Abstract
Study Objectives:
Left ventricular hypertrophy (LVH) is associated with augmented risk for mortality in patients with coronary artery disease (CAD). These patients often have obstructive sleep apnea (OSA). We aimed to evaluate the relationship between OSA and the left ventricular mass index (LVMI) in men with CAD.
Methods:
Consecutive patients with CAD were recruited and underwent overnight portable monitoring for the assessment of OSA. LVMI was ascertained using high-resolution echocardiography. Univariate and multivariate regression analyses were conducted to explore the associations between the OSA parameters and the LVMI levels.
Results:
Of the 1,053 examined male patients with CAD, 425 (40.4%) had moderate-to-severe OSA (respiratory event index ≥ 15 events/h). The prevalence of LVH (LVMI > 125 g/m2) was 36.0% (n = 379). The mean LVMI values increased with increasing OSA severity (P < .001). Patients with respiratory event index ≥ 30 events/h had 2.30 (95% confidence interval 1.50–3.54, P < .001) times increased risk of LVH than those without OSA (respiratory event index < 5 events/h) independent of confounders. The minimum oxygen saturation levels were the strongest factor correlated with LVMI (β = −0.299, P = .004) of several OSA indices. Patients with minimum oxygen saturation < 70% had an adjusted odds ratio of 3.62 (95% confidence interval 1.81–7.25, P < .001) for LVH development compared with those with minimum oxygen saturation ≥ 90%.
Conclusions:
OSA severity was associated with a higher likelihood of LVH in men with CAD, which is partially related to severe nocturnal intermittent hypoxemia. Aggressive effort at managing OSA among patients with CAD may further reduce the cardiovascular risk.
Citation:
Huang Z, Wang L, Liu Y, et al. Impact of obstructive sleep apnea on left ventricular mass index in men with coronary artery disease. J Clin Sleep Med. 2020;16(10):1675–1682.
Keywords: left ventricle, sleep apnea, coronary artery, oxygen saturation, hypoxemia
BRIEF SUMMARY
Current Knowledge/Study Rationale: Left ventricular hypertrophy is associated with augmented risk for mortality in patients with coronary artery disease. These patients often have obstructive sleep apnea, but little is known regarding the relationship between obstructive sleep apnea and left ventricular hypertrophy in this population.
Study Impact: We showed that the severity of obstructive sleep apnea is associated with a higher likelihood of left ventricular hypertrophy in a large population of men with coronary artery disease, which is partially related to severe nocturnal intermittent hypoxemia. Presumably, overnight oximetry and periodical measurement for left ventricular mass index in patients with coronary artery disease and coexisting obstructive sleep apnea may facilitate to identify those who warrant an optimal therapeutic strategy.
INTRODUCTION
Obstructive sleep apnea (OSA) affects 20–30% of the adult population1 and approximately 45% of patients with coronary artery disease (CAD).2 The comorbid OSA is associated with an increased risk for cardiovascular adverse outcomes including mortality.3 However, there is limited information regarding the implication of OSA for the biomarkers that reflect poor prognosis of patients with CAD.
Left ventricular hypertrophy (LVH), defined by a critical increase in the left ventricular (LV) mass, is associated with a higher risk of mortality and sudden cardiac death in patients with CAD, compared with those without LVH.4–6 The body surface area-adjusted LV mass, namely left ventricular mass index (LVMI), is a useful marker for evaluating LVH and is known to cause an additive risk on sudden cardiac death beyond LV function and conventional risk factors.6,7 As the risk of recurrent cardiovascular events and the prevalence of OSA remains high despite contemporary therapies in patients with CAD, we should explore whether OSA could predispose these patients to LVH.
Several,8–12 but not all,13–16 studies have reported the independent detrimental effect of untreated OSA on LV geometrics that leads to LVH. Most of these studies are small,8 involving limited presentation of patients with obesity12 or those referred to sleep clinics.10,14 Little is known regarding LVMI associated with sleep apnea in patients with CAD. In addition, as LVH in women mainly occurs after menopause and involves the menopausal time and changes in the estrogen and progesterone levels, this may lead to many confounding factors.17,18 Therefore, we aimed to analyze the possible association between OSA and the LVMI levels or the presence of LVH in a large male population with CAD.
METHODS
Study population
We prospectively enrolled patients with CAD from July 2015 through September 2019. Patients were admitted to the Department of Cardiology of our hospital with angina pectoris, dyspnea, or suspected CAD. Consenting male patients who had a confirmed diagnosis of CAD after clinical stabilization and considered as being in a stable CAD condition were included. Patients who met one of the following criteria were excluded: (1) previously diagnosed with OSA and received continuous positive airway pressure therapy or other modalities; (2) had overt pulmonary disease, end-stage renal failure, advanced cancer and any deteriorated conditions; (3) required oxygen supplement during sleep; (4) received intensive care due to susceptibility of malignant arrhythmia and/or hemodynamic instability; (5) expected not to tolerate sleep study due to severe neurological, memory, behavioral, and perceptual disorders; and (6) used hypnotic agents, opiates, muscle relaxants, and any medications that may influence the actual respiratory recordings. Of the 2,357 male patients who were initially screened, 477 (163 had overt pulmonary disease, 16 had end-stage renal failure, 140 received intensive care, 94 underwent OSA treatment, and 64 had neuropsychiatric disorders or received hypnotics and other medication) were excluded. Of the remaining 1,880 patients, 1,496 patients had a confirmed diagnosis of CAD after evaluation, but 443 lacked echocardiographic, anthropometric data and/or essential serum tests. In total, 1,053 eligible patients were included for the final data analysis. The study was approved by the Clinical Ethics Committee of Guangdong Provincial People’s Hospital, and written informed consent was obtained from all participants.
