Abstract
Study Objectives:
Reduced gray matter volume in the posterior cingulate cortex (PCC) has recently been found in patients with non–rapid eye movement (NREM) parasomnia, providing a neuroanatomical substrate for the arousal state dissociation. It remains unclear whether PCC changes in NREM parasomnias might also play a role in cognitive or affective dysfunction in these patients. The aim of this exploratory study was to investigate neurobehavioral correlates of PCC abnormalities in patients with NREM parasomnia.
Methods:
The Reinforcement Sensitivity Theory of Personality Questionnaire and the Stress Coping Questionnaire were used to assess personality and stress coping in 15 patients with NREM parasomnia and 15 age- and sex-matched healthy controls. Patients’ left PCC gray matter volume was quantified with voxel-based morphometry on 3 Tesla T1-weighted magnetic resonance imaging data.
Results:
In the Reinforcement Sensitivity Theory of Personality Questionnaire, increased trait reactivity of the behavioral inhibition system and goal-drive persistence contributed most to the discrimination of patients and controls. In the Stress Coping Questionnaire, patients showed an increased negative coping trait (ie, anxious rumination) related to an increase in adjusted left PCC volume.
Conclusions:
The results suggest subclinical behavioral abnormalities in patients with NREM parasomnias. Such traits might trigger maladaptive emotion regulation processes related to a relative PCC volume increase. The findings encourage further longitudinal studies on this topic, which can provide insights into the causal relations underlying the PCC volume–behavior correlation. Such future studies will have a more direct implication for the clinical management of patients with NREM parasomnias.
Citation:
Ramm M, Urbanek A, Failing A, et al. Increased behavioral inhibition trait and negative stress coping in non–rapid eye movement parasomnias. J Clin Sleep Med. 2020;16(10):1737–1744.
Keywords: somnambulism, personality, stress coping, reinforcement sensitivity
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although significant progress has been made in recent years, the pathophysiological processes contributing to the phenomenon of non–rapid eye movement parasomnias are still not well understood. Previous studies were not able to reconcile electrophysiological and neuroimaging findings pointing towards a critical role of the cingulate cortex with studies showing psychopathological changes.
Study Impact: In the present study we tested for subclinical psychological abnormalities, which we interpreted in the context of a widely accepted neuropsychological theory. Furthermore, posterior cingulate cortex volume was measured and related to psychological traits. This study brings together the neuroimaging approach and the psychological perspective contributing to the understanding of the complex pathological processes in non–rapid eye movement parasomnias. Further longitudinal studies are needed to prove the clinical significance of our findings.
INTRODUCTION
Non–rapid eye movement (NREM) parasomnias refer to behaviors with altered self-awareness initiated during incomplete arousal from slow-wave sleep.1 Behavioral manifestations include confusional arousals, sleepwalking, sleep terrors, and sleep-related eating disorder.2
The pathophysiological processes contributing to the behavioral phenomena of NREM parasomnia remain largely unknown.3 The finding of a coexistence of local NREM sleeplike and wakelike electroencephalographic patterns during a confusional arousal episode suggested a sleep–wake dissociation as one important pathophysiological mechanism in NREM parasomnias.4 Using single-photon emission computed tomography during a parasomnia episode, increased cerebral blood flow was found in the cerebellum and in the posterior cingulate cortex (PCC), whereas blood flow was reduced in frontal and parietal brain regions.5 Recently, a neuroimaging study using voxel-based morphometry identified reduced gray matter volume in the left dorsal PCC (Brodmann area 23) and posterior midcingulate cortex (Brodmann area 24) in patients with NREM parasomnia.6 The authors suggested that morphometric changes in the PCC might contribute to sleep state dissociation between motor and cingulate cortices, on the one hand, and frontal and parietal association cortices, on the other hand. This interpretation is congruent with the current theory that the PCC is a key structure in the default mode network7 involved in the regulation of arousal and awareness. Increasing evidence also suggests a crucial role for the PCC in cognition. The PCC was shown to be involved in internally directed thoughts8 and environmental change detection.9 Other evidence indicates that the PCC supports attention focus regulation, possibly controlling the balance between internal and external attention.10 Furthermore, changes in the PCC and the default mode network were found in a range of neurological and psychiatric disorders—for example, Alzheimer disease, autistic spectrum disorders, schizophrenia, depression, and attention-deficit/hyperactivity disorder (for review, see reference 11). More specifically, abnormal PCC function, in particular a reduced deactivation under cognitive load, has been shown to be a correlate of lower attention performance and psychopathology.7 For instance, in depression, abnormal PCC functional connectivity was suggested to play an important role in the pathophysiology of depression.12 Moreover, an increased functional connectivity between the PCC and subgenual cingulate was related to excessive rumination, which forms a key feature of behavioral dysfunction in depression.13 In sum, there is much evidence that PCC abnormality may not only be a neuroanatomical substrate of NREM parasomnias but is also related to daytime behavioral abnormalities.
