Abstract
Study Objectives:
The aim of this study was to compare the risk of undiagnosed sleep disorders among medical patients with chronic obstructive pulmonary disease (COPD) compared with those without COPD.
Methods:
In a prospective cohort study, hospitalized medical ward patients without a known sleep disorder were screened, using validated questionnaires, for sleep disorders, such as obstructive sleep apnea and insomnia. Daily sleep duration and efficiency in the hospital were measured via wrist actigraphy. Participants were classified into two groups: those with a primary or secondary diagnosis of COPD and those without a history of COPD diagnosis. Sleep outcomes were compared by COPD diagnosis.
Results:
From March 2010 to July 2015, 572 patients completed questionnaires and underwent wrist actigraphy. On admission, patients with COPD had a greater adjusted risk of obstructive sleep apnea (adjusted odds ratio 1.82, 95% confidence interval 1.12–2.96, P = .015) and clinically significant insomnia (adjusted odds ratio 2.07, 95% confidence interval 1.12–3.83, P = .021); no differences were observed for sleep quality or excess sleepiness on admission. After adjustment, compared with patients without COPD, patients with COPD averaged 34 fewer minutes of nightly sleep (95% confidence interval 4.2–64.0 minutes, P = .026), as well as 22.5% lower odds of normal sleep efficiency while in the hospital (95% confidence interval 3.3%–37.9%, P = .024). No statistically significant differences were observed for in-hospital sleep quality, soundness, or ease of falling asleep.
Conclusions:
Among hospitalized patients in medical wards, those with COPD have higher risk of OSA and insomnia and worse in-hospital sleep quality and quantity compared with those without COPD.
Citation:
Stewart NH, Walters RW, Mokhlesi B, Lauderdale DS, Arora VM. Sleep in hospitalized patients with chronic obstructive pulmonary disease: an observational study. J Clin Sleep Med. 2020;16(10):1693–1699.
Keywords: chronic obstructive pulmonary disease, hospitalizationobstructive sleep apnea, insomnia, sleep efficiency
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep complaints are highly prevalent in patients with chronic obstructive pulmonary disease (COPD). To date, no study has characterized the risk of undiagnosed sleep disorders in hospitalized patients with COPD.
Study Impact: Among hospitalized medical patients, those with COPD have a higher risk of obstructive sleep apnea and insomnia and shorter duration of sleep per night during hospitalization compared with those without COPD. Understanding the prevalence of sleep complaints in the hospitalized COPD population can better inform the health care of these patients.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects more than 24 million adults in the United States and is the third leading cause of morbidity and mortality in the United States.1,2 It results in significant costs to the health care system, particularly emergency department visits and hospitalizations.2,3 COPD readmissions are a target for Medicare penalties, and many disease management programs targeting hospitalized COPD patients have been introduced as a result.3–5
One underrecognized but known risk for acute COPD exacerbations is poor baseline sleep quality.6 Higher baseline poor sleep symptoms, as assessed by the Pittsburgh Sleep Quality Index, are related to subsequent symptom-based exacerbations over an 18-month follow-up period.6 In addition to poor sleep, a common unrecognized factor that can exacerbate COPD is concomitant obstructive sleep apnea (OSA). OSA is a common sleep disorder, affecting approximately one in four adults, and up to 90% of patients with OSA remain undiagnosed.7–10 The term overlap syndrome refers to COPD and OSA in the same patient. These patients have more frequent nocturnal oxygen desaturations, hypoxemia, hypercapnia, and dysrhythmias than those without combined COPD and OSA.11,12 Importantly, patients with overlap syndrome have higher rates of comorbidities and mortality and lower daily vitality than those with COPD alone.11,13,14
Recognizing overlap syndrome in hospitalized COPD patients may be especially important to helping improve their symptoms and reduce readmissions. In fact, hospitalization has been described as a missed opportunity to address sleep disorders attributable to the high prevalence of undiagnosed sleep disorders in this population. A recent study of hospitalized general medical patients showed that 40% are at high risk of OSA and had worse in-hospital sleep quantity and quality.15 Despite growing evidence linking sleep disturbances in patients with COPD and outcomes, no studies have yet characterized the risk of underlying sleep disorders, shorter sleep duration, or worse sleep quality among hospitalized patients with COPD. The aim of this study was to compare the risk of undiagnosed sleep disorders and in-hospital sleep duration and sleep efficiency among medical inpatients with COPD compared with those without COPD.