Clinical, angiographic, and laboratory data collection
The patients’ demographics, anthropometrics, medical history, medication, and daytime sleepiness data assessed by Epworth Sleepiness Scale were recorded.19 The number of diseased vessels (defined as ≥ 50% angiographic stenosis of the left anterior descending artery, left circumflex branch, and/or right coronary artery) was used to evaluate the CAD severity.20 The Gensini score21 and the number of stents were also recorded. Fasting venous blood was sampled for lipid profiles, glycosylated hemoglobin, fasting blood glucose, and creatinine. All laboratory tests were performed using standard measurement methods (ISO 9000 Quality Management and Assurance Standards) in the laboratory department of our hospital.
Nocturnal respiratory events study
All eligible patients underwent overnight respiratory portable monitoring (Alice PDx, Philips [China] Investment Co., Shanghai, China) during the first 24–72 h after admission to the hospital to facilitate the diagnosis of OSA. The parameters examined included oxygen saturation (Sao2), airflow and respiratory effort measured by pulse oximetry, nasal pressure, and respiratory inductance plethysmography, respectively. Nocturnal respiratory events were evaluated and scored according to the American Academy of Sleep Medicine guidelines.22 An obstructive apnea was defined as a ≥ 90% reduction in nasal airflow lasting ≥ 10 s with continuing abdominal and thoracic movements. An obstructive hypopnea was defined as a ≥ 30% reduction in airflow lasting ≥ 10 s, associated with oxygen desaturation of more than 3% from baseline, in combination with abdominal and thoracic movements. The respiratory event index (REI) was calculated as the number of episodes of obstructive apnea and hypopnea per hour of recording and was used to categorize the different OSA groups using cut-off values of 5, 15, and 30 events/h.
The indices of nocturnal hypoxemia of OSA included oxygen desaturation index, ie, the sum of the average number of ≥ 3% oxygen desaturations per recorded sleep hour; minimum Sao2 levels (minSao2), indicative of the severity of hypoxemia; mean Sao2 levels, reflecting nocturnal average Sao2; recording time spent with Sao2 < 90%, indicative of the duration of hypoxemia; and the hypoxic burden, determined by the area under the respiratory event-associated oxygen desaturation curve from the pre-event baseline.23 All recordings were scored manually or calculated by a trained sleep technologist and were reviewed by another investigator. A recorded time of at least 4 h was considered effective for interpretation of respiratory events and hypoxic sequelae.
Transthoracic echocardiographic assessment of the left ventricular mass
Echocardiographic studies were performed using GE Vivid E9 (GE Vingmed Ultrasound, Horten, Norway) with an M5S probe (2–4 MHz) or Philips IE33 (Philips Healthcare, Andover, MA) with an S5-1 probe (2.5–3.5 MHz). In accordance with the guidelines of the American Society of Echocardiography,24 transthoracic echocardiographic images (M-mode, 2-dimension, color Doppler) were obtained from a perpendicular projection across the heart with patients lying in a modified left lateral decubitus position. The main indicators were left and right atrium diastolic diameters, LV end-systolic (LVDs) and end-diastolic diameter (LVDd), interventricular septum thickness at end-diastole (IVSd), and LV posterior wall thickness at end-diastole (PWTd), which were measured in the parasternal long-axis view. The relative wall thickness (RWT) was calculated by the ratio of sum of IVSd and PWTd to LVDd. LV ejection fraction was evaluated using the biplane Simpson’s method. The Devereux formula25 was used to calculate the left ventricular mass (LVM) and the LVMI, as follows:
Where BSA is body surface area. We utilized an LVMI value of 125 g/m2 as a cut-off for LVH, which was supported by previous evidence.6,26 LV geometric pattern was classified into normal (RWT ≤ 0.42 and no LVH), concentric remodeling (RWT > 0.42 and no LVH), eccentric hypertrophy (RWT ≤ 0.42 and LVH), and concentric hypertrophy (RWT > 0.42 and LVH).27 Echocardiographic evaluation was performed during the index hospitalization by an experienced cardiologist who was blinded to patients’ information regarding respiratory events.
Statistical analysis
Baseline data between groups were compared using the unpaired t test for normally distributed variables, the Mann-Whitney U test for continuous variables that were not normally distributed, and the chi-square test or Fisher exact test for categorical variables. The association between the LVMI levels and OSA variables was analyzed with linear regression. Confounders included in the multivariate models were the ones that are correlated with LVMI or clinically relevant factors. The continuous variables including LVMI and OSA indices were log transformed using the base-10 log due to a skewed distribution. Univariate and multivariate logistic analyses were used to analyze the association between the LVH and REI groups. To examine the relationship between nocturnal nadir Sao2 and LVH, further analyses were conducted using the minSao2 levels across different Sao2 thresholds. The statistically significant level was set at P < .05. Statistical analyses were computed using SPSS version 19.0 (IBM Corp., Armonk, NY).
RESULTS
Characteristics
Of the 1,053 participants with CAD, 273 (25.9%) had a normal REI (defined as an REI < 5 events/h), whereas 355 (33.7%), 238 (22.6%), and 187 (18.7%) had mild (REI 5–14.9 events/h), moderate (REI 15–29.9 events/h), and severe (REI ≥ 30 events/h) OSA, respectively. Subjective sleepiness as assessed by the Epworth Sleepiness Scale score did not differ between the OSA severity groups (P = .736), and the majority of patients with OSA did not report excessive daytime sleepiness with an Epworth Sleepiness Scale score < 10. LVH was found in 379 men (36.0% of the study population). A high prevalence of comorbidities of hypertension (58.1%), diabetes mellitus (25.6%), and hyperlipidemia (11.1%) was observed. The prevalence of the acute coronary syndrome was 26.8%, whereas ST (the flat segment of the electrocardiogram between the end of S wave and the start of T wave)-elevation myocardial infarction was found in 27 men (2.6%).
