and adolescents in the United States suggesting that exposure also occurs at young ages. These NHANES 2007–2010 data confirming that >80% of the general U.S. population is exposed to DEET could be used to derive internal dose exposure estimates.
4.2. Determinants of DEET exposure
We observed that adjusted GM concentrations of DCBA were dependent upon season of the year, race/ethnicity, household income and age. The differences in concentrations among the various demographic groups examined may reflect lifestyle differences in the use of DEET containing products, likely the primary source of exposure to DEET. Also, because trace levels of DEET have been detected in water intended for human consumption (ATSDR, 2015; Calza et al., 2011), and DHMB (but not DCBA) was also detected in Italian natural river waters (Calza et al., 2011), we can’t rule out that exposure to DEET or its transformation products from contaminated drinking water may occur. Among NHANES participants, we found that regardless of household income, survey cycle or race/ethnicity, adjusted GM concentrations of DCBA were higher in the warmer months than in the colder months. These findings are consistent with the increased use of DEET in warm weather when people likely spend more time outdoors for recreational activities than during colder weather (Chan and Ryan, 2009); also pests are more abundant with higher seasonal temperatures (Meineke et al., 2013), thus additional protection may be needed to keep away insects and other bugs when temperatures are higher in the spring/summer months. Furthermore, the higher concentrations of DEET in surface water and wastewater during summer months may too provide additional evidence of consumers’ increased use of DEET-containing products in the warm season (Aronson et al., 2012).
Interestingly, NHANES participants’ adjusted GM concentrations of DCBA were almost two times higher in 2009–2010 than in 2007–2008. Because we used the same analytical approach to analyze all samples, these results cannot be explained by differences in analytical methods. Instead, we posit that these findings may be related to differences in sampling locations per survey cycle. Each year, NHANES visits approximately 15 different localities (CDC, 2013). Perhaps in 2009–2010 compared to 2007–2008, the selected locations included warmer climate regions of the country, areas closer to outdoor recreation spaces (e.g., state parks) or other areas where people may use more frequently DEET-containing products than in other parts of the country. To ensure participants’ full privacy, NCHS does not release to the public certain details (e.g., residence) that, together with other information, could lead to participants’ identification (CDC, 2010), and we did not include a variable related to residential location in our analysis. Future NHANES data will be useful to evaluate whether potential exposure trends exist and whether the concentration patterns we observed during these four years continue in years to come.
NHANES participants, regardless of race/ethnicity, not only had significantly higher adjusted GM concentrations of DCBA, but also were more likely to exhibit concentrations above the 95th percentile in summer than in the winter months. In particular, non-Hispanic whites were about 10 times more likely in summer than in winter to have DCBA concentrations above the 95th percentile. Mexican Americans and non-Hispanic blacks were also more likely to present DCBA concentrations above the 95th percentile in summer than in winter, but the differences were not statistically significant. Non-Hispanic whites were about three times more likely than non-Hispanic blacks to have DCBA concentrations above the 95th percentile only during the summer season. The reason for such differences is unknown, but concurrent application of DEET and sunscreen can increase dermal penetration of DEET in vitro and in animal models (reviewed in Rodriguez and Maibach, 2016), and exposure to benzophenone-3, a widely used sunscreen agent, is reportedly higher among non-Hispanic white than non-Hispanic black Americans (Calafat et al., 2008). Therefore, we speculate that, in the summer months, non-Hispanic whites may experience higher percutaneous absorption of DEET from increased concurrent use of sunscreen and DEET-containing products than non-Hispanic blacks. Although children had adjusted DCBA GM concentrations higher than adults, perhaps parents may apply DEET-containing insect repellent regularly to protect their children from insect bites while not applying as often repellent on themselves; age was not significantly associated with having concentrations above the 95th percentile. Our data suggest that, compared with other demographic groups, non-Hispanic whites in summer months have potentially higher exposures to DEET than other population groups.
4.3. Selection of DEET biomarkers
Generating high-quality biomonitoring data requires state-of-the-art analytical chemistry methods as well as controlled sampling protocols and quality control/quality assurance procedures (Angerer et al., 2007; Calafat and Needham, 2009; Koch and Calafat, 2009; Needham et al., 2005). In addition, the proper interpretation of biomonitoring data requires an understanding of the toxicokinetics of the target compounds (Calafat et al., 2006, 2008; Koch and Calafat, 2009). NHANES 1999–2002 includes exposure data for DEET, based on the urinary concentrations of DEET itself (CDC, 2009). Although the frequency of detection and concentration ranges of DEET in NHANES 1999–2002 and NHANES 2007–2010 are quite similar, the NHANES 2007–2010 data for DCBA suggest that about 84% of the U.S. general population is exposed to DEET, a much greater percentage than suspected based on the previous NHANES data, based only on DEET measurements. Furthermore, these data also suggest that for background exposures, DEET is a rather poor biomarker because it metabolizes by oxidation and conjugation, before urinary excretion in humans (ATSDR, 2015; Selim et al., 1995; Usmani et al., 2002; Wu et al., 1979). By contrast, DCBA, a major oxidative metabolite of DEET, appears to be a sensitive and specific indicator of DEET exposure. In fact, 81% of those classified as exposed to DEET would have been misclassified as unexposed based on urinary concentrations of DEET only. The fact that the urinary concentrations of DEET oxidative metabolites are orders of magnitude higher than DEET confirms animal and human data suggesting that most DEET recovered in the urine is in the form of oxidative metabolites (ATSDR, 2015; Selim et al., 1995; Usmani et al., 2002; Wu et al., 1979), and only a small percentage was in the form of DEET or its phase II conjugates. Therefore, we recommend that future biomonitoring studies, particularly those focused on environmental exposures, rely on DCBA or other oxidative (i.e., phase I) metabolites and not solely on DEET.