METHODS
Study design
We enrolled patients from an ongoing prospective study of in-hospital sleep in general medicine inpatients at the University of Chicago Medical Center.15,16 Eligible patients included those admitted to general medicine wards who were community-dwelling adults aged ≥ 50 years and cognitively intact (as defined by the Short Portable Mental Status Questionnaire).15 Excluded patients had known sleep disorders (by self-report or chart review), were in respiratory isolation, had been transferred from the intensive care unit (ICU), were already 72 hours into hospitalization before eligibility screening, or had been readmitted within 2 weeks. Patients were also excluded if they were immobile, on bed rest, or unable to use wrist actigraphy accurately or safely. These exclusion criteria were used to define a sample of patients without known sleep disorders and whose actigraphy data could be interpreted. Using the International Classification of Diseases 9th Revision coding (codes 490–496), we defined two groups: those with COPD as a primary or secondary diagnosis and those without a history of COPD. The University of Chicago Institutional Review Board approved this study, and written consent was obtained from all participants.
Data collection
Risk of sleep disorders
The risk of undiagnosed sleep disorders was measured during initial patient interview with a trained research assistant using the Berlin Questionnaire, which includes 11 items specific to snoring, fatigue, and high blood pressure.17 The Berlin Questionnaire, considered the most accurate screening questionnaire to predict diagnosis of OSA,18,19 has been shown to have validity evidence with polysomnography in a variety of patient populations.20 Risk of OSA using the Berlin score is determined through three categories with 11 total questions; high risk is demonstrated when two or more categories have a (+) score. Risk of insomnia was measured during the initial patient interview using the Insomnia Severity Index (ISI), which grades insomnia as mild, moderate, or severe through seven questions with responses on a 0 to 4 scale, with a total score of 0 to 28 (< 8 is no clinical insomnia) and has been previously validated.21 Excessive daytime sleepiness was measured on intake using the Epworth Sleepiness Scale), which includes responses on an additive scale never (0) to high (3) to create total score. A score of > 9 is indicative of excessive sleepiness.22
Self-reported sleep quality in hospital and before hospitalization
Patient-reported daily sleep quality at night during the hospitalization was assessed using the Karolinska Sleep Diary, which includes questions regarding quality, ease, and soundness of sleep the previous night, including number of awakenings.23 Self-reported sleep quality throughout the month before hospital admission was measured on intake using the Pittsburgh Sleep Quality Index.24
Objective sleep duration and efficiency
Enrolled study patients wore a wrist activity monitor (Actiwatch 2; Respironics Inc., Murrysville, Pennsylvania) to collect objective sleep-wake activity data, which have been validated to calculate sleep duration and efficiency in field and workplace studies.25,26 Through actigraphy, sleep duration (in minutes) and sleep efficiency (100 × [minutes of actual sleep/sleep onset-sleep offset]) can be estimated. Sleep onset and offset can also be determined via self-report. Consented patients wore wrist actigraphy initiated on the day of enrollment until the day of hospital discharge. Data were downloaded and analyzed using Actiware 5 (Respironics, Inc.). To confirm the accuracy of sleep interval, nightly sleep onset and awakening were based on patient report logged in the Karolinska Sleep Diary. Sleep duration was determined by total time spent asleep during the sleep interval, and sleep efficiency was defined as the percentage of time spent asleep during the sleep interval.
Demographics, health status, disease severity, and patient outcomes
Demographic information, including age, race, ethnicity, and biologic sex, was collected. Diagnoses, health status variables, and number of hospitalizations and rehospitalizations were also collected using International Classification of Diseases-9 coding. These data were obtained from inpatient interviews, chart reviews, and administrative data collected as part of an ongoing study of admitted general medicine patients to the University of Chicago Medical Center.16,27
Data analysis
Study data were collected and managed using REDCap (Research Electronic Data Capture) hosted at The University of Chicago.28,29 Patient demographic and disease severity variables were merged with the ongoing data set within the REDCap database.