Comparisons of baseline data between the LVMI groups
The levels of LVMI ranged from 50 to 315 g/m2, with a mean value of 120 g/m2. The patients were divided into 2 groups using a cut-off value of LVMI 125 g/m2. The patients with LVH were significantly heavier, had lower estimated glomerular filtration rate, lower left ventricular ejection fraction, higher proportion of diabetes mellitus, dyslipidemia, hypertension, and use of antihypertensives (Table 1). There were no significant differences in age, in the levels of some lipid profiles, glycosylated hemoglobin, acute coronary syndrome, ST-elevation myocardial infarction, CAD severity based on the number of diseased vessels, and atrial fibrillation between the 2 groups.
Table 1.
Baseline characteristics.
| Variables | All (n = 1,053) | LVMI < 125 g/m2 (n = 674) | LVMI ≥ 125 g/m2 (n = 379) | P Value |
|---|---|---|---|---|
| Age, year | 61.6 ± 9.5 | 61.5 ± 9.5 | 61.7 ± 9.6 | .985 |
| BMI, kg/m2 | 25.2 ± 3.2 | 24.9 ± 3.2 | 25.6 ± 3.2 | < .001 |
| BSA, m2 | 1.91 ± 0.16 | 1.90 ± 0.15 | 1.93 ± 0.16 | .009 |
| ESS score | 4.5 ± 4.0 | 4.4 ± 3.7 | 4.8 ± 4.5 | .008 |
| Diabetes mellitus, n (%) | 270 (25.6) | 155 (23.0) | 115 (30.3) | .009 |
| Dyslipidemia, n (%) | 117 (11.1) | 86 (12.8) | 31 (8.2) | .023 |
| Hypertension, n (%) | 612 (58.1) | 340 (50.4) | 272 (71.8) | < .001 |
| Stage 1 | 106 (10.1) | 58 (8.6) | 48 (12.7) | |
| Stage 2 | 209 (19.8) | 128 (19.0) | 81 (21.4) | |
| Stage 3 | 297 (28.2) | 154 (22.8) | 143 (37.7) | |
| ACS, n (%) | 282 (26.8) | 163 (24.2) | 119 (31.4) | .110 |
| STEMI, n (%) | 27 (2.6) | 13 (1.9) | 14 (3.7) | .082 |
| Number of diseased vessels, n | 1.7 ± 1.0 | 1.6 ± 1.0 | 1.7 ± 1.0 | .166 |
| Gensini score | 25 (10 – 50) | 24 (9 – 48) | 29 (11 – 56) | .012 |
| Number of stents | 0.9 ± 0.8 | 0.9 ± 0.8 | 0.8 ± 0.7 | .007 |
| Atrial fibrillation, n (%) | 53 (5.0) | 29 (4.3) | 24 (6.3) | .148 |
| Antihypertensives, n (%) | 959 (91.1) | 603 (89.5) | 356 (93.9) | .004 |
| Antiplatelets, n (%) | 978 (92.9) | 632 (93.8) | 346 (91.3) | .227 |
| Anticoagulants, n (%) | 44 (4.2) | 15 (2.2) | 29 (7.7) | < .001 |
| Statins, n (%) | 980 (93.1) | 637 (94.5) | 343 (90.5) | .027 |
| Total cholesterol, mmol/L | 4.3 ± 1.2 | 4.4 ± 1.3 | 4.3 ± 1.2 | .560 |
| LDL-C, mmol/L | 2.8 ± 0.9 | 2.8 ± 0.8 | 2.8 ± 0.9 | .182 |
| HDL-C, mmol/L | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | < .001 |
| Triglycerides, mmol/L | 1.8 ± 1.4 | 1.7 ± 1.4 | 1.8 ± 1.4 | .904 |
| HbA1c, % | 6.4 ± 1.4 | 6.3 ± 1.3 | 6.5 ± 1.4 | .177 |
| FBG, mmol/L | 6.2 ± 2.6 | 6.2 ± 2.5 | 6.4 ± 2.9 | .142 |
| Creatinine, µmol/L | 90.3 (79.9 – 102.2) | 88.3 (79.6 – 99.1) | 94.4 (82.4 – 109.5) | .007 |
| eGFR, mL/min | 83.5 (70.5, 97.4) | 85.7 (73.6 – 98.5) | 78.7 (63.9 – 93.7) | < .001 |
| EF, % | 58.9 ± 12.5 | 61.9 ± 9.9 | 53.5 ± 14.6 | < .001 |
| LA, mm | 37.1 ± 5.5 | 35.3 ± 4.4 | 40.2 ± 5.8 | < .001 |
| LVDd, mm | 50.2 ± 7.5 | 47.3 ± 5.1 | 55.7 ± 8.0 | < .001 |
| LVDs, mm | 33.7 ± 9.3 | 30.5 ± 6.3 | 39.4 ± 10.9 | < .001 |
| IVSd, mm | 10.6 ± 1.9 | 10.0 ± 1.5 | 11.6 ± 2.2 | < .001 |
| PWTd, mm | 10.2 ± 1.7 | 9.6 ± 1.3 | 11.2 ± 1.9 | < .001 |
| RWT | 0.42 ± 0.09 | 0.42 ± 0.08 | 0.42 ± 0.10 | .950 |
| LVM, g | 231.3 ± 78.9 | 186.7 ± 34.2 | 310.9 ± 73.9 | < .001 |
| LVMI, g/m2 | 112.5 (94.0 – 138.4) | 99.7 (86.7 – 111.0) | 149.9 (136.1 – 173.3) | < .001 |
Values are given as the mean ± SD or median (interquartile range); n is the number of participants. Antihypertensives include angiotensin II receptor antagonist, angiotensin-converting enzyme inhibitors, alpha blockers, beta blockers, calcium channel blocker, and diuretics. ACS = acute coronary syndrome, BMI = body mass index, BSA = body surface area, CAD = coronary artery disease, eGFR = estimated glomerular filtration rate, EF = ejection fraction, ESS = Epworth Sleepiness Scale, FBG = fasting blood glucose, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein cholesterol, IVSd = interventricular septum thickness at end-diastole, LA = left atrium diameter, LDL-C = low-density lipoprotein cholesterol, LVDd = left ventricular diastolic diameter, LVDs = left ventricular systolic diameter, LVM = left ventricular mass, LVMI = left ventricular mass index, PWTd = LV posterior wall thickness at end-diastole, RWT = relative wall thickness, STEMI = ST (the flat segment of the electrocardiogram between the end of S wave and the start of T wave)-elevation myocardial infarction.