Epidemiological studies showed that NREM parasomnias are associated, among others, with anxiety14 and depression,15 but little is known about the role psychopathology plays in the pathophysiology of NREM parasomnias. Patients with NREM parasomnias often name psychological stress as an important trigger for their episodes, although the association between perceived stress level and the severity of NREM parasomnia has not yet been systematically investigated. However, a considerable number of patients with NREM parasomnia report life-changing events at the onset of the disorder, suggesting that stress may somehow play a role.16 So far, behavioral abnormalities in the subclinical range have rarely been studied. Perogamvros et al17 investigated temperament and character traits in, among others, patients with NREM parasomnia (ie, sleepwalking) using the Temperament and Character Inventory.18 They found higher scores on the novelty-seeking scale and a lower self-directedness, which indicated an increase in reward sensitivity and impulsivity. On the other hand, trend effects for a greater anticipatory worry about and dependence on social attachment were observed. In sum, the empirical evidence for subclinical personality changes in NREM parasomnias is limited and their relationship to the recently found volumetric changes in the PCC has not been explored.
In the current study, we tested for abnormalities in psychological traits in patients with a NREM parasomnia compared with matched healthy controls (HCs). We used well-established questionnaires to assess personality and, specifically, distinct aspects of stress coping. Based on previous psychopathological findings,14,15 we hypothesized that patients with NREM parasomnias show increased scores in personality scales that are associated with anxiety, worry, and negative stress. As suggested previously,17 these patients might additionally present an increased reward sensitivity. In the context of an established neuropsychological framework, the Reinforcement Sensitivity Theory of Personality (RST), we predicted increased scores in behavioral inhibition and behavioral approach traits. Moreover, we hypothesized that patients use positive coping strategies less often and negative coping strategies more often than controls. Finally, PCC is not only critically involved in the arousal dysregulation in patients with NREM parasomnias3,5 but the PCC might also play a role in depression,12 particularly in anxious rumination.13 Following our previous finding of morphometric changes in the PCC of patients with NREM parasomnias,6 we now tested whether these changes were related to scores on the personality and stress coping scales.
METHODS
Participants
Fifteen patients with current NREM parasomnia (females: 7; mean age: 29.7 ± 8.5 years) were recruited from the Institute of Sleep Medicine and Neuromuscular Disorders, University Hospital Muenster, Germany. Patients underwent high-resolution magnetic resonance imaging and a standard psychometric assessment. All patients were screened for other sleep disorders using at least 2 nights of cardiorespiratory video polysomnography. The patients fulfilled the diagnostic criteria for an NREM parasomnia according to the International Classification of Sleep Disorders, Third Edition.2 Exclusion criteria were medication use that is known to influence NREM sleep, substance use, insufficient sleep syndrome, or a sleep-related breathing or movement disorder. Moreover, patients with comorbid neurological disorders (except for restless-legs syndrome) or structural brain lesions were excluded.
As a control group, 15 age- and sex-matched HCs (female: 7; mean age: 29.7 ± 8.5 years) with no history of parasomnia symptoms or other psychiatric or neurological disorders completed the questionnaires. All participants gave written informed consent. The study was approved by the local ethics committee.
Magnetic resonance imaging data acquisition
Magnetic resonance imaging measurements were performed on a 3-Tesla whole-body magnetic resonance scanner (Magnetom TIM Trio; Siemens, Erlangen, Germany) using a 12-channel head coil. All participants underwent the same magnetic resonance imaging protocol, including whole-brain T1, T2, proton density-weighted, and fluid-attenuated inversion-recovery sequences. For voxel-based morphometry a coronal T1-weighted 3D magnetization prepared rapid gradient echo sequence (MPRAGE) was used (TR: 1800 ms; TE: 2.18 ms; inversion time: 900 ms; slice thickness: 1.2 mm; matrix: 256 × 204 pixels; flip angle: 9°; field of view: 220 × 165 mm).
Image post-processing
Voxel-based segmentation of the gray matter compartment was achieved using the standard version of the diffeomorphic anatomical registration using exponentiated lie algebra toolbox (DARTEL)19,20 of the statistical parametric mapping software package (SPM12; Wellcome Department of Cognitive Neurology, London, United Kingdom) implemented in Matlab 9.2 (MathWorks, Inc, Sherborn, MA).
In order to guarantee the highest level of spatial accuracy to localize the volume cluster of the PCC, identified by statistical parametric mapping in a previous study, the individual T1-weighted image was normalized to the intrinsic T1-weighted template and the obtained deformation fields were inverted and applied to the PCC gray matter segmentation in MNI (Montreal Neurological Institute) space.21 The obtained PCC gray matter volume in the individual patients’ space was adjusted for total intracranial volume.