Given skewed data distributions, continuous variables are presented as median and interquartile range (IQR). Categorical variables are presented as frequency and percentage. Multivariable logistic regression models were used to compare the odds of potential underlying sleep disorder (measured by Berlin and ISI), excessive daytime sleepiness (Epworth Sleepiness Scale) and poor self-reported sleep quality (measured by the Pittsburgh Sleep Quality Index) between groups. Nightly sleep duration obtained from actigraphy was analyzed using a linear mixed-effects model to account for repeated measurement of patients during their hospital stay. Model assumptions were evaluated using histograms, Q-Q plots, and scatterplots of random effects residuals. Sleep efficiency could range from 0% to 100%.
These percentages were converted to a proportion and estimated using a mixed-effects beta model. Given that this model used the logit link, it compared the odds of normal sleep efficiency between groups. Nightly sleep quality, ease of falling asleep, and sleep soundness (measured by the Karolinska Sleep Diary) were evaluated using a mixed-effects proportional-odds model with a cumulative logit link and multinomial conditional response distribution. The tenability of the proportional odds assumption was evaluated using the Score test. For all mixed-effects models, the need for additional random slopes was tested using the likelihood ratio test. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina), with P < .05 used to indicate statistical significance.
RESULTS
From March 2010 to July 2015, 572 individuals at a single center completed questionnaires and had corresponding wrist actigraphy data. Of these, 131(22.9%) had a COPD diagnosis. Thirty-five patients had an admitting diagnosis of COPD, the remaining 96 patients a history of COPD. Median age was 62 years (IQR: 56–73 years), and median length of stay across groups was 4 days (IQR: 3–6 days). Compared with patients without COPD, patients with COPD were slightly younger and were more likely to be African American, to have congestive heart failure, and require oxygen within the first 24 hours of admission (Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| Total Cohort (N = 572) | COPD (n = 131) | No COPD (n = 441) | P Value | |
|---|---|---|---|---|
| Age (y) | 62 (56–73) | 61 (55–71] | 63 (57–74) | .027 |
| BMI (kg/m2) | 26 (22–32) | 26 (22–31] | 26 (22–32) | .711 |
| Length of stay (days) | 4 (3–6) | 4 (2–6] | 4 (3–6) | .343 |
| Wrist actigraphy (days) | 1 (1–2) | 1 (1–2] | 1 (1–2) | .181 |
| Biological sex | ||||
| Male | 258 (45.1) | 52 (39.7) | 206 (46.7) | .156 |
| Female | 314 (54.9) | 79 (60.3) | 235 (53.3) | |
| Race | ||||
| White | 124 (22.1) | 22 (17.1) | 102 (23.7) | .070 |
| African American | 415 (74.1) | 105 (81.4) | 310 (71.9) | |
| Other | 21 (3.8) | 2 (1.6) | 19 (4.4) | |
| Comorbidities | ||||
| Prior hospitalization | 174 (39.7) | 33 (32.4) | 141 (42.0) | .082 |
| CHF | 79 (13.8) | 29 (22.1) | 50 (11.4) | .002 |
| ESRD | 64 (11.2) | 11 (8.4) | 53 (12.1) | .242 |
| Diabetes | 162 (28.3) | 41 (31.3) | 121 (27.4) | .389 |
| Charlson Comorbidity Index | ||||
| 0 | 131 (28.5) | 16 (15.7) | 115 (32.1) | <.001 |
| 1 | 103 (22.4) | 39 (38.2) | 64 (17.9) | |
| 2 | 72 (15.7) | 22 (21.6) | 50 (14.0) | |
| 3 | 86 (18.7) | 13 (12.8) | 73 (20.4) | |
| ≥4 | 68 (14.8) | 12 (11.8) | 56 (15.6) | |
| Oxygen requirement | ||||
| Present on admission | 12 (2.1) | 2 (1.5) | 10 (2.3) | 1.000 |
| Within first 24 h | 176 (31.4) | 63 (48.8) | 113 (26.2) | <.001 |
| No need w/in 24 h | 157 (89.2) | 56 (88.9) | 101 (89.4) | .920 |
| >4% Desaturation | 367 (64.4) | 86 (65.7) | 281 (64.0) | .731 |
Data are presented as median [interquartile ratio, IQR] or frequency (%).
BMI = body mass index, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, ESRD = end stage renal disease.