REI, oxygen desaturation index, minSao2, recording time spent with Sao2 < 90%, and hypoxic burden were significantly different between the LVMI groups (Table 2). The total recording time did not differ significantly between the 2 groups. Moderate-to-severe OSA (REI ≥ 15 events/h) was more common among men with increased LVMI.
Table 2.
Comparisons of nocturnal respiratory parameters.
| Variables | All (n = 1,053) | LVMI < 125 (n = 674) | LVMI ≥ 125 (n = 379) | P Value |
|---|---|---|---|---|
| Total recording time, h | 8.2 (7.5 – 8.9) | 8.3 (7.6 – 8.9) | 8.1 (7.3 – 8.8) | .112 |
| 3% ODI, events/h | 13.6 (6.0 – 27.3) | 10.0 (3.8 – 21.2) | 17.1 (6.5 – 31.9) | < .001 |
| REI, events/h | 12.7 (6.1 – 26.2) | 10.9 (5.6 – 22.0) | 16.1 (6.8 – 32.3) | < .001 |
| OSA severity, n (%) | < .001 | |||
| REI < 5 events/n | 273 (25.9) | 201 (29.8) | 72 (19.0) | |
| REI 5–14.9 events/h | 355 (33.7) | 232 (34.4) | 123 (32.5) | |
| REI 15–29.9 events/h | 238 (22.6) | 148 (22.0) | 90 (23.7) | |
| REI > 30 events/h | 187 (17.8) | 93 (13.8) | 94 (24.8) | |
| MinSao2, % | 86 (81 – 88) | 86 (81 – 89) | 85 (80 – 88) | < .001 |
| Mean Sao2, % | 94 (93 – 95) | 94 (93 – 95) | 94 (93 – 95) | .362 |
| T90Sao2, % | 3.0 (0.2 – 15.6) | 2.0 (0.1 – 11.7) | 4.2 (0.4 – 24.4) | < .001 |
| Hypoxic burden, %min/h | 7.9 (2.4 – 24.7) | 6.4 (2.0 – 18.5) | 9.5 (2.5 – 28.2) | .001 |
n is number of participants. LVMI = left ventricular mass index, minSao2 = minimum oxygen saturation levels, mean Sao2 = mean of nocturnal oxygen saturation levels, ODI = 3% oxygen desaturation index, OSA = obstructive sleep apnea, REI = respiratory event index, T90Sao2 = total recording time spent with oxygen saturation ≤ 90%.
Associations between OSA variables and LVMI levels
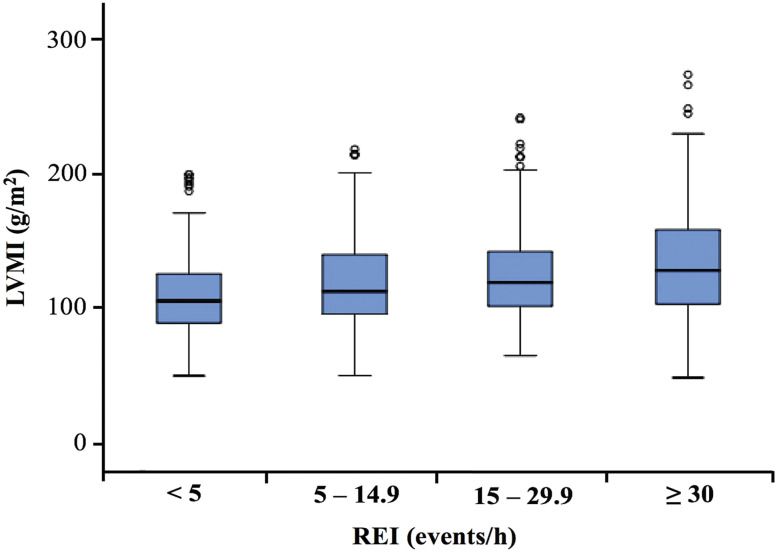
The mean LVMI levels were increased in accordance with the severity of OSA from 114.0 g/m2 in men with an REI < 5 to 130.3 g/m2 in those with an REI ≥ 30 (P < 0.001). The distribution of LVMI levels in the OSA groups is demonstrated in Figure 1.
Figure 1. Distribution of LVMI in different OSA groups.
The LVMI levels increased with increasing OSA severity (P < .001). The mean LVMI value was 114.0 g/m2, 117.5 g/m2, 125.0 g/m2, and 130.3 g/m2 among patients with REI < 5, 5–14.9, 15–29.9, and ≥ 30 events/h, respectively. LVMI, left ventricular mass index; OSA, obstructive sleep apnea; REI, respiratory event index.