Psychometric evaluation
The RST is a suitable neuropsychological framework to quantify behavioral abnormalities. According to its main assumption, personality reflects the sensitivity of underlying neurobehavioral systems to specific kinds of reinforcing stimuli.22 The RST relies on a large body of neurophysiological studies of learning and emotion in animals and humans, and trait measures of RST have been applied in a range of psychiatric and neurological disorders,23,24 suggesting the RST to be a framework for research on personality–psychopathology associations.25 In its most recent version, the RST differentiates 3 neurobehavioral systems: the fight-flight-freeze system (FFFS; related to fear) responds to all aversive stimuli and promotes avoidance and escape behavior, mediated by the amygdala, subgenual anterior cingulate cortex, and the periaqueductal gray26; the behavioral approach system (BAS; related to reward orientation) is activated by all forms of appetitive stimuli and supports approach behavior, controlled by dopaminergic reward regions27; the behavioral inhibition system (BIS; related to anxiety) signals all forms of goal conflict (eg, coactivation of BAS and FFFS in an approach-avoidance conflict), implemented by a septo-hippocampal network including the amygdala, anterior cingulate cortex and PCC and ventromedial prefrontal cortex. The BIS resolves a goal conflict by weighting relevant information and then giving rise to either BAS or FFFS activity.
The RST of Personality Questionnaire
The RST of Personality Questionnaire (RST-PQ) is a personality questionnaire that aims to assess traits according to the RST systems (ie, BAS, FFFS, and BIS). The RST is based on the central assumption that human personality reflects the reactivity of underlying neurobehavioral systems to different kinds of reinforcing stimuli (eg, rewarding or punishment stimuli).
The RST-PQ has been shown to be among the most valid measures to assess traits according to the revised RST.28 Exploratory and confirmatory factor analyses of the 78 items revealed a robust 6-factor structure29: 2 unitary defensive factors, the FFFS and the BIS; and 4 BAS factors (BAS1, Reward Interest; BAS2, Goal-Drive Persistence; BAS3, Reward Reactivity; BAS4, Impulsivity). Within the 2 defensive factors, the FFFS items measure facets of active avoidance, flight, and freeze, whereas the BIS items refer to facets of behavioral disengagement, obsessive thoughts, cautious risk assessment, motor planning interruption, and worry. The BIS scale shows high positive correlations with the State-Trait Anxiety Inventory,30 suggesting convergent validity.
The German coping questionnaire
The Stress Coping Questionnaire (SVF-120)31 is the only well-evaluated questionnaire for the multidimensional assessment of situation-invariant coping styles as traits in the German language.32
The SVF-120 consists of 120 items that refer to 20 subscales: 1 (trivialize stressor), 2 (self-aggrandizement by comparison with others), 3 (denial of guilt), 4 (distraction), 5 (substitute gratification), 6 (self-affirmation), 7 (recreation), 8 (situation control), 9 (reaction control), 10 (positive self-instructions), 11 (need for social support), 12 (avoidance), 13 (escape), 14 (social withdrawal), 15 (rumination), 16 (resignation), 17 (attitude of self-pity), 18 (self-blame), 19 (aggression), and 20 (pharmacotherapy).
Most primary scales are summarized into 1 secondary factor for negative coping (NEG; subtests 13–18) and 3 secondary factors for positive coping (POS)—that is, cognitive reinterpretation (POS1; subtests 01–03), distraction (POS2; subtests 04–07), and control of stressors (POS3; subtests 08–10).
Reliability is good for primary scales; Cronbach’s α ranges from 0.76 to to 0.92. The secondary scales were proven to have a Cronbach’s α ranging from 0.87 to 0.95. Divergent and convergent validity have been proved.
Additional questionnaires
Daytime functioning was evaluated using the Epworth Sleepiness Scale (ESS) and the Beck Depression Inventory (BDI). The ESS score ranges from 0 to 24; an ESS score >10 is interpreted as increased daytime sleepiness. BDI scores >13 indicate a depressive syndrome.
Statistical analysis
Statistical analysis was carried out with IBM SPSS Statistics Software (version 25.0; IBM Corporation, Armonk, NY). Multivariate analysis of covariance was conducted to analyze group differences across the outcome measures of the RST-PQ and the SVF-120, respectively. Age and sex were included as covariates in all models. All measures met the assumptions of the general linear model. Pearson correlational analyses between the abnormal behavioral measures and the left PCC gray matter volume were performed.
For the statistical analysis, P was set at .05. Note that for correlational analysis with the psychometric measures, the critical P value was Bonferroni-adjusted to .005 (P = .05/10).