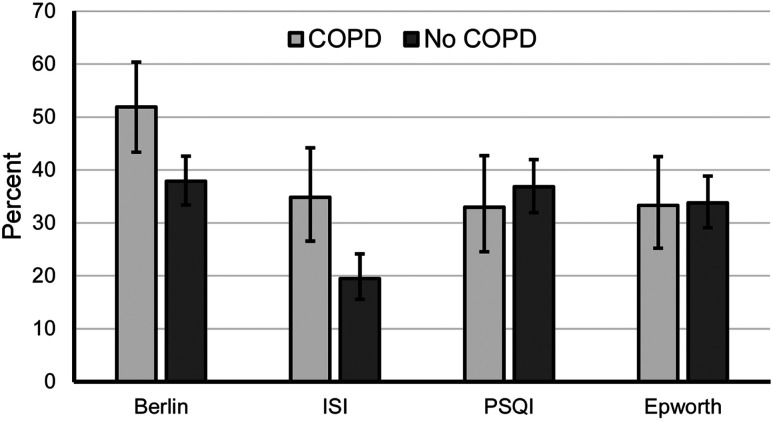
Patients with COPD had a greater unadjusted risk of OSA via Berlin and rate of clinically significant insomnia via ISI (Figure 1). Associations remained statistically significant after adjustment for covariates (adjusted odds ratio [aOR] 1.82, 95% confidence interval [CI] 1.12–2.96, P = .015, aOR 2.15, 95% CI 1.23–3.75, P = .007). No statistically significant unadjusted differences were observed when comparing self-reported sleep quality via the Pittsburgh Sleep Quality Index (P = .482) or excessive daytime sleepiness via Epworth (P = .927) in patients with or without COPD (Figure 1). These results remained after adjustment for covariates (aOR .92, 95% CI .52–1.62, P = .767 and aOR .90, 95% CI .52–1.58, P = .724, respectively).
Figure 1. Questionnaire evaluation of sleep disorders.
Error bars represent confidence intervals. %COPD vs % no COPD: Berlin (51.9% vs 37.9%, P = .005); Insomnia Severity Index (34.9% vs 19.5%, P = .001); Pittsburgh Sleep Quality Index (33.0% vs 36.8%, P = .482); Epworth (33.3% vs 33.8%, P = .927). COPD = chronic obstructive pulmonary disease.
Patients at high risk for sleep apnea, determined by Berlin, averaged 17.1% longer length of stay compared with low-risk patients (5.0 days vs 4.3 days, 95% CI: 0.8–36.0%, P = .039). Patients with clinical insomnia, determined by ISI, averaged 13.5% longer length of stay compared with patients with nonclinical insomnia (5.0 days vs 4.4 days, 95% CI: 6.8% shorter to 38.3% longer, P = .207). The interaction with COPD was not statistically significant; interaction P = .414 for Berlin, and interaction P = .531 for ISI.
Using wrist actigraphy obtained from 825 nights from individuals, median sleep duration was 316 minutes (IQR 193–393) for patients with COPD compared with 325 minutes (IQR 237–416) for patients without COPD (P = .044). An unconditional (ie, no predictor) linear mixed-effects model indicated approximately 33% of variability in sleep time was available to predict via between-patient covariates (eg, COPD, age, sex). A fixed linear effect of time indicated patients averaged an 8.7-minute decrease in sleep each day in hospital (95% CI 0.7–16.6 minutes, P = .033) irrespective of COPD. No between-patient differences for this decrease were indicated given nonsignificant random linear slope variance (–2∆LL [df = 2] = 2.3, P = .317). After adjusting for covariates (age, sex, race, body mass index [BMI], prior hospitalization, congestive heart failure [CHF], end-stage renal disease [ESRD], and diabetes mellitus), patients with COPD averaged 34.1 fewer minutes of sleep compared with patients without COPD (95% CI 4.2–64.0 minutes, P = .026) (Table 2). Normality and homoscedasticity of residuals were tenable for this final model.
Table 2.
Actigraphy sleep time linear mixed-effects model results.