5. Conclusions
We measured the urinary concentrations of DEET and two of its oxidative metabolites (DCBA, DHMB) in the general U.S. population. DCBA was detectable in most persons examined, DHMB in less than one-fourth of participants, and DEET was detected in only 3% of them. Protection against vector borne diseases by application of insect repellent is of public health relevance. Behavioral measures, such as wearing protective clothes and avoiding activities at peak exposure times and places when bugs are most active, can also reduce the risk against insect bites. Insect repellents such as DEET, however, provide reasonably long-lasting protection for outdoor activities and during disease outbreaks, special events or gatherings, natural disasters, or other conditions that may affect a person’s health from exposure to mosquitoes, ticks, and other arthropods bites.
The NHANES 2007–2010 data demonstrating Americans’ exposure to DEET can be used to establish a nationally representative baseline assessment of exposure to this insect repellent. Furthermore, the significantly higher frequency of detection and urinary concentrations of DCBA than of DEET confirms the validity of DCBA as a biomarker for DEET exposure assessment and suggests widespread prevalence of exposure to DEET, including children as young as 6 years of age. More importantly, these NHANES data suggest that background exposure to DEET would be underestimated by using DEET as the sole urinary biomarker and strongly support using additional biomarkers, specifically DCBA or other oxidative metabolites.
Supplementary Material
Acknowledgments
We thank Charles Chambers for technical assistance. This work was supported by the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- DCBA
3-(Diethylcarbamoyl)-benzoic acid
- DEET
N,N-diethyl-m-toluamide
- DHMB
N,N-diethyl-3-(hydroxymethyl)benzamide
- GM
Geometric mean
- LOD
Limit of detection
- NCHS
National Centers for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds ratio
Footnotes
Conflict of interest
The authors declare they have no competing financial interests.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Study approval
The Institutional Review Board of the National Centers for Health Statistics of the Centers for Disease Control and Prevention reviewed and approved the study protocol.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.envint.2016.04.021.
References
- Angerer J, Ewers U, Wilhelm M, 2007. Human biomonitoring: state of the art. Int. J. Hyg. Environ. Health 210, 201–228. [DOI] [PubMed] [Google Scholar]
- Aronson D, Weeks J, Meylan B, Guiney PD, Howard PH, 2012. Environmental release, environmental concentrations, and ecological risk of N,N-diethyl-m-toluamide (DEET). Integr. Environ. Assess. Manag 8, 135–166. [DOI] [PubMed] [Google Scholar]
- ATSDR, 2015. Draft Toxicological Profile for DEET. Agency for Toxic Substances and Disease Registry, Atlanta, GA: (http://www.atsdr.cdc.gov/toxprofiles/tp185.pdf. Available February 10, 2016). [PubMed] [Google Scholar]
- Calafat AM, Needham LL, 2009. What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ. Health Perspect 117, 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye XY, Silva MJ, Kuklenyik Z, Needham LL, 2006. Human exposure assessment to environmental chemicals using biomonitoring. Int. J. Androl 29, 166–170. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Ye X, Reidy JA, Needham LL, 2008. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect 116, 893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Silva MJ, Samandar E, Preau JL, Jia LT, Needham LL, 2011. Selecting adequate exposure biomarkers of diisononyl and diisodecyl phthalates: data from the 2005–2006 National Health and Nutrition Examination Survey. Environ. Health Perspect 119, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Environmental Protection Agency, 2000. N,N-diethyl-meta-toluamide (DEET) — risk characterization document. http://www.cdpr.ca.gov/docs/risk/rcd/deet.pdf (Available February 2, 2016).
- Calza P, Medana C, Raso E, Giancotti V, Minero C, 2011. N,N-diethyl-m-toluamide transformation in river water. Sci. Total Environ 409, 3894–3901. [DOI] [PubMed] [Google Scholar]
- CDC, 2009. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences. (http://www.cdc.gov/exposurereport/. Available June 24, 2015). [Google Scholar]
- CDC, 2010. How the National Health and Nutrition Examination Survey Keeps Your Information Strictly Confidential. http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/english_confidentiality.pdf (Available February 1, 2016).
- CDC, 2011. NHANES Environmental Chemical Data Tutorial. National Center for Health Statistics; (http://www.cdc.gov/nchs/tutorials/environmental/index.htm. Available March 1, 2015). [Google Scholar]
- CDC, 2013. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. Centers for Disease Control and Prevention; (http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. Available January 22, 2016). [Google Scholar]
- CDC, 2015a. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, February 2015. Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences, Atlanta, GA: (http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. Available April 20, 2015). [Google Scholar]
- CDC, 2015b. Protection against mosquitoes, ticks, & other arthropods. http://wwwnc.cdc.gov/travel/yellowbook/2016/the-pre-travel-consultation/protection-againstmosquitoes-ticks-other-arthropods (Available February 2, 2016).
- Chan CB, Ryan DA, 2009. Assessing the effects of weather conditions on physical activity participation using objective measures. Int. J. Environ. Res. Public Health 6, 2639–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo SD, Watkinson AJ, Murby EJ, Kolpin DW, Sandstrom MW, 2007. Is there a risk associated with the insect repellent DEET (N,N-diethyl-m-toluamide) commonly found in aquatic environments? Sci. Total Environ 384, 214–220. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Kato K, Silva MJ, Reidy JA, Malek NA, Hurtz D, Caudill SP, Needham LL, Barr DB, Calafat AM, 2004. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di(2-ethylhexyl) phthalate. Environ. Health Perspect 112, 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Angerer J, 2007. Di-isononylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int. J. Hyg. Environ. Health 210, 9–19. [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM, 2009. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 364, 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Preuss R, Angerer J, 2006. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure — an update and latest results. Int. J. Androl. 29, 155–165. [DOI] [PubMed] [Google Scholar]
- Kuklenyik P, Baker SE, Bishop AM, Morales A, Calafat AM, 2013. On-line solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry approach to quantify N,N-diethyl-m-toluamide and oxidative metabolites in urine. Anal. Chim. Acta 787, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Koch HM, Gries W, Schutze A, Langsch A, Bruning T, Otter R, 2014. Urinary metabolite excretion after oral dosage of bis(2-propylheptyl) phthalate (DPHP) to five male volunteers — characterization of suitable biomarkers for human biomonitoring. Toxicol. Lett 231, 282–288. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Cantonwine DE, Anzalota Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, Baker SE, Alshawabkeh AN, Cordero JF, Meeker JD, 2014. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 13, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meineke EK, Dunn RR, Sexton JO, Frank SD, 2013. Urban warming drives insect pest abundance on street trees. PLoS One 8, e59687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2012. Exposure Science in the 21st Century: A Vision and a Strategy. National Academies Press (US), Washington (DC) (http://www.nap.edu/catalog/13507/exposure-science-in-the-21st-century-a-vision-and-a. Available May 28, 2015). [PubMed] [Google Scholar]
- Needham LL, Patterson DG, Barr DB, Grainger J, Calafat AM, 2005. Uses of speciation techniques in biomonitoring for assessing human exposure to organic environmental chemicals. Anal. Bioanal. Chem 381, 397–404. [DOI] [PubMed] [Google Scholar]
- Rodriguez J, Maibach HI, 2016. Percutaneous penetration and pharmacodynamics: wash-in and wash-off of sunscreen and insect repellent. J. Dermatol. Treat 27, 11–18. [DOI] [PubMed] [Google Scholar]
- Selim S, Hartnagel RE, Osimitz TG, Gabriel KL, Schoenig GP, 1995. Absorption, metabolism, and excretion of N,N-diethyl-m-toluamide following dermal application to human volunteers. Fundam. Appl. Toxicol 25, 95–100. [DOI] [PubMed] [Google Scholar]
- Swale DR, Sun B, Tong F, Bloomquist JR, 2014. Neurotoxicity and mode of action of N,N-diethyl-meta-toluamide (DEET). PLoS One 9, e103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA, 2015. DEET. http://www.epa.gov/insect-repellents/deet (Available January 12, 2016). [Google Scholar]
- Usmani KA, Rose RL, Goldstein JA, Taylor WG, Brimfield AA, Hodgson E, 2002. In vitro human metabolism and interactions of repellent N,N-diethyl-m-toluamide. Drug Metab. Dispos 30, 289–294. [DOI] [PubMed] [Google Scholar]
- Wiles D, Yee J, Castillo U, Russell J, Spiller H, Casavant M, 2014. A lethal case of DEET toxicity due to intentional ingestion. J. Anal. Toxicol 38, 696–698. [DOI] [PubMed] [Google Scholar]
- Wu A, Pearson LM, Shekoski DL, Soto RJ, Stewart RD, 1979. High resolution gas chromatography/mass spectrometric characterization of urinary metabolites of N,N-diethyl-m-toluamide (DEET) in man. J. High Resolut. Chromatogr 2, 558–562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.