RESULTS
Participants
Table 1 presents demographic and clinical data of the participants. Age and sex were matched between patients with NREM parasomnia and the controls. Seven patients described an increased self-reported sleep propensity as reflected by an ESS score >10. Two patients showed a mild depressive syndrome as indexed by a score ≥13 on the BDI.
Table 1.
Demographics and clinical data.
| NREM Parasomnia (n = 15) | Healthy Controls (n = 15) | |
|---|---|---|
| Demographics | ||
| Age, years | 29.7 ± 8.5 | 29.7 ± 8.5 |
| Sex (female/male), n/n | 7/8 | 7/8 |
| Clinical data | ||
| Disease onseta | 9/2/4 | — |
| NREM subtypesb | 6/–/4/5 | — |
| Frequency of episodes per month | 8.4 ± 7.9 | — |
| Epworth Sleepiness Scale | 8.9 ± 4.7 | — |
| Beck Depression Inventory | 8.1 ± 5.6 | — |
Data are presented as means ± SDs unless otherwise indicated. NREM = non–rapid eye movement. –, indicates not included in the sample.
Disease onset: number of patients with onset in childhood/adolescence/adulthood.
NREM subtypes: number of patients with sleep walking/sleep terror/confusional arousal/all subtypes.
The RST-PQ
Figure 1A presents the results of the RST-PQ. A multivariate analysis of covariance revealed that patients and controls differed in the combination of the 6 RST-PQ factors (Pillai’s trace; V = 0.57, F6, 21 = 4.7, P = .003). Scores on the RST-PQ factors were significantly increased in women compared with men (Pillai’s trace; V = 0.61, F6, 22 = 4.7, P = .004).
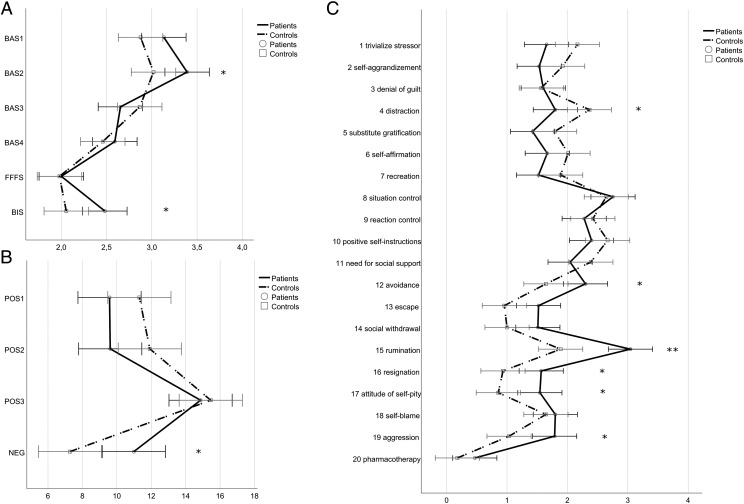
Figure 1. Mean scores.
(A) Mean scores of the Reinforcement Sensitivity Theory of Personality Questionnaire factors. BAS1, Reward Interest; BAS2, Goal-Drive Persistence; BAS3, Reward Reactivity; BAS4, Impulsivity; FFFS, Fight-Flight-Freeze System; BIS, Behavioral Inhibition System. (B) Mean scores of the secondary coping factors. POS1, Cognitive Reinterpretation; POS2, Distraction/Compensation; POS3, Control/Self-Instruction. (C) Mean scores of the primary coping scales. Higher scores reflect a higher frequency of that behavior or an increased trait. Error bars represent the 95% confidence interval.*Significant at the .05 level, **significant at the .01 level.
A subsequent discriminant function analysis revealed a significant discriminant function (canonical R2 = .57). Patients and controls were significantly discriminated by a linear combination of the 6 factors [λ = 0.4; χ2(6) = 21.1; P = .002]. A total of 93.3% of the patients and controls were correctly classified. The BAS2 score (“Goal-Drive Persistence”; r = .41) and BIS score (r = .36) contributed most and the FFFS score (r = .01) contributed least to group discrimination.
The German coping questionnaire
Figure 1B shows the results of the SVF-120. A multivariate analysis of covariance yielded significant differences between patients and controls across the 4 secondary coping factors (Pillai’s trace; V = 0.40, F4, 23 = 3.9, P = .015). Moreover, sex showed a significant effect on the measures (Pillai’s trace; V = 0.38, F4, 23 = 3.8, P = 0.023).