In the univariate regression models, there was a linear association between variables of sleep apnea and the LVMI levels. Correlated factors of LVMI, plus age and body mass index (BMI), were retained for inclusion in the multiple linear regression models. Therefore, multivariate regression analyses adjusted for age, BMI, hypertension, use of antihypertensives, estimated glomerular filtration rate, diabetes mellitus, and dyslipidemia were performed to assess the contribution of OSA variables on the levels of LVMI. REI (β = 0.286, P = .001), oxygen desaturation index (β = 0.285, P < .001), minSao2 (β = −0.311, P = .003), and recording time spent with Sao2 < 90% (β = 0.149, P = .007) remained significantly associated with the LVMI levels after adjustment for the confounders of age and BMI and other possible confounders (Table 3).
Table 3.
Linear regression analysis with dependent variable LVMI.
| Independent Variables | Unadjusted | Adjusted for Age and BMI | Adjusted for Age, BMI, eGFR, Hypertension, Antihypertensives, Diabetes Mellitus, Dyslipidemia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | (95% CI) | P value | β | (95% CI) | P value | β | (95% CI) | P value | |
| MinSao2 | −0.449 | (−0.70 to −0.30) | < .001 | −0.391 | (−0.60 to −0.18) | < .001 | −0.311 | (−0.52 to −0.10) | .003 |
| REI | 0.376 | (0.23–0.52) | < .001 | 0.289 | (0.13–0.45) | < .001 | 0.286 | (0.16–0.39) | .001 |
| ODI | 0.432 | (0.30–0.57) | < .001 | 0.360 | (0.21–0.51) | < .001 | 0.285 | (0.14–0.43) | < .001 |
| T90Sao2 | 0.194 | (0.09–0.30) | < .001 | 0.168 | (0.06–0.28) | .003 | 0.149 | (0.04–0.26) | .007 |
| Hypoxic burden | 0.190 | (0.08–0.30) | .001 | 0.128 | (0.01–0.24) | .029 | 0.084 | (−0.03–0.20) | .136 |
n is number of participants. Each line in table represents a separate regression model. Variables including LVMI, minSao2, REI, 3%ODI, T90Sao2 have been log transformed using the base-10 log. BMI = body mass index, CI = confidence interval, eGFR = estimated glomerular filtration rate, LVMI = left ventricular mass index, minSao2 = minimum oxygen saturation levels, ODI = 3% oxygen desaturation index. REI = respiratory event index, T90Sao2 = total recording time spent with oxygen saturation ≤ 90%.
Association between severity of OSA and LVH
In logistic regression models, patients were stratified into 4 categories based on REI. Patients with REI ≥ 30 events/h had 2.30 (95% confidence interval 1.50–3.54, P < .001) times increased risk of LVH than those without OSA (REI < 5 events/h) independent of confounders (Table 4). Given that the minSao2 levels had the strongest association with LVMI levels, we further analyzed how different severest nadir Sao2 levels were associated with the likelihood of LVH. The severity of hypoxemia was subdivided into several groups with a varying degree of nocturnal nadir Sao2 thresholds. There was a dose-response relationship between increasing severity of minSao2 and the likelihood of LVH, which remained after adjusting for confounders. The adjusted odds ratio was 3.62 (95% confidence interval 1.81–7.25, P < .001) for LVH for men with minSao2 < 70% when compared with those with minSao2 ≥ 90% (Table 5).
Table 4.
Estimates of the risk for LVH (LVMI > 125 g/m2) by REI.
| Independent Variables | Unadjusted | Adjusted for Age and BMI | Adjusted for Age, BMI, eGFR, Hypertension, Antihypertensives, Diabetes Mellitus, Dyslipidemia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P value | OR | (95% CI) | P value | OR | (95% CI) | P value | |
| REI < 5 events/h | 1.00 | 1.00 | 1.00 | ||||||
| REI 5–14.9 events/h | 1.48 | (1.05–2.09) | .027 | 1.43 | (1.01–2.03) | .043 | 1.36 | (0.95–1.95) | .098 |
| REI 15–29.9 events/h | 1.70 | (1.17–2.47) | .006 | 1.52 | (1.03–2.24) | .034 | 1.33 | (0.89–1.99) | .167 |
| REI ≥ 30, events/h | 2.82 | (1.90–4.18) | < .001 | 2.38 | (1.58–3.58) | < .001 | 2.30 | (1.50–3.54) | < .001 |
BMI = body mass index, CI = confidence interval, eGFR = estimated glomerular filtration rate, LVH = left ventricular hypertrophy, LVMI = left ventricular mass index, OR = odds ratio, REI = respiratory event index.
Table 5.
Estimates of the risk for LVH (LVMI > 125 g/m2) by minSao2.
| Independent Variables | Unadjusted | Adjusted for Age and BMI | Adjusted for Age, BMI, eGFR, Hypertension, Antihypertensives, Diabetes Mellitus, Dyslipidemia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | P value | OR | (95% CI) | P value | OR | (95% CI) | P value | |
| MinSao2 ≥ 90% | 1.00 | 1.00 | 1.00 | ||||||
| MinSao2 85–89% | 1.60 | (1.13–2.27) | .009 | 1.51 | (1.06–2.15) | .023 | 1.55 | (1.07–2.22) | .019 |
| MinSao2 80–84% | 2.09 | (1.40–3.12) | < .001 | 1.82 | (1.20–2.76) | .005 | 1.88 | (1.24–2.86) | .003 |
| MinSao2 70–79% | 2.53 | (1.61–3.97) | < .001 | 2.12 | (1.32–3.42) | .002 | 2.40 | (1.49–3.84) | < .001 |
| MinSao2 < 70% | 3.75 | (1.95–7.22) | < .001 | 3.07 | (1.56–6.05) | < .001 | 3.62 | (1.81–7.25) | < .001 |
BMI = body mass index, CI = confidence interval, eGFR = estimated glomerular filtration rate, LVH = left ventricular hypertrophy, LVMI = left ventricular mass index, minSao2, minimum oxygen saturation levels, OR = odds ratio.