| 95% CI for Coefficient | |||||
|---|---|---|---|---|---|
| Coefficient (min) | SE | Lower | Upper | P Value | |
| COPD vs no COPD | −34.13 | 15.20 | −64.04 | −4.22 | .026 |
| Linear time (0 = baseline) | −9.30 | 4.05 | −17.27 | −1.34 | .022 |
| Age | 1.68 | .64 | .41 | 2.94 | .010 |
| Male vs female | 27.81 | 13.31 | 1.62 | 54.00 | .038 |
| Race | |||||
| White vs other | 35.51 | 37.62 | −38.53 | 109.56 | .346 |
| Black vs other | 28.72 | 36.07 | −42.27 | 99.71 | .427 |
| White vs Black | 6.79 | 15.71 | −24.13 | 37.71 | .666 |
| BMI | .19 | 0.45 | −1.08 | .70 | .677 |
| Prior hospitalization | −7.29 | 14.63 | −36.08 | 21.50 | .619 |
| CHF | 2.97 | 19.11 | −34.64 | 40.58 | .877 |
| ESRD | 5.71 | 21.60 | −36.81 | 48.22 | .792 |
| Diabetes | −10.52 | 14.77 | −39.59 | 18.56 | .477 |
| Intercept | 204.88 | 47.67 | — | — | — |
BMI = body mass index, CHF = congestive heart failure, CI, confidence interval, COPD = chronic obstructive pulmonary disease, ESRD = end-stage renal disease, SE, standard error.
Random intercept variance = 7,033.99, SE = 1,309.63. Residual variance = 13,274, SE = 1,085.71. The reference category for the fixed effect of any categorical predictor is identified following the “vs” For example, the patients with a COPD diagnosis averaged 34.13 fewer minutes of sleep per night compared with patients with no COPD diagnosis. Linear time 0 equals first study day.
Median sleep efficiency was 71.8% (IQR 54.6%–84.3%) for patients with COPD compared with 76.6% (IQR 62.8%–86.2%) for patients without COPD (P = .012). We found no statistically significant change in sleep efficiency across days (OR 0.98, 95% CI 0.93–1.04, P = 0.488) irrespective of COPD; a random linear time slope could not be estimated. This result indicated that the sleep efficiency of patients fluctuated across days, an absence of systematic or random change. After controlling for age, sex, race, BMI, prior hospitalizations, CHF, and ESRD, patients with COPD had a 22.5% lower odds of normal sleep efficiency relative to patients without COPD (95% CI 3.3%–37.9%, P = .024) (Table 3).
Table 3.
Sleep efficiency mixed-effects beta model results.
| 95% CI for OR | ||||||
|---|---|---|---|---|---|---|
| Coefficient | SE | OR | Lower | Upper | P Value | |
| COPD vs no COPD | −.25 | .11 | .78 | .62 | .97 | .024 |
| Linear time (0 = baseline) | −.03 | .03 | .97 | .92 | 1.03 | .347 |
| Age | .01 | .00 | 1.01 | 1.00 | 1.02 | .009 |
| Male vs female | .21 | .10 | 1.23 | 1.01 | 1.49 | .037 |
| Race | ||||||
| White vs other | .30 | .28 | 1.35 | .78 | 2.33 | .282 |
| Black vs other | .23 | .27 | 1.26 | .75 | 2.12 | .390 |
| White vs Black | .07 | .12 | 1.07 | .85 | 1.35 | .552 |
| BMI | .00 | .00 | 1.00 | 1.00 | 1.01 | .473 |
| Prior hospitalization | .09 | .11 | 1.10 | .88 | 1.36 | .405 |
| CHF | −.05 | .14 | .95 | .72 | 1.26 | .742 |
| ESRD | .17 | .16 | 1.19 | .86 | 1.64 | .291 |
| Diabetes | −.02 | .11 | .98 | .79 | 1.22 | 0.881 |
| Intercept | −.17 | .36 | — | — | — | — |
COPD = chronic obstructive pulmonary disease. BMI = body mass index. CHF = congestive heart failure, CI, confidence interval, ESRD = end stage renal disease, OR, odds ratio, SE, standard error.
Random intercept variance = 0.44, SE = 0.07. Scale = 6.84, SE = 0.62. Sleep efficiency was modeled as a proportion with 0 = no sleep efficiency and 1 = perfect sleep efficiency. In a beta model, we are predicting the probability of the outcome coded 1 (ie, perfect sleep efficiency); fixed effects are interpreted similarly to logistic regression. The reference category for the fixed effect for any categorical predictor is identified following the “vs” For example, the odds of reporting normal sleep efficiency were 22% lower for patients with a COPD diagnosis relative to those with no COPD diagnosis (ie, [1–0.78]*100).
Finally, using the Karolinska Sleep Quality Index, after adjusting for covariates, no statistically significant differences between patients were found for self-reported sleep quality (aOR 0.92, 95% CI 0.63–1.34, P = .660), sleep soundness (aOR 1.04, 95% CI .70–.56, P = .840), or ease of falling asleep (aOR 0.91, 95% CI 0.59–1.40, P = .679).