A subsequent discriminant function analysis revealed a significant discriminant function (canonical R2 = .37). Patients and controls were significantly discriminated by a linear combination of the 4 factors [λ = 0.6; χ2(4) = 12.1; P = .017]. A total of 80% of the patients and controls were correctly classified. NEG (“negative coping”; r = .69) contributed most to group discrimination, followed by POS2 (“distraction”; r = −0.48). POS1 (“cognitive reinterpretation”; r = −.3) and POS3 (“control of stressors”; r = −.1) contributed least. Importantly, the factor “group” affects positive coping and negative coping in opposite directions, indicating that patients use negative coping strategies more often and positive coping strategies less often than the control group.
A second multivariate analysis of covariance with the subtests corresponding to negative coping revealed a significant effect of group (patients vs controls; Pillai’s trace; V = 0.53, F6, 21 = 3.9, P = .009). The discriminant function analysis showed that “rumination” (r = .81) contributed most to group discrimination, followed by “attitude of self-pity” (r = .59) and “resignation” (r = .58). “Self-blame” (r = .17) contributed least to group discrimination (Figure 1C).
Correlations analyses
First, we tested whether those psychological traits where we found abnormalities were related to the frequency of NREM parasomnia episodes or scores of the ESS and BDI, as those questionnaires evaluate typical daytime symptoms. Age at disease onset had no influence on any of the psychometric measures. Interestingly, we found a positive correlation between the frequency of NREM parasomnia episodes and the BIS score, which however, did not survive Bonferroni correction (r = .50; P = .07). We found that z-transformed BIS score positively correlated with BDI (r = .63; P = .016) and ESS (r = .76; P = .001). In addition, z-transformed NEG score was strongly related to BDI (r = .68; P = .008) and ESS (r = .69; P = .004).
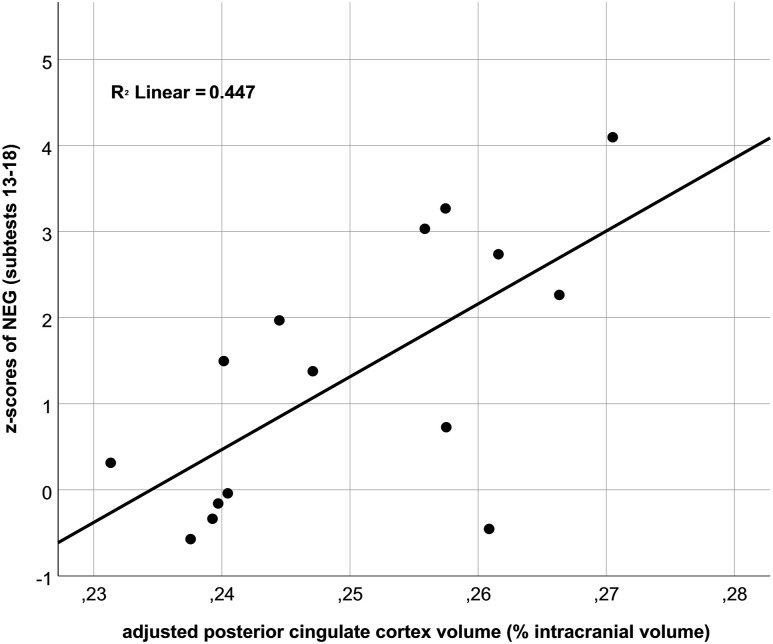
Second, we investigated correlations between left total intracranial volume–adjusted PCC volume and psychological traits as assessed by the RST-PQ and the SVF-120, but also the ESS and BDI as markers of disease severity. Indeed, increased NEG score was related to an increase in PCC volume on a trend level (r = .67; P = .006; Figure 2). Notably, this correlation was similar for primary scales of negative coping—that is, “resignation” (r = .68; P = .006), “flight” (r = .64; P = .011), “social withdrawal” (r = .68; P = .006), and “rumination” (r = .5; P = .058). Moreover, PCC volume was significantly related to the ESS (r = .55; P = .033), while the correlation between PCC volume and BDI was not significant (r = .41; P = .14). Notably, when including ESS scores as a covariate, we still observed a high correlation between PCC volume and NEG score (r = .48; P = .08), which, however, was not significant.
Figure 2. Scatterplot.
Scatterplot of the relationship between gray matter volumes of the posterior cingulate cortex in percentage of intracranial brain volumes and mean z-transformed scores of the negative coping factor (“NEG”) representing the sum score of the primary scales 13–18.
DISCUSSION
In this study we explored personality traits and different aspects of stress coping in patients with NREM parasomnia compared with HCs. Moreover, we tested the association between those traits and PCC volume. Indeed, we found that patients with NREM parasomnias differed from HCs in a combination of RST traits, showing abnormalities in the sensitivity to reward and to goal conflicts. With respect to stress coping, patients with NREM parasomnia specifically presented an increased trait to using negative coping strategies, which was related to a relative increase in PCC gray matter volume.