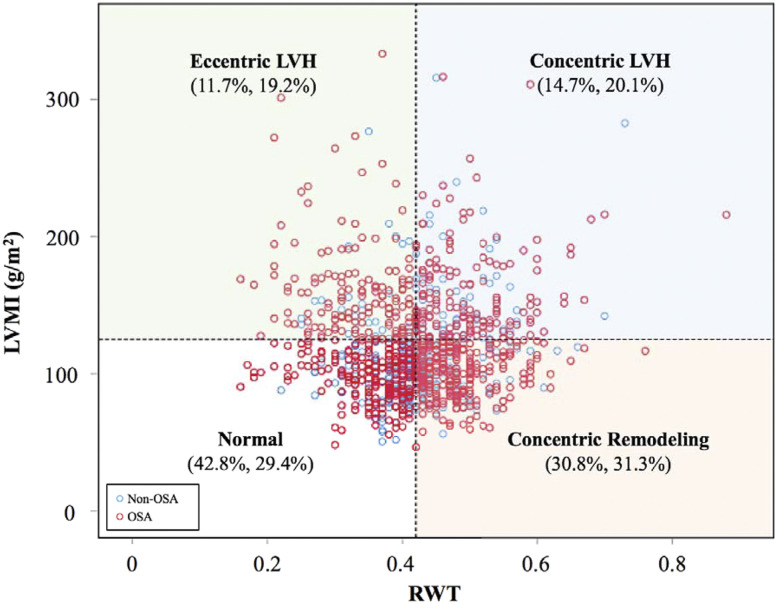
In Figure 2, the frequency distribution among the 4 LV geometric patterns in patients with CAD with or without OSA was compared (χ2 = 20.9; P < .001). Overall, LV geometric remodeling was present in 67.1% of patients with CAD. Concentric LV remodeling, concentric LVH, and eccentric LVH occurred in 31.1% (n = 328), 18.7% (n = 197), and 17.3% (n = 182) of the participants, respectively. Notably, patients with OSA had a similar percentage of concentric and eccentric LVH (20.1% vs 19.2%).
Figure 2. Distribution of LV geometrics in patients with CAD with or without OSA.
The LVMI distribution (g/m2) versus RWT is presented. There were 4 different LV geometric patterns, including normal, concentric remodeling, eccentric LVH, and concentric LVH, based on the normal/high RWT and the absence/presence of LVH. The vertical and horizontal reference lines indicate the RWT upper limit (0.42) and LVH cut-off (125 g/m2), respectively. The numbers in brackets under each geometrical pattern represent the percentage without and with OSA, respectively. CAD, coronary artery disease; LV, left ventricle; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; OSA, obstructive sleep apnea; RWT, relative wall thickness.
DISCUSSION
Our study provides novel insights into LV geometrical alteration in patients with OSA following diagnosis of CAD. The key findings were that there was a significant relationship between the OSA severity and the LVMI levels and that a severely elevated REI and severe nocturnal hypoxemia were correlated with a higher likelihood of LVH even after eliminating the effects of confounders in a large population of men with CAD.
Our findings were consistent with those of previous studies.8–11 Arias et al8 found that increased LVMI was observed in patients with OSA compared with controls after adjusting for BMI, hypertension, and diabetes mellitus. However, the sample size was limited to 42 men (27 with OSA and 15 healthy).8 Significant increase in LVM in OSA was reported in another 2 studies,9,10 but the patients in the OSA groups were more obese or hypertensive than the controls.9,10 Javaheri et al11 showed that OSA severity and nocturnal hypoxemia were significantly correlated with increased LVMI in a large community-based individuals aged ≤ 65 years, as opposed to our population-based sample of men with CAD. In contrast, LVM adjusted for height instead of body surface area was used to determine LVMI in that study.11 Indeed, LVM per height index is not an adequate method for LVM normalization and may misclassify very tall or short individuals.28 A recent meta-analysis29 reported that increased LVM was a significant feature of patients with OSA without cardiovascular comorbidities. In contrast, OSA was still an independent risk factor for LVH in our patients with CAD. Therefore, LVM should be routinely monitored in patients with OSA and those with OSA plus CAD to optimize risk stratification.
There are also conflicting data in which no association between OSA and LVM has been found.13–16 Varol et al13 reported that no significant difference in LVM between 18 healthy participants, 25 with mild to moderate OSA, and 21 with severe OSA. Niroumand et al16 found that LVMI was higher in patients with OSA, but this association was insignificant after eliminating effects of coexisting obesity, aging, and hypertension. Moreover, another 2 studies did not find a significant difference between OSA and controls with respect to LVM.14,15 The small sample size, selection of study population, and methodology approaches may be related to the discrepancies in our findings.
LVH is associated with an increased myocardial oxygen consumption, impaired coronary blood-flow reserve, and reduced coronary blood supply due to hypertrophic ventricle and its atherogenic factors.4 These factors would predispose individuals to a susceptibility to ventricular arrhythmia and sudden coronary plaque rupture.7 This may partially explain the repeating cardiovascular mortality despite the advanced performed strategies of secondary prevention of CAD in those with LVH. In our study, severe OSA seemed to carry an increased likelihood of experiencing LVH in patients with CAD. The association between OSA and LVH could be confounded by the presence of aging, hypertension, obesity, diabetes, and dyslipidemia. However, participants with OSA in our study did not have a high proportion of these comorbidities and their potential effects on LVMI were adjusted in the multivariate models. Notably, minSao2 remained associated with LVMI and was the most influential parameter correlated with LVH in patients with CAD. These results were partially in discrepancy with the corresponding reported by Javaheri et al,11 who showed that the REI and hypoxemia were correlated with LVMI. However, REI remained the most contributing factor in contrast to minSao2 in our study.