DISCUSSION
In this prospective observational study, we demonstrated, among patients hospitalized in medical wards in a tertiary urban medical center, that those with a diagnosis of COPD have higher odds of being screened as being at high risk for OSA and clinically significant insomnia and experience worse in-hospital sleep quantity and quality as measured by wrist actigraphy compared with those without COPD. Specifically, patients with COPD averaged more than half an hour less sleep per night during hospitalization than their counterparts without COPD, irrespective of underlying comorbidities. Although no clinically significant difference in sleep efficiency between patients with and without COPD was noted (71.8% vs 76.6%, respectively), patients with COPD had 22.5% lower odds of normal sleep efficiency while in the hospital (95% CI 3.3%–37.9%, P = .024). Aside from sex and age, no other covariates were significantly associated with sleep time (Table 2). All patients, regardless of comorbidities, averaged more than 8 minutes less sleep each night with each consecutive day in the hospital. No statistically significant differences were observed for in-hospital sleep quality, soundness, or ease of falling asleep.
In this study, we also demonstrated the feasibility of screening for risk of sleep disorders during hospitalization using previously validated tools. Our results suggest that the prevalence of OSA in COPD patients may be > 1.5 times the estimated prevalence of OSA within the general adult population.8 It is important to keep in mind that these patients underwent interviews to assess risk, not in-laboratory polysomnography, the gold standard for OSA diagnosis. This higher in-hospital prevalence may be explained by the association between OSA and other acute medical diagnoses associated with receiving hospital care.30 It is possible the association between screening high risk for OSA with a COPD diagnosis suggests that the “overlap syndrome” is a contributing factor. In addition to OSA, we observed an association between screening high risk of insomnia and COPD among hospitalized patients. This finding is concerning because increased risk of insomnia also places patients at increased risk for other deleterious medical conditions, including myocardial infarction and obesity.31,32 It is also possible the higher risk of insomnia emanating from poorly controlled symptom management of COPD is interfering with sleep. We also demonstrated that patients screening at higher risk for OSA via Berlin spent nearly 1 day more in hospital than those screening at high risk for insomnia via ISI. Although not statistically significant, 1 day more of hospitalization carries increased costs and clinical significance. Because of their known high risk for readmission, hospitalization is a missed opportunity to screen COPD patients for sleep disorders. Future work to elucidate whether formally diagnosing and treating underlying sleep disorders in hospitalized COPD patients can improve their health is warranted.
The lower than normal sleep efficiency and duration we observed in hospitalized patients with COPD may have various causes and may relate to the altered physiology of breathing during sleep, or it could be associated with known causes of poor sleep in hospitalized patients.33 Although increased risk and a sleep difference of approximately 30 minutes in patients with COPD may seem small, they have potential clinical implications and could impact patient outcomes.6 Poor sleep in the hospital setting has also been associated with poor hospital outcomes, such as delirium and hyperglycemia.34,35 Our results are in concert with prior studies assessing sleep in hospitalized patients, which have primarily reported on nighttime hospital disruptions and sleep environment in the ICU.33,36 Alarm fatigue, activities of patient care, and patient pain levels during hospitalization have all been shown to impact inpatient sleep.33,37 Future work to understand whether improving the sleep environment for COPD patients can improve their hospital stay and underlying illness(s) is merited.
Our study also has implications for screening and educating COPD patients on sleep disorders during the hospitalization period. For example, an analysis of patients with COPD from the Long-Term Oxygen Treatment cohort found a greater risk of undiagnosed OSA to be associated with poor outcomes.38 Interestingly, prior studies have demonstrated a multifactorial approach to reducing readmission rates within the COPD population, including increasing patient education, involving a multidisciplinary team, and noninvasive ventilation in appropriate patients.39–43 During the post-hospital period, patients are most vulnerable and at greatest risk for readmission.44 Because COPD patients are often rehospitalized for clinical conditions other than COPD, poor sleep has been suggested as an underlying contributor to this “post-hospital syndrome.”44 Given the great interest by various stakeholders on reducing readmission for COPD exacerbations to control costs and improve health, it is important to test whether sleep-based interventions as part of a multifactorial approach can reduce readmissions.