In the RST-PQ, patients with NREM parasomnias showed higher scores on the BAS2 scales (Goal-Drive Persistence), indicating increased sensitivity for specific aspects of reward. Notably, in the RST-PQ, reward sensitivity is multidimensional including a goal-planning facet representing a behavioral restraint (temporal bridging when reward is not immediately available) and a pleasure/nonplanning facet (ie, rapid, impulsive responding when the subject is close to reinforcer).33 Patients did not differ from controls on the other 3 BAS factors (ie, “reward interest,” “reward reactivity,” and “impulsivity”), suggesting that, in NREM parasomnia, specifically the goal-planning aspect of the BAS trait is enhanced. Notably, the differentiation of the BAS into subfactors is not supported by the RST in terms of distinct neurobiological underpinnings,22 questioning the neuropsychological implication of a selective increase in 1 BAS subscale for the evaluation of BAS functioning in patients with NREM parasomnias. However, an increased BAS trait would be in line with a previous study that used a different psychometric measure and found higher scores for the Temperament and Character Inventory novelty-seeking scale in sleepwalking patients.17 The Temperament and Character Inventory novelty-seeking scale assesses the tendency for appetitive approach behavior in response to novel and reward cues,18 reflecting a similar theoretical concept as the BAS in the revised RST, both partly related to dopaminergic neurotransmission.27,34 In sum, our results on the BAS scale of the RST-PQ only moderately support the notion that patients with NREM parasomnias show changes in the BAS trait and its associated dopaminergic reward regions.
Compared with the inconclusive results of the BAS scale, patients showed higher scores on the BIS scale (but normal FFFS scores). Generally, BIS activity promotes the inhibition of prepotent conflicting behaviors, an increase in arousal, and the engagement of risk-assessment processes (biased cognition) to resolve a goal conflict. Moreover, BIS hyperactivity has consistently been associated with anxiety and anxiety disorders, but positive associations have also been found with depressive symptoms.35 Accordingly, it has been suggested that BIS hyperactivity represents a nonspecific component of both anxiety and depressive disorders (eg, negative affectivity).36 Notably, reports regarding the association between affective and anxiety disorders and NREM parasomnias are inconsistent,37,38 suggesting that neurobehavioral abnormalities are more likely observed on the level of subclinical psychological traits rather than on the level of disorders.
Our finding on the BIS scale is in line with the results of the SVF-120 specifically showing that patients with NREM parasomnias more extensively use negative coping strategies (increased score on the NEG scale). On the level of subscales, “anxious rumination” best discriminated patients with NREM parasomnia from HCs, which is perfectly in line with the previous study showing a trend for higher scores on the “anticipatory worry” and “dependence of social attachment” Temperament and Character Inventory subscales.17 The results suggest that patients cannot disengage from negative thoughts, which they perceive more often (as suggested by higher BIS trait). Dysfunctional coping, such as anxious rumination, might promote negative affectivity, which forms a key feature of depressive disorders.39 Moreover, increased anxiety has not only been related to a cognitive bias and negative affect but also to an increase in cortical activity as measured by event-related potentials.40 Thus, we speculate that a dysregulated arousal, as it has been described in a range of psychopathologies,41 might be 1 common factor predisposing to an NREM parasomnia as well as daytime psychological changes (ie, anxious rumination). This interpretation is supported by our correlational analyses showing that BIS trait was not only related to the estimated frequency of episodes (on a trend level) but also to ESS and BDI scores, both evaluating possible daytime symptoms in NREM parasomnias.