Patients with CAD and significant hypoxemia due to OSA (ie, those in the minSao2 < 80%) had a 2- to 3-fold higher odds of having LVH than those with mild hypoxemia (minSao2 ≥ 90%). Previous large-scale studies reported that decline in nocturnal Sao230 and nadir oxygen saturation (minSao2 < 78%),31 but not the REI, were independently associated with an increased risk of atrial fibrillation and sudden cardiac death onset.30,31 Severe nocturnal hypoxemia from OSA denoted as minSao2 ≤ 85% was shown to be an independent predictor of major adverse cardiovascular events after over 4 years of follow-up.32 However, evidence on the severity threshold of nocturnal nadir Sao2 in OSA is uncertain regarding its prediction of poor cardiovascular outcomes. Therefore, we subdivided the threshold of minSao2 in a consecutive manner. Strikingly, a significant dose-response relationship between the severity of nocturnal hypoxemia and LVH was noted. Although hypoxic burden may be a reliable estimate of nocturnal hypoxemia as it captures duration and depth of respiratory-related desaturation, we did not find a significant association between this metric and LVMI. This could be attributed to its inability to distinguish the short and deep desaturations from the long and shallow ones.1 For example, 2 min of 5% desaturation and 10 min of 1% desaturation per hour would be similarly represented. Therefore, the hypoxic burden was likely to dilute the effect of an existing severe nadir oxygen saturation (ie, minSao2) on LVMI. Meanwhile, in patients with CAD who are vulnerable to hypoxia, the OSA-related severe minSao2 may aggravate the response of the left ventricle to myocardial ischemia. In this case, the minSao2 levels could be a more appropriate index of nocturnal hypoxic stress in assessing LVH risk in patients with CAD.
Plausible mechanisms underlying an association between OSA severity and elevated LVMI levels include the following. First, large negative intrathoracic pressure swings due to forceful inspiration against an occluded upper airway could lead to increased LV transmural pressure and afterload33. Second, hypoxia-induced sympathetic activation may generate repetitive surges of nocturnal hypertension.34 These consequences, in combination with increased heart rate response to hypoxia, may promote myocardial oxygen demand and confer a high risk of cardiac ischemia, arrhythmia, and, chronically LVH. Meanwhile, systemic inflammation plays an essential role in intermittent hypoxia-induced LV remodeling. Inflammatory response and free radical generation could result in an imbalance between myocardial oxidation and antioxidant activity, leading to myocardial injury and susceptibility to myocardial ischemia.35 The pathological consequences are related to disorganization and accumulation of disrupted myocardial fibers, causing LV perivascular and interstitial fibrosis, hypertrophy, and passive stiffness.36 In fact, it is somewhat difficult to delineate how the aforementioned mechanisms affect the LV mass in patients with OSA, due to their complex interactions with existed comorbidities.
Our findings might suggest the potential relationship between severe OSA and cardiovascular adverse outcomes in patients with CAD, whereas LVH appears to be a morphological intermediate. Exploration of high LVMI levels may enable the identification of the more “vulnerable” phenotypes of patients with OSA who may benefit more from timely and targeted therapeutic interventions, such as nocturnal heart rate and blood pressure management. Additionally, overnight monitoring of nadir Sao2, apart from conventional OSA indices, would probably be a cost-effective tool of risk stratification and progression observation among patients with OSA. However, these assumptions need to be further examined by large-scale trials.
The strengths of this study were the relatively large sample size, comprehensive assessment of sleep apnea parameters, and analyses performed in patients with CAD, especially the association among CAD, OSA, and LVMI, which has not been widely examined in the literature. Nevertheless, the study had limitations, which include the cross-sectional design, such that reported associations do not prove causality. The representative sample of men makes it unlikely to generalize the observed results in women. However, prior evidence, including a subgroup of women, revealed similar findings between the 2 sexes.11 Ambulatory blood pressure monitoring was lacking to assess its nocturnal effect on LVMI, but hypertension stages and antihypertensives were considered as confounders to evaluate the risk for LVH. We did not consider the ratio of early diastolic transmitral flow velocity to early diastolic mitral annular velocity (E/e′) and more specific markers of LV diastolic function to avoid over adjustment from highly collinear variables with a focus on LVMI. The latter was not calculated from measures of cardiac magnetic resonance, a gold standard of LVM evaluation. However, its cost tends to be a limiting factor in a large-scale study, while echocardiography has its advantages including direct visualization, cost-effectiveness, and high accessibility.
In conclusion, the severity of OSA was significantly associated with elevated LVMI levels in a large population of men with CAD. OSA with a severely elevated REI and severe nocturnal hypoxemia were independently associated with a higher likelihood of having LVH. Presumably, overnight oximetry and periodical measurement for LVMI in patients with CAD and coexisting OSA may facilitate to identify those who warrant an optimal therapeutic strategy.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Work for this study was performed in the Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China. This study was funded by a grant from the Science and Technology Special Funding of Guangdong Provincial People’s Hospital (No.2017bq03). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the patients for their participation and acknowledge the technical assistance (Philips Alice PDX, GE Vivid E9, and Philips IE33) provided by Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention.
ABBREVIATIONS
- BMI
body mass index
- CAD
coronary artery disease
- LV
left ventricular
- LVH
left ventricular hypertrophy
- LVM
left ventricular mass
- LVMI
left ventricular mass index
- minSaO2
minimum oxygen saturation
- OSA
obstructive sleep apnea
- REI
respiratory event index
- SaO2
oxygen saturation
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CH, Sethi R, Li R, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133(21):2008–2017. 10.1161/CIRCULATIONAHA.115.019392 [DOI] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. 10.1016/S0140-6736(08)61622-0 [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. 10.1056/NEJM199005313222203 [DOI] [PubMed] [Google Scholar]
- 5.Sundström J, Lind L, Arnlöv J, Zethelius B, Andrén B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103(19):2346–2351. 10.1161/01.CIR.103.19.2346 [DOI] [PubMed] [Google Scholar]
- 6.Laukkanen JA, Khan H, Kurl S, et al. Left ventricular mass and the risk of sudden cardiac death: a population-based study. J Am Heart Assoc. 2014;3(6):e001285. 10.1161/JAHA.114.001285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinier K, Dervan C, Singh T, et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8(8):1177–1182. 10.1016/j.hrthm.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias MA, García-Río F, Alonso-Fernández A, Mediano O, Martínez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112(3):375–383. 10.1161/CIRCULATIONAHA.104.501841 [DOI] [PubMed] [Google Scholar]
- 9.Koga S, Ikeda S, Urata J, Kohno S. Effect of nasal continuous positive airway pressure in men on global left ventricular myocardial performance in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2008;101(12):1796–1800. 10.1016/j.amjcard.2008.02.083 [DOI] [PubMed] [Google Scholar]
- 10.Dursunoğlu N, Dursunoğlu D, Kiliç M. Impact of obstructive sleep apnea on right ventricular global function: sleep apnea and myocardial performance index. Respiration. 2005;72(3):278–284. 10.1159/000085369 [DOI] [PubMed] [Google Scholar]
- 11.Javaheri S, Sharma RK, Wang R, et al. Association between obstructive sleep apnea and left ventricular structure by age and gender: the multi-ethnic study of atherosclerosis. Sleep. 2016;39(3):523–529. 10.5665/sleep.5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujante P, Abreu C, Moreno J, et al. Obstructive sleep apnea severity is associated with left ventricular mass independent of other cardiovascular risk factors in morbid obesity. J Clin Sleep Med. 2013;9(11):1165–1171. 10.5664/jcsm.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varol E, Akcay S, Ozaydin M, Ozturk O, Cerci SS, Sahin U. Influence of obstructive sleep apnea on left ventricular mass and global function: sleep apnea and myocardial performance index. Heart Vessels. 2010;25(5):400–404. 10.1007/s00380-009-1225-3 [DOI] [PubMed] [Google Scholar]
- 14.Tugcu A, Guzel D, Yildirimturk O, Aytekin S. Evaluation of right ventricular systolic and diastolic function in patients with newly diagnosed obstructive sleep apnea syndrome without hypertension. Cardiology. 2009;113(3):184–192. 10.1159/000193146 [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Cho G-Y, Shin C, et al. Impact of obstructive sleep apnea on left ventricular diastolic function. Am J Cardiol. 2008;101(11):1663–1668. 10.1016/j.amjcard.2008.01.056 [DOI] [PubMed] [Google Scholar]
- 16.Niroumand M, Kuperstein R, Sasson Z, Hanly PJ. Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med. 2001;163(7):1632–1636. 10.1164/ajrccm.163.7.2007014 [DOI] [PubMed] [Google Scholar]
- 17.Miya Y, Sumino H, Ichikawa S, et al. Effects of hormone replacement therapy on left ventricular hypertrophy and growth-promoting factors in hypertensive postmenopausal women. Hypertens Res. 2002;25(2):153–159. 10.1291/hypres.25.153 [DOI] [PubMed] [Google Scholar]
- 18.Ebong IA, Watson KE, Goff DC Jr, et al. Age at menopause and incident heart failure: the multi-ethnic study of atherosclerosis. Menopause. 2014;21(6):585–591. 10.1097/GME.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 20.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366(16):1467–1476. 10.1056/NEJMoa1110717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 22.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019;40(14):1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography . Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. 10.1067/mje.2002.120202 [DOI] [PubMed] [Google Scholar]
- 25.Jafary FH. Devereux formula for left ventricular mass--be careful to use the right units of measurement. J Am Soc Echocardiogr. 2007;20(6):783. 10.1016/j.echo.2007.02.034 [DOI] [PubMed] [Google Scholar]
- 26.Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165(15):1788–1793. 10.1001/archinte.165.15.1788 [DOI] [PubMed] [Google Scholar]
- 27.Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727–754. 10.1016/j.echo.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 28.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56(1):91–98. 10.1161/HYPERTENSIONAHA.110.150250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, Li H, Liu X, et al. Left ventricular remodeling and dysfunction in obstructive sleep apnea: systematic review and meta-analysis. In press. Herz. 2019. 10.1007/s00059-019-04850-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. 10.1016/j.jacc.2006.08.060 [DOI] [PubMed] [Google Scholar]
- 31.Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–616. 10.1016/j.jacc.2013.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie J, Sert Kuniyoshi FH, Covassin N, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5(8):e003162. 10.1161/JAHA.115.003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107(13):1822–1826. 10.1161/01.CIR.0000061758.05044.64 [DOI] [PubMed] [Google Scholar]
- 34.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. 10.1093/sleep/31.6.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng J, Zhang D, Chen B. Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012;16(2):283–294. 10.1007/s11325-011-0519-8 [DOI] [PubMed] [Google Scholar]
- 36.Castro-Grattoni AL, Alvarez-Buvé R, Torres M, et al. Intermittent hypoxia-induced cardiovascular remodeling is reversed by normoxia in a mouse model of sleep apnea. Chest. 2016;149(6):1400–1408. 10.1016/j.chest.2015.11.010 [DOI] [PubMed] [Google Scholar]