This study has several limitations. Owing to its observational nature, we cannot address direction of causality. This was a single institution study, representing English-speaking patients admitted to a general medicine ward, which limits the ability to generalize findings. Unlike the gold standard for OSA diagnosis (ie, polysomnography), actigraphy is unable to assess sleep architecture; however, it can provide more robust information on sleep duration across multiple days. Under- and over-diagnosis of COPD remains a challenge in health services research, particularly if International Classification of Diseases coding instead of spirometry is used, as in our study.45,46 Many COPD patients are initially admitted to the ICU, and this study excluded ICU patients and ICU transfers. Because we excluded the sickest of the COPD patients, this study may in fact underestimate risk of sleep disorders in patients with COPD. Because only 6% of study patients were admitted for COPD exacerbation, steroid effect on sleep was not evaluated. Several challenges exist with these data. The data include survey data, which are self-reported, and therefore subject to response and social desirability biases; however, we used validated questionnaires. Temporal issues such as recall bias are of concern owing to the question of accurate recall of details of sleep quality a month before hospital admission. Administrative data can also present potential coding discrepancies and questions of accuracy and validity of diagnoses.47 Furthermore, it should be noted that overdiagnosis of COPD in hospitalized patients could occur in up to a third of patients diagnosed with COPD, based on confirmatory spirometry testing.48 Daytime hypercapnia was not evaluated in these patients. Confounding was considered during analysis. In controlling for comorbid conditions, we attempted to control for demographics and severity of illness. The possibility of omitted confounders is a consideration. Goodness-of-fit of the model was tested with intake survey data and actigraphy, but not with the Karolinska questionnaire owing to ordinal nature of the data.
This study is the first to compare the risk of undiagnosed sleep disorders among medical inpatients with COPD and report on in-hospital self-reported and objective sleep of patients with COPD. Hospitalized patients with COPD are at higher risk than patients without COPD for underlying sleep disorders, such as insomnia and OSA. Hospitalized COPD patients also obtain more than 30 minutes less sleep per night compared with other hospitalized patients. Given the known association between sleep disorders and COPD, as well as the potential contributor of sleep loss to risk of readmission, future work to diagnose and treat underlying sleep disorders for these patients is warranted.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript as submitted. This study was performed at the University of Chicago Medical Center. Nancy H. Stewart. Ryan W. Walters, Babak Mokhlesi, and Diane S. Lauderdale have no conflicts of interest or financial support to report. Vineet M. Arora has no conflicts of interest and is funded by NHLBI K24HL136859.
ACKNOWLEDGMENTS
The authors thank Dr. David Meltzer and Ainoa Coltri of the University of Chicago Hospitalist Project and Samantha Anderson of the University of Chicago Division of General Internal Medicine.
ABBREVIATIONS
- aOR
adjusted odds ratio
- BMI
body mass index
- CHF
congestive heart failure
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- ESRD
end-stage renal disease
- ICU
intensive care unit//
- IQR
interquartile range
- ISI
Insomnia Severity Index
- OSA
obstructive sleep apnea
REFERENCES
- 1.https://www.nhlbi.nih.gov/files/docs/factbook/FactBook2012.pdf. Accessed September 30, 2020.
- 2.https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. Accessed September 30, 2020.
- 3.Feemster LC, Au DH. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634–639. 10.1164/rccm.201308-1541PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. 10.1016/j.chest.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. 10.1378/chest.14-2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shorofsky M, Bourbeau J, Kimoff J, et al. Impaired sleep quality in COPD is associated with exacerbations: the CanCOLD Cohort Study. Chest. 2019;156(5):852–863. 10.1016/j.chest.2019.04.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: results from the national sleep foundation sleep in America 2005 poll. Chest. 2006;130(3):780–786. 10.1378/chest.130.3.780 [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. 10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 12.Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12(4):367–372. 10.1016/j.sleep.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 13.Starr P, Agarwal A, Singh G, et al. Obstructive sleep apnea with chronic obstructive pulmonary disease among Medicare beneficiaries. Ann Am Thorac Soc. 2019;16(1):153–156. 10.1513/AnnalsATS.201712-932OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XL, Dai HP, Zhang H, Gao B, Zhang L, Han T, Wang C. Obstructive sleep apnea in patients with fibrotic interstitial lung disease and COPD. J Clin Sleep Med. 2019;15(12):1807–1815. 10.5664/jcsm.8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shear TC, Balachandran JS, Mokhlesi B, et al. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10(10):1061–1066. 10.5664/jcsm.4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists. Ann Intern Med. 2002;137(11):866–874. 10.7326/0003-4819-137-11-200212030-00007 [DOI] [PubMed] [Google Scholar]
- 17.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 18.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. 10.1097/ALN.0b013e31816d91b5 [DOI] [PubMed] [Google Scholar]
- 19.Fedson AC, Pack AI, Gislason T. Frequently used sleep questionnaires in epidemiological and genetic research for obstructive sleep apnea: a review. Sleep Med Rev. 2012;16(6):529–537. 10.1016/j.smrv.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Senaratna CV, Perret JL, Matheson MC, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–124. 10.1016/j.smrv.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 23.Åkerstedt T, Hume K, Minors D, Waterhouse J. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79(1):287–296. 10.2466/pms.1994.79.1.287 [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 26.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. 10.5665/sleep.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185–2186. 10.1111/j.1532-5415.2011.03644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Minor BL, et al.REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correia LCL, Souza AC, Garcia G, et al. Obstructive sleep apnea affects hospital outcomes of patients with non-ST-elevation acute coronary syndromes. Sleep. 2012;35(9):1241–1245A. 10.5665/sleep.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124(19):2073–2081. 10.1161/CIRCULATIONAHA.111.025858 [DOI] [PubMed] [Google Scholar]
- 32.Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. 10.1016/j.jacc.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grossman MN, Anderson SL, Worku A, et al. Awakenings? patient and hospital staff perceptions of nighttime disruptions and their effect on patient sleep. J Clin Sleep Med. 2017;13(2):301–306. 10.5664/jcsm.6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188–193. 10.2337/dc16-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altman MT, Knauert MP, Murphy TE, Ahasic AM, Chauhan Z, Pisani MA. Association of intensive care unit delirium with sleep disturbance and functional disability after critical illness: an observational cohort study. Ann Intensive Care. 2018;8(1):63. 10.1186/s13613-018-0408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–738. 10.1164/rccm.201411-2099CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller A, Roth T, Roehrs T, Yaremchuk K. Correlation between sleep disruption on postoperative pain. Otolaryngol Head Neck Surg. 2015;152(5):964–968. 10.1177/0194599815572127 [DOI] [PubMed] [Google Scholar]
- 38.Donovan LM, Feemster LC, Udris EM, et al. Poor outcomes among patients with chronic obstructive pulmonary disease with higher risk for undiagnosed obstructive sleep apnea in the LOTT cohort. J Clin Sleep Med. 2019;15(1):71–77. 10.5664/jcsm.7574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galli JA, Krahnke JS, James Mamary A, Shenoy K, Zhao H, Criner GJ. Home non-invasive ventilation use following acute hypercapnic respiratory failure in COPD. Respir Med. 2014;108(5):722–728. 10.1016/j.rmed.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson T, North M, Bourne SC. Reducing hospital admissions and improving the diagnosis of COPD in Southampton City: methods and results of a 12-month service improvement project. NPJ Prim Care Respir Med. 2014;24(1):14035. 10.1038/npjpcrm.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Press VG, Au DH, Bourbeau J, et al. Reducing chronic obstructive pulmonary disease hospital readmissions: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2019;16(2):161–170. 10.1513/AnnalsATS.201811-755WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coughlin S, Liang WE, Parthasarathy S. Retrospective assessment of home ventilation to reduce rehospitalization in chronic obstructive pulmonary disease. J Clin Sleep Med. 2015;11(6):663–670. 10.5664/jcsm.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzma AM, Meli Y, Meldrum C, et al. Multidisciplinary care of the patient with chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008;5(4):567–571. 10.1513/pats.200708-125ET [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. 10.1164/rccm.201804-0621CI [DOI] [PubMed] [Google Scholar]
- 46.Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, ninth revision, clinical modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87–93. 10.1378/chest.11-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho T-W, Ruan S-Y, Huang C-T, Tsai Y-J, Lai F, Yu C-J. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from National Health Insurance claim data in Taiwan. Int J Chron Obstruct Pulmon Dis. 2018;13:3055–3063. 10.2147/COPD.S174265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynn N, Johnson N, Ferreira TBD. Multidisciplinary team approach to reduce COPD readmissions in a tertiary care center. Am J Respir Crit Care Med. 2018;197:A4977. [Google Scholar]