With regard to common factors, the phenomenology of NREM parasomnia episodes and daytime behavioral changes should share common features. Indeed, during an NREM parasomnia episode, patients regularly show motivated behavior, accompanied by an increase in autonomic arousal, emotional response, and the perception of a strong urgency for their behavior.42 These features might be associated with increased activity in the cingulate cortex in NREM parasomnias,4,5 probably due to its roles in emotion and goal-directed behavior.43,44 The strong motivation that may be observed during parasomnia episodes would be in line with daytime behavior in terms of an increased BAS trait. Moreover, negative emotions might represent a sleep manifestation of FFFS activity (fear) indexing the strengthened processing of punishment stimuli as a consequence of BIS hyperactivity. In sum, phenomenological similarities of nighttime and daytime behavioral tendencies in terms of increased BIS (and possibly BAS) trait would agree with the notion that behavior during the wake state continues in sleep.45
In our previous study we found a volume decrease in the dorsal PCC and midcingulate cortex.6 As the PCC is crucially involved in the regulation of arousal, attentional focus, internal thought, and emotion11 we specifically tested whether PCC structural changes might not only reflect a correlate of the nighttime sleep-state dissociation but are also related to daytime psychological traits. Indeed, we found a correlation between PCC volume and an increased negative coping trait that almost reached significance (using the conservative Bonferroni α-level correction for multiple testing). Thus, patients with NREM parasomnias not only described a more exaggerated use of negative coping strategies (NEG scale) but this also correlated with an increased PCC volume. Notably, after partial regression of the ESS score, the correlation remained high, although it did not reach statistical significance, which indicates that the trait–PCC volume association is not sufficiently explained by a third factor, “sleepiness.” The positive correlation was an unexpected finding, as a previous study also found that anterior cingulate volumes were positively related to emotion regulation capacities.46 However, an increase in depressive mood symptoms, closely related to anxious rumination, has been observed in healthy participants with larger PCC volumes.47 Moreover, increased connectivity of the default mode network was shown to be related to the quantity of anxious rumination in depressed patients.13 Also, the positive correlation between PCC volume and negative coping (ie, anxious rumination) is in agreement with the critical role of the PCC in the default mode network mediating internally directed attention, such as mind wandering.7,48
How can the finding of a positive correlation in our patients be explained in the context of a reduced PCC volume in patients with NREM parasomnias in a previous study6? We do not have a conclusive explanation for this. However, we suggest that the increased PCC volume in patients using more negative coping strategies might be relative to a generally reduced volume in patients with NREM parasomnia. The condition of NREM parasomnia, possibly related to a PCC dysfunction and/or sleep fragmentation, might trigger (maladaptive) emotion regulation processes that cause compensatory PCC recruitment and thus an increase in tissue volume. This interpretation is highly speculative and is only one of several explanations for our results. Investigating these patients in the course of their disease probably advances our knowledge of the relationship between PCC function, behavioral abnormalities at daytime, and NREM parasomnia.
The study has several limitations. First, as this was an exploratory study, our sample of patients with NREM parasomnia was small. Due to the sample size we were not able to separately analyze subtypes of NREM parasomnias. Thus, averaging those subtypes might have affected the results. Second, due to our clear a priori hypothesis, we restricted our analysis to the part of the PCC where we previously found volumetric changes in patients with NREM parasomnia.6 Future studies should further investigate whether this trait–volume association is indeed specific to the PCC and, more importantly, whether it is specific to patients with an NREM parasomnia. Last, this was a cross-sectional study, which does not allow to conclude causal relations between behavioral findings and brain morphometry.
In conclusion, results of the present study suggest specific psychological traits, not fulfilling the diagnostic criteria for a disease, in patients with NREM parasomnias. The association between negative coping trait (ie, anxious rumination) and an increased PCC volume would be consistent with a common factor underlying nighttime phenomenology and daytime behavior. In the context of a decreased PCC volume in those patients, this correlation might be best explained by compensatory emotion regulation processes triggered by the NREM parasomnia itself. However, so far, this remains speculative due to a lack of longitudinal studies. Currently, our results do not have an implication for the clinical management of NREM parasomnias. However, this interesting line of investigation is currently under development and might become a focus for clinicians in the near future. Future studies could clarify how nighttime and behavioral daytime symptoms in NREM parasomnias interact and whether such findings have the potential to further improve the treatment.
DISCLOSURE STATEMENT
The authors have seen and approved the manuscript. Work for this study was performed at the Department of Neurology, Institute of Translational Neurology, formerly the Institute of Sleep Medicine and Neuromuscular Disorders, University Hospital Muenster, Muenster, Germany. AH received speaker honoraria from UCB Pharma GmbH, Bioprojet GmbH, and Jazz Pharmaceuticals and Medice. PY received speaker honoraria from Sanofi-Genzyme GmbH, Sanofi-Genzyme Europe, UCB, BioMarin, Neuro-Consil, Löwenstein Medical, Medice, Inspire, Vanda, Bayer Vital, and Cortex, as well as honoraria for advisory agreements from Genzyme GmbH, Sanofi-Genzyme Europe, BioMarin, Medice, Vanda, and Inflectis. MR received travel support from Bioprojet GmbH. BH received personal fees from Otsuka, Mundipharma, UCB, Janssen Cilag, Lündbeck, AbbVie, Lilly, Axovant, Benevolent, and as consultant for Roche und Takeda. AU, AF, and CS declare no conflict of interest. None of the authors report any conflict of interest concerning the content of this work.
ABBREVIATIONS
- BAS
behavioral approach system
- BDI
Beck Depression Inventory
- BIS
behavioral inhibition system
- ESS
Epworth Sleepiness Scale
- FFFS
fight-flight-freeze system
- HC
healthy control
- NEG
negative coping
- NREM
non–rapid eye movement
- PCC
posterior cingulate cortex
- POS
positive coping
- RST
Reinforcement Sensitivity Theory
- RST-PQ
Reinforcement Sensitivity Theory of Personality Questionnaire
- SVF-120
Stress Coping Questionnaire
REFERENCES
- 1.Broughton RJ. Sleep disorders: disorders of arousal? Enuresis, somnambulism, and nightmares occur in confusional states of arousal, not in “dreaming sleep”. Science. 1968;159(3819):1070–1078. 10.1126/science.159.3819.1070 [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3.Castelnovo A, Lopez R, Proserpio P, Nobili L, Dauvilliers Y. NREM sleep parasomnias as disorders of sleep-state dissociation. Nat Rev Neurol. 2018;14(8):470–481. 10.1038/s41582-018-0030-y [DOI] [PubMed] [Google Scholar]
- 4.Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21(5):502–506. 10.1111/j.1365-2869.2012.01003.x [DOI] [PubMed] [Google Scholar]
- 5.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356(9228):484–485. 10.1016/S0140-6736(00)02561-7 [DOI] [PubMed] [Google Scholar]
- 6.Heidbreder A, Stefani A, Brandauer E, et al. Gray matter abnormalities of the dorsal posterior cingulate in sleep walking. Sleep Med. 2017;36:152–155. 10.1016/j.sleep.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 7.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. 10.1038/nn1727 [DOI] [PubMed] [Google Scholar]
- 8.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999;11(1):80–93. 10.1162/089892999563265 [DOI] [PubMed] [Google Scholar]
- 9.Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol. 2009;19(18):1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31(9):3217–3224. 10.1523/JNEUROSCI.5626-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. 10.1016/j.biopsych.2011.10.035 [DOI] [PubMed] [Google Scholar]
- 13.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircanski K, Craske MG, Epstein AM, Wittchen HU. Subtypes of panic attacks: a critical review of the empirical literature. Depress Anxiety. 2009;26(10):878–887. 10.1002/da.20603 [DOI] [PubMed] [Google Scholar]
- 15.Lam SP, Fong SY, Ho CK, Yu MW, Wing YK. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374–1382. 10.4088/JCP.v69n0904 [DOI] [PubMed] [Google Scholar]
- 16.Ohayon MM, Guilleminault C, Priest RG. Night terrors, sleepwalking, and confusional arousals in the general population: their frequency and relationship to other sleep and mental disorders. J Clin Psychiatry. 1999;60(4):268–276; quiz 277. 10.4088/JCP.v60n0413 [DOI] [PubMed] [Google Scholar]
- 17.Perogamvros L, Aberg K, Gex-Fabry M, Perrig S, Cloninger CR, Schwartz S. Increased Reward-Related Behaviors during Sleep and Wakefulness in Sleepwalking and Idiopathic Nightmares. PLoS One. 2015;10(8):e0134504. 10.1371/journal.pone.0134504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloninger, CR. The temperament and character inventory (TCI): A guide to its development and use. St. Louis, MO: Center for Psychobiology of Personality; 1994. [Google Scholar]
- 19.Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3(3):165–189. 10.1002/hbm.460030303 [DOI] [Google Scholar]
- 20.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Andersson JL, Friston KJ. Image registration using a symmetric prior—in three dimensions. Hum Brain Mapp. 2000;9(4):212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray JA, McNaughton N. The Neuropsychology of Anxiety. An Enquiry into the Functions of the Septo-hippocampal System. Oxford University Press: Oxford, UK; 2000. [Google Scholar]
- 23.Gonen T, Sharon H, Pearlson G, Hendler T. Moods as ups and downs of the motivation pendulum: revisiting reinforcement sensitivity theory (RST) in bipolar disorder. Front Behav Neurosci. 2014;8:378. 10.3389/fnbeh.2014.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurent R, van Wouwe NC, Turchan M, et al. Motivational sensitivities linked to impulsive motor errors in Parkinson’s disease. J Int Neuropsych Soc. 2018;24(2):128–138. 10.1017/S1355617717000741 [DOI] [PubMed] [Google Scholar]
- 25.Bijttebier P, Beck I, Claes L, Vandereycken W. Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clin Psychol Rev. 2009;29(5):421–430. 10.1016/j.cpr.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 26.Kennis M, Rademaker AR, Geuze E. Neural correlates of personality: an integrative review. Neurosci Biobehav Rev. 2013;37(1):73–95. 10.1016/j.neubiorev.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 27.Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22(3):491–517; discussion 518-569. 10.1017/S0140525X99002046 [DOI] [PubMed] [Google Scholar]
- 28.Corr PJ. Reinforcement Sensitivity Theory of Personality Questionnaires: structural survey with recommendations. Pers Individ Dif. 2016;89:60–64. 10.1016/j.paid.2015.09.045 [DOI] [Google Scholar]
- 29.Corr PJ, Cooper AJ. The Reinforcement Sensitivity Theