Abstract
Study Objectives:
Sleep is an important recovery period for athletes. Women, including athletes, have reported sleep disturbances around menses. Thus, the aim of this study was to assess the changes in objective sleep parameters in the nights during menses and in the midfollicular phase of the menstrual cycle of young female athletes.
Methods:
Female collegiate athletes with regular menstrual cycles were recruited. The participants underwent home electroencephalogram monitoring during the first and second nights after the onset of menses (M1 and M2, respectively) and during one night between the 7th and the 10th night after menses onset (midfollicular phase).
Results:
Data from 45 athletes were analyzed. The total sleep time was significantly reduced, and sleep onset latency was significantly prolonged in M2 compared with those in the night during the midfollicular phase. Sleep efficiency was significantly reduced in M1 compared with that in the night during the midfollicular phase. Changes in the percentage of deep sleep across menstrual cycles differed among the participants with and without menstrual symptoms or concerns for sanitary products; moreover, such participants spent a lower percentage of time in deep sleep in M1 compared with the other nights.
Conclusions:
Collegiate female athletes with regular menstrual cycles are likely to have trouble falling asleep, tend to sleep less, and when concerned about sanitary products, have less deep sleep during menses. Even in young female athletes with regular menstrual cycles, sleep can be disturbed during menses. Interventions to restore or improve sleep should be considered.
Citation:
Koikawa N, Takami Y, Kawasaki Y, et al. Changes in the objective measures of sleep between the initial nights of menses and the nights during the midfollicular phase of the menstrual cycle in collegiate female athletes. J Clin Sleep Med. 2020;16(10):1745–1751.
Keywords: electroencephalogram, menstruation, sports, women
BRIEF SUMMARY
Current Knowledge/Study Rationale: Female athletes have poorer self-reported sleep quality than their male counterparts; however, limited data are available regarding electroencephalogram-based sleep parameters during the menstrual cycle of female athletes.
Study Impact: We assessed the changes in objective sleep measures based on electroencephalographic monitoring during and after menses in collegiate female athletes and established that they are likely to have trouble falling asleep, tend to sleep less, and when concerned about sanitary products, have less deep sleep during menses. Even in young female athletes with regular menstrual cycles, sleep can be disturbed during menses.
INTRODUCTION
Sleep plays a crucial role in recovery after exercise1,2; however, few studies have investigated the scientific aspects of sleep in athletes.3,4 In general, women are more likely to be dissatisfied with their sleep quality.5,6 Indeed, female athletes showed poorer self-reported sleep quality than their male counterparts in some studies.7,8 Although the reasons for these differences remain unclear and are thought to be multifactorial, menstruation and its related symptoms and issues may be one such reason.9
In studies evaluating self-reported sleep in women, sleep disturbances were reported during the last few premenstrual days and the initial few days after the onset of menses.10,11 In studies objectively evaluating sleep in women, electroencephalogram (EEG)-based sleep parameters during the midfollicular (MF) phase and those during the mid- or late-luteal phase were generally compared, and no differences were noted in most sleep parameters except for a minor reduction in rapid eye movement (REM) sleep during the mid- or late-luteal phase.12–14 Because sleep disturbances were reported during the initial few days after menses onset10,11 and menstruation is an important predictor of short sleep duration in female athletes,15 objective assessments of sleep during menses are of interest; however, few studies have assessed EEG-based sleep parameters during menses and have reported inconsistent results.16–18 In addition, no specific data in athletes are available. Thus, we aimed to assess the changes in objective sleep measures based on EEG monitoring during and after menses in collegiate female athletes. Our specific hypotheses included that collegiate female athletes are more likely to have poor objective sleep quality during menses and that such poor sleep quality can be obvious in participants with some symptoms and concerns related to menses and/or sanitary products.
METHODS
Participants
Healthy female collegiate athletes with regular menstrual cycles defined according to the Japanese Society of Obstetrics and Gynecology (ie, menstrual cycles between 25 and 38 days long and variation of each cycle within 6 days) were recruited from the Juntendo University School of Health and Sports Science. The exclusion criteria included the use of any drugs, including hormonal contraceptives, premenstrual dysphoric disorder, dysmenorrhea, other gynecological pathologies that may interfere with sleep patterns, shift work, and transmeridian travel within the past 3 months and during study periods. This study was approved by the Research Ethics Committee of Juntendo University. Informed consent was obtained from all participants.
Sleep-related baseline assessments
Sleep-related baseline assessments using the following tools were conducted once at the time of enrollment for all participants. The Pittsburgh Sleep Quality Index was used to assess self-reported sleep quality over a 1-month period.19,20 The total score of the seven components ranged from 0 to 21; a total score ≥ 6 indicates poor sleep quality.19,20 The Epworth Sleepiness Scale21 is an established tool for evaluating self-reported sleepiness over a few weeks to months.22 In this study, significant self-reported sleepiness was defined as an Epworth Sleepiness Scale score ≥ 11. Restless legs syndrome was assessed based on the international criteria and a positive answer to all five interview questions.23 Moreover, the participants were asked the following questions: (1) Have you snored during the past one month? Answer: “No,” “<1 night per week,” “1 or 2 nights per week,” or “>2 nights per week”; and (2) Have you displayed apneas during sleep? Answer: “No,” “<1 night per week,” “1 or 2 nights per week,” or “>2 nights per week.”
Sleep monitoring
All participants underwent overnight home sleep monitoring using a two-channel portable EEG device (ZA-9. Proassist, Ltd., Osaka, Japan), which consists of two pairs of electrodes connected to a transmitter and a receiver; this provides EEG, electro-oculogram, and submental electromyogram. The home EEG monitoring was set up by the participants themselves in their usual sleep environments. Sleep parameters obtained from this device showed a high degree of agreement with those from polysomnography.24,25
All data were manually scored by an experienced sleep technologist based on widely used criteria.26 For sleep stages, the percentages of REM sleep, light sleep (ie combined N1 and N2), and deep sleep (ie, N3) per total sleep time (TST) were determined. TST was calculated as the total sleep period minus the time spent awake during the sleep period. Sleep-onset latency was defined as the time from bedtime to sleep onset. Sleep efficiency was evaluated as the TST as a percentage of the total time in bed. Wake after sleep onset was calculated as the total wakefulness time between initial sleep onset and final sleep offset. Arousal index was calculated as the number of microarousals per hour of sleep.
The participants underwent EEG monitoring during the following four occasions: (1) the first night at any time during the menstrual cycle to adapt to the EEG monitoring system; the obtained data were not scored or used (ie, test EEG monitoring session); (2) the first night after menses onset (ie, M1); (3) the second night after menses onset (ie, M2); and (4) one night between the 7th and 10th nights after menses onset (ie, MF phase). The MF phase was chosen because in previous studies, while objectively evaluating sleep in women, EEG-based sleep parameters during the MF phase and those during the midluteal phase were generally compared, and no differences were noted except for a minor reduction in REM sleep during the midluteal phase12–14; moreover, it is generally easier to capture MF than the midluteal phase. After the TEST, the order of starting from MF to M1/2 (ie, MF first) or from M1/2 to MF (ie, menses first) was dependent on the participants. We asked all participants to perform each EEG monitoring during the league’s regular season.
Menstruation-related data
All participants answered the following questions: (1) Do you have any unpleasant symptoms during menses? “Yes” or “No.” If “Yes,” what types of symptoms do you have? (2) What types of sanitary products did you use during the M1 and M2? (3) What types of sanitary products do you regularly use? Do you have any of the following concerns when you sleep during menses: (a) menstrual blood leak, (b) stuffiness around the crotch associated with sanitary products, and (c) dislodgement of sanitary products from underwear?
Statistical analysis
The continuous variables were summarized using the mean and standard deviation or median (interquartile range) as appropriate. The categorical variables are shown as percentages. Variations in sleep parameters across occasions were assessed using repeated-measures one-way analysis of variance, and the Tukey test for multiple comparisons. Sleep-onset latency and wake after sleep onset were natural log-transformed because they were non-normally distributed. To assess the effect of the order (early first or late first) on sleep parameters and to assess the effects of menses-related symptoms or issues on the alteration of sleep parameters, the first-order interactions in two-way repeated-measures analysis of variance models were examined by entering interaction terms between the instances of EEG monitoring (ie, time) and order (ie, time-by-order interaction) and by entering interaction terms between the instances of EEG monitoring and subgroups (ie, time-by-subgroup interaction). If significant time-by-subgroup interactions were found, repeated-measures analysis of variance was performed within each subgroup. P < .05 was considered statistically significant. All analyses were conducted using SPSS v.23 (SPSS Inc., Chicago, Illinois).
RESULTS
Participant characteristics
Overall, 52 healthy female collegiate athletes with regular menstrual cycles were enrolled. All had been instructed to record their menstrual cycles regularly by their team, which allowed us to confirm a regular menstrual cycle and no use of oral contraceptives. The data from seven female athletes could not be used because they could not complete all EEG monitoring occasions for personal reasons. Thus, data from 45 female athletes were finally analyzed. Their characteristics are summarized in Table 1. None had restless legs syndrome symptoms. Four (8.8%) reported snoring < 1 night per week, two (4.4%) reported snoring 1 or 2 nights per week for the previous one month, and none reported experiencing apnea during sleep. Thirty-one participants (68.9%) reported unpleasant symptoms during menses; 27 (60.0%) reported menses-related pain during the initial 2 days of menses, and five (11.1%) reported general fatigue within a day before and/or on the first day of menses. All participants used sanitary pads during sleep in M1 and M2, and they used the same sanitary pads regularly. Thirty-six participants (80.0%) had concerns regarding menstrual blood leak, nine (20.0%) reported stuffiness around the crotch associated with sanitary products, and 13 (28.9%) experienced the dislodgement of sanitary products from their underwear.
Table 1.
Participant characteristics.
| N = 45 | |
|---|---|
| Age, y | 20.2 ± 1.1 |
| Height, cm | 162.0 ± 5.7 |
| Weight, kg | 56.1 ± 5.7 |
| BMI, kg/m2 | 21.4 ± 1.6 |
| Age at first menstruation, y | 12.6 ± 1.7 |
| Menstrual cycle, days | 28.2 ± 3.7 |
| Duration of menses, days | 5.8 ± 1.0 |
| Type of sports | |
| Soccer (%) | 15 (33.3) |
| Track and field (%) | 12 (26.7) |
| Kendo (%) | 9 (20.0) |
| Basketball, % | 8 (17.8) |
| Softball, % | 1 (2.2) |
| PSQI score | 4.5 ± 1.8 |
| % of athletes with poor sleep quality, % | 13 (28.9) |
| ESS score | 10.3 ± 3.3 |
| % of athletes with sleepiness (%) | 21 (46.7) |
Continuous data are summarized using the mean ± standard deviation. BMI = body mass index, ESS = Epworth Sleepiness Scale, PSQI = Pittsburgh Sleep Quality Index.
Sleep parameters in M1, M2, and MF
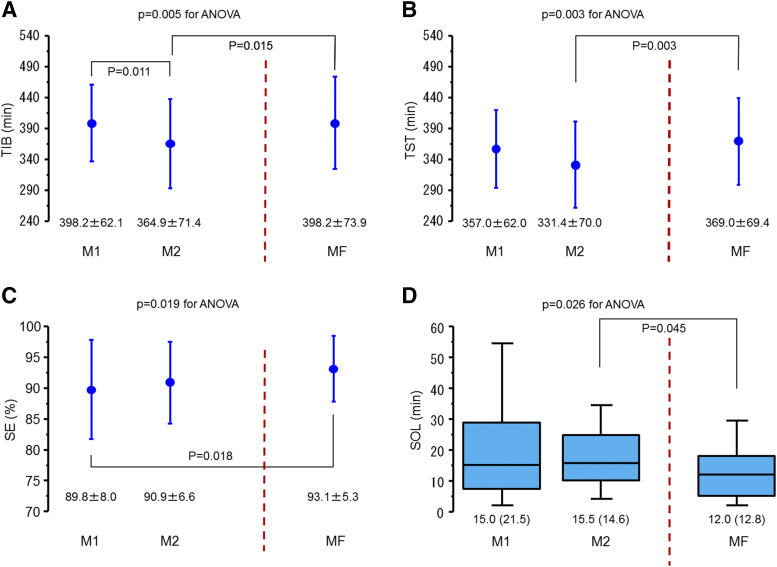
Total time in bed varied significantly across the menstrual cycle and was significantly reduced in M2 compared with M1 and MF; no difference was observed between M1 and MF (Figure 1A). No time-by-order interaction was found (P = .318). TST varied significantly across the menstrual cycle; TST was significantly reduced in M2 compared with MF, but no difference was observed between M1 and MF (Figure 1B). No time-by-order interaction was found (P = .234). Sleep-onset latency varied significantly across the menstrual cycle and was significantly prolonged in M2 compared with MF; no difference was observed between M1 and MF (Figure 1C). No time-by-order interaction was found (P = .453). SE varied significantly across the menstrual cycle and was significantly reduced in M1 compared with MF; no difference was observed between M2 and MF (Figure 1D). No time-by-order interaction was found (P = .487). Wake after sleep onset, percentages of N3 and REM sleep, and ArI were similar across the menstrual cycle (Table 2). No time-by-order interactions were noted (P = .532, .215, .089, and .472, respectively).
Figure 1. Changes in TIB, TST, SE, and SOL across M1/2 and MF.
(A) TIB varied significantly (P for ANOVA, .005) across the menstrual cycle and was significantly reduced in M2 compared with M1 and MF. Values represent the mean ± standard deviation (SD). (B) TST varied significantly (P for ANOVA, 0.003) across the menstrual cycle and was significantly reduced in M2 compared with MF. Values represent the mean ± SD. (C) SE varied significantly (P for ANOVA, 0.019) across the menstrual cycle and was significantly reduced in M1 compared with MF. Values represent the mean ± SD. (D) SOL varied significantly (P for ANOVA, 0.026) across the menstrual cycle and was significantly reduced in M2 compared with MF. Values represent the median and interquartile range. ANOVA, analysis of variance; M1/2, first and second night after menses onset; MF, midfollicular phase; SE, sleep efficiency; SOL, sleep-onset latency; TIB, time in bed; TST, total sleep time.
Table 2.
Changes in WASO, percentage of REM, and N3 sleep across M1/2 and MF.
| M1 | M2 | MF | P for ANOVA | |
|---|---|---|---|---|
| WASO, min | 9.0 (11.6) | 9.5 (11.0) | 7.0 (8.0) | 0.194 |
| % of REM sleep, % | 26.8 ± 6.6 | 25.7 ± 5.9 | 27.3 ± 4.2 | 0.273 |
| % of N3 sleep, % | 24.2 ± 9.1 | 24.6 ± 8.1 | 24.6 ± 6.5 | 0.939 |
| ArI, events/h of sleep | 7.4 ± 2.8 | 6.7 ± 2.5 | 6.7 ± 2.3 | 0.063 |
Continuous data are summarized using the mean ± standard deviation or median (interquartile range). No differences in each pairwise comparison. ANOVA = analysis of variance, ArI = arousal index, M1/2 = first and second night after menses onset, MF = midfollicular phase, N3 = deep non-REM sleep, REM = rapid eye movement, WASO = wake after sleep onset.
Subgroup analyses
Although no significant within-subgroup changes in the percentage of N3 sleep were found in the athletes with or without unpleasant symptoms during menses, a significant time-by-subgroup interaction between the changes in the percentages of N3 sleep and the presence/absence of unpleasant symptoms during menses was observed (P = .038) (Table 3). No time-by-subgroup interactions were found between the changes in all sleep parameters and the presence or absence of concerns regarding menstrual blood leak or stuffiness around the crotch associated with sanitary products (Table S1 and Table S2); however, a significant interaction between changes in the percentage of N3 sleep and the presence/absence of concerns regarding dislodgement of sanitary products from underwear was found (P = .038) (Table 4). Likewise, changes in the percentage of N3 sleep within participants concerned with the dislodgement of sanitary products from underwear differed significantly across the menstrual cycle, with a lower percentage of N3 sleep in M1 than in other phases (Table 4).
Table 3.
Subgroup analysis in participants with and without unpleasant symptoms during menses.
| M1 | M2 | MF | P for Interaction | ||
|---|---|---|---|---|---|
| TIB, min | Yes (n = 31) | 404.7 ± 69.0 | 358.6 ± 78.3 | 391.3 ± 78.9 | .150 |
| No (n = 14) | 383.8 ± 41.7 | 378.9 ± 53.0 | 413.4 ± 61.4 | ||
| TST, min | Yes (n = 31) | 361.5 ± 68.2 | 321.7 ± 75.4 | 362.9 ± 74.1 | .136 |
| No (n = 14) | 346.8 ± 46.2 | 352.6 ± 50.9 | 382.4 ± 57.6 | ||
| SOL, min | Yes (n = 31) | 14.5 (15.8) | 16.5 (19.3) | 9.0 (9.8) | .221 |
| No (n = 14) | 15.0 (24.5) | 11.5 (18.0) | 14.5 (23.0) | ||
| SE, % | Yes (n = 31) | 89.4 ± 7.7 | 89.9 ± 7.3 | 93.4 ± 5.3 | .332 |
| No (n = 14) | 90.5 ± 8.9 | 93.1 ± 4.1 | 92.7 ± 5.5 | ||
| WASO, min | Yes (n = 31) | 10.0 (12.5) | 8.0 (10.3) | 7.0 (7.9) | .585 |
| No (n = 14) | 7.5 (10.0) | 10.3 (12.0) | 6.3 (8.0) | ||
| % of REM sleep, % | Yes (n = 31) | 27.9 ± 6.2 | 25.7 ± 5.5 | 27.0 ± 4.5 | .084 |
| No (n = 14) | 24.2 ± 7.1 | 25.6 ± 6.9 | 27.9 ± 3.4 | ||
| % of N3 sleep, % | Yes (n = 31) | 22.1 ± 8.9 | 23.5 ± 6.6 | 24.8 ± 6.7 | .038 |
| No (n = 14) | 28.8 ± 7.8 | 27.0 ± 10.7 | 24.2 ± 6.3 | ||
| Ar I, events/h of sleep | Yes (n = 31) | 7.7 ± 2.7 | 7.1 ± 2.5 | 7.0 ± 1.8 | .865 |
| No (n = 14) | 6.7 ± 2.9 | 5.7 ± 2.3 | 6.0 ± 3.2 |
Continuous data are summarized using the mean ± standard deviation or median (interquartile range). ArI = arousal index, M1/2 = first and second night after menses onset, MF = midfollicular phase, N3 = deep non-REM sleep, REM = rapid eye movement, SE = sleep efficiency, SOL = sleep onset latency, TST = total sleep time, WASO = wake after sleep onset.
Table 4.
Subgroup analysis in participants with and without concerns regarding dislodgement of sanitary products from underwear.
| M1 | M2 | MF | P for Interaction | ||
|---|---|---|---|---|---|
| TIB, min | Yes (n = 13) | 406.9 ± 69.5 | 366.9 ± 67.1 | 380.0 ± 93.1 | .307 |
| No (n = 32) | 394.7 ± 59.7 | 364.1 ± 74.1 | 405.6 ± 64.9 | ||
| TST, min | Yes (n = 13) | 371.2 ± 58.1 | 331.3 ± 63.9 | 357.5 ± 85.2 | .333 |
| No (n = 32) | 351.2 ± 63.5 | 331.4 ± 72.9 | 373.6 ± 62.8 | ||
| SOL, min | Yes (n = 13) | 11.5 (19.5) | 12.5 (17.3) | 8.5 (10.4) | .872 |
| No (n = 32) | 15.0 (21.0) | 16.5 (14.3) | 12.8 (14.8) | ||
| SE, % | Yes (n = 13) | 91.6 ± 6.0 | 90.8 ± 9.1 | 94.4 ± 4.4 | .555 |
| No (n = 32) | 89.0 ± 8.6 | 90.9 ± 5.4 | 92.6 ± 5.6 | ||
| WASO, min | Yes (n = 13) | 9.5 (13.5) | 9.5 (10.3) | 5.5 (8.1) | .534 |
| No (n = 32) | 8.8 (11.5) | 8.8 (13.0) | 7.0 (9.0) | ||
| % of REM sleep, % | Yes (n = 13) | 27.6 ± 6.5 | 25.7 ± 5.9 | 27.2 ± 4.5 | .799 |
| No (n = 32) | 26.4 ± 6.7 | 25.7 ± 6.0 | 27.3 ± 4.1 | ||
| % of N3 sleep, % | Yes (n = 13)* | 21.7 ± 8.7 | 26.4 ± 7.7 | 26.9 ± 8.1 | .038 |
| No (n = 32) | 25.2 ± 9.2 | 23.8 ± 8.3 | 23.6 ± 5.6 | ||
| Ar I, events/h of sleep | Yes (n = 13) | 8.3 ± 3.2 | 7.7 ± 2.7 | 6.9 ± 1.7 | .231 |
| No (n = 32) | 7.0 ± 2.5 | 6.3 ± 2.4 | 6.6 ± 2.6 |
Continuous data are summarized using the mean ± standard deviation or median (interquartile range). *P < 0.05 for analysis of variance within subgroups. ArI = arousal index, M1/2 = first and second night after menses onset, MF = midfollicular phase; N3 = deep non-REM sleep, REM = rapid eye movement, SE = sleep efficiency, SOL = sleep onset latency, TST = total sleep time, WASO = wake after sleep onset.
DISCUSSION
Menstrual cycle and the associated variations of reproductive hormones, particularly progesterone, which is low in the follicular phase and increases during the luteal phase, may play some roles in sleep variations.27 Thus, in previous studies in which EEG-based sleep parameters were evaluated, data from the MF phase and those from the mid- or late-luteal phase were generally compared.28 Not all studies, however, found effects of the menstrual cycle on sleep or found only small effects, including a small reduction in REM sleep during the mid- or late-luteal phases,12–14 possibly owing to individual variabilities in sleep and issues across menstrual cycles. Conversely, women reported sleep disturbance during the initial few days of menses in addition to the late-luteal phase.10,11 A few small-scale studies have performed EEG-based objective sleep assessments during menses.16–18 In a study comparing eight women with premenstrual syndrome and eight age-matched control women without premenstrual syndrome, both groups experienced less deep sleep and more intermittent awakening during menses and in the premenstrual phases. In a study assessing EEG-based sleep parameters throughout a full cycle in nine healthy women, no significant changes were noted in any sleep parameters during menses compared with the other phases.17 In a study comparing 10 women with dysmenorrhea and 8 women with normal menstrual cycles, the latency to deep sleep was longer in menses than in the midluteal phase, there was less REM sleep in menses than in the midluteal and MF phases, and these associations were more prominent in women with dysmenorrhea.18 Small sample size and individual variations of menstruation and related symptoms were possible reasons for these findings. From this perspective, a strength of the present study is the relatively large number of homogeneous (ie, all participants were collegiate female athletes with regular menstrual cycles) participants enrolled. Furthermore, no data are available regarding objective sleep assessment during menses in collegiate female athletes. Thus, this is the first study assessing objective sleep quality and quantity in menses and in the MF phase. In addition, the results of the present study highlight that improving the quality of sleep during menses can be an important issue in conditioning for female athletes.
Results of the subgroup analysis in which participants with concerns regarding sanitary products had less deep sleep during menses and the results of a previous study18 in which impairments of sleep quality in menses were prominent in symptomatic participants lead to the consideration of interventions to relieve symptoms or the use of individually fit sanitary products. In a randomized crossover trial comparing objective sleep parameters between nights with sanitary pads designed for night use and nights with those designed for day use,29 however, no differences were found in any objective sleep parameters between use of those two sanitary pads. Nevertheless, the effects of use of other sanitary products (other types of pads, tampons, and menstrual cups, etc.) should be assessed in further studies. Controlling menstruation with oral contraceptives might be another option against sleep disturbances during menses; however, in athletes, oral contraceptives may reduce insulin-like growth factor levels, which is an important bone trophic hormone; thus, oral contraceptives are not good for bone health.30 A recent meta-analysis31 reported that longer sleep duration resulted in beneficial effects on subsequent sports-specific performance measures,32 whereas napping, sleep hygiene, and postexercise recovery strategies provided inconsistent results, suggesting that sleep interventions or sleep disturbances may play important roles, but not all athletes or all types of intervention may be beneficial in their sport-specific performance. The data suggest that menses may not influence the performance of young female athletes33,34; however, this may not be true in some female athletes, presumably those with self-reported or objective sleep disturbances in menses. Whether interventions for sleep disturbance during menses provide benefits for sports-specific performance should be evaluated in further studies.
Our study has some limitations. First, a lack of overnight polysomnography is a major limitation. When sleep-related baseline assessments were conducted, only two participants reported snoring 1 or 2 nights per week, whereas none reported experiencing apnea during sleep or having restless legs syndrome symptoms; the presence or absence of sleep disorders such as sleep apnea and sleep-related movement disorders were not confirmed formally. Conversely, the strength of our study is the inclusion of TEST to exclude the first night’s effect of sleep recordings, and although the order of recordings in M1/M2 and MF were based on the participants’ selection, no time-by-order interactions were confirmed. Second, we lacked data on productive hormone levels and body temperatures. Because all our participants have regular menstrual cycles based on regularly recorded cycles, and we focused on the first and second days after menses onset and the seventh and tenth nights after menses onset, their phases were adequately captured. Third, in the present study, all participants used sanitary pads in M1 and M2. Variations in sleep parameters when tampon and other sanitary products are used remain to be elucidated. Thus, the results of some subgroup analyses based on the response to questions regarding menstruation-related issues may not be applicable if sanitary products other than a sanitary pad were used.
CONCLUSIONS
In conclusion, collegiate female athletes with regular menstrual cycles have difficulty falling asleep and sleep less in the nights during menses. Sleep quality could be impaired because of a few symptoms and concerns related to menses and/or sanitary products. Sleep assessment is emphasized in young female athletes, even those with a regular menstrual cycle. Some interventions should be considered, particularly alternative sanitary products, and further studies should investigate whether their sleep Is restored or improved by those interventions.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was performed at Juntendo University. This study was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Program for the Strategic Research Foundation at Private Universities, 2014–2018, Japanese Center for Research on Women in Sport, Juntendo University, Project for Fostering, Survey Research for the Strategic Strengthening of Female Athletes 2017–2018 by the Japan Sports Agency and by the Juntendo University Young Investigator Joint Project Award 2015 (K1517). These funding sources did not have any other roles in this study. Fusae Kawana and Takatoshi Kasai are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare. Fukuda Denshi, Nanako Shiroshita, and Takatoshi Kasai are affiliated with a department endowed by Paramount Bed. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ABBREVIATIONS
- EEG
electroencephalogram
- M1/2
first or second night after menses onset
- MF
midfollicular
- PMS
premenstrual syndrome
- REM
rapid eye movement
- TST
total sleep time
REFERENCES
- 1.Myllymäki T, Rusko H, Syväoja H, Juuti T, Kinnunen ML, Kyröläinen H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur J Appl Physiol. 2012;112(3):801–809. 10.1007/s00421-011-2034-9 [DOI] [PubMed] [Google Scholar]
- 2.Samuels C. Sleep, recovery, and performance: the new frontier in high-performance athletics. Neurol Clin. 2008;26(1):169–180, ix-x. 10.1016/j.ncl.2007.11.012 [DOI] [PubMed] [Google Scholar]
- 3.Simpson NS, Gibbs EL, Matheson GO. Optimizing sleep to maximize performance: implications and recommendations for elite athletes. Scand J Med Sci Sports. 2017;27(3):266–274. 10.1111/sms.12703 [DOI] [PubMed] [Google Scholar]
- 4.Leeder J, Glaister M, Pizzoferro K, Dawson J, Pedlar C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J Sports Sci. 2012;30(6):541–545. 10.1080/02640414.2012.660188 [DOI] [PubMed] [Google Scholar]
- 5.Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53(3):741–748. 10.1016/S0022-3999(02)00333-1 [DOI] [PubMed] [Google Scholar]
- 6.Lindberg E, Janson C, Gislason T, Björnsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387. 10.1093/sleep/20.6.381 [DOI] [PubMed] [Google Scholar]
- 7.Koikawa N, Shimada S, Suda S, Murata A, Kasai T. Sex differences in subjective sleep quality, sleepiness, and health-related quality of life among collegiate soccer players. Sleep Biol Rhythms. 2016;14(4):377–386. 10.1007/s41105-016-0068-4 [DOI] [Google Scholar]
- 8.Kawasaki Y, Kasai T, Koikawa N, et al. Sex differences in factors associated with poor subjective sleep quality in athletes. J Sports Med Phys Fitness. 2020;60(1):140–151. 10.23736/S0022-4707.19.09875-X [DOI] [PubMed] [Google Scholar]
- 9.Baker FC, Lee KA. Menstrual cycle effects on sleep. Sleep Med Clin. 2018;13(3):283–294. 10.1016/j.jsmc.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 10.Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. 10.1016/S0022-3999(03)00067-9 [DOI] [PubMed] [Google Scholar]
- 11.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(20):2370–2376. 10.1001/archinte.165.20.2370 [DOI] [PubMed] [Google Scholar]
- 12.Baker FC, Sassoon SA, Kahan T, Palaniappan L, Nicholas CL, Trinder J, Colrain IM. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. 10.1111/j.1365-2869.2012.01007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. 10.1155/2010/259345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver HS, Werth E, Dijk D-J, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3(1):1–11. 10.1016/j.jsmc.2007.10.003 [DOI] [Google Scholar]
- 15.Silva MR, Paiva T. Risk factors for precompetitive sleep behavior in elite female athletes. J Sports Med Phys Fitness. 2019;59(4):708–716. 10.23736/S0022-4707.18.08498-0 [DOI] [PubMed] [Google Scholar]
- 16.Parry BL, Mendelson WB, Duncan WC, Sack DA, Wehr TA. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30(3):285–303. 10.1016/0165-1781(89)90020-6 [DOI] [PubMed] [Google Scholar]
- 17.Driver HS, Dijk DJ, Werth E, Biedermann K, Borbély AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. [DOI] [PubMed] [Google Scholar]
- 18.Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol. 1999;277(6):E1013–E1021. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 20.Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, Kamei Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97(2-3):165–172. 10.1016/S0165-1781(00)00232-8 [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 22.Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10(5):556–565. 10.1016/j.sleep.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 23.Allen RP, Picchietti DL, Garcia-Borreguero D, et al. International Restless Legs Syndrome Study Group . Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. 10.1016/j.sleep.2014.03.025 [DOI] [PubMed] [Google Scholar]
- 24.Nonoue S, Mashita M, Haraki S, et al. Inter-scorer reliability of sleep assessment using EEG and EOG recording system in comparison to polysomnography. Sleep Biol Rhythms. 2017;15(1):39–48. 10.1007/s41105-016-0078-2 [DOI] [Google Scholar]
- 25.Kanemura T, Kadotani H, Matsuo M, et al. Evaluation of a portable two-channel electroencephalogram monitoring system to analyze sleep stages. J Oral Sleep Med. 2016;2:101–108. [Google Scholar]
- 26.Berry RB, Brooks R, Gamaldo C, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. 10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8(6):613–622. 10.1016/j.sleep.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 28.Lee KA, Shaver JF, Giblin EC, Woods NF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep. 1990;13(5):403–409. [PubMed] [Google Scholar]
- 29.Hachul H, Andersen ML, Tufik S. Sleep quality based on the use of different sanitary pads during menstruation. Int J Gynaecol Obstet. 2011;115(1):57–60. 10.1016/j.ijgo.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 30.De Souza MJ, Nattiv A, Joy E, et al. Expert Panel . 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289. 10.1136/bjsports-2013-093218 [DOI] [PubMed] [Google Scholar]
- 31.Bonnar D, Bartel K, Kakoschke N, Lang C. Sleep interventions designed to improve athletic performance and recovery: a systematic review of current approaches. Sports Med. 2018;48(3):683–703. 10.1007/s40279-017-0832-x [DOI] [PubMed] [Google Scholar]
- 32.Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34(7):943–950. 10.5665/SLEEP.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoene RB, Robertson HT, Pierson DJ, Peterson AP. Respiratory drives and exercise in menstrual cycles of athletic and nonathletic women. J Appl Physiol. 1981;50(6):1300–1305. 10.1152/jappl.1981.50.6.1300 [DOI] [PubMed] [Google Scholar]
- 34.Tsampoukos A, Peckham EA, James R, Nevill ME. Effect of menstrual cycle phase on sprinting performance. Eur J Appl Physiol. 2010;109(4):659–667. 10.1007/s00421-010-1384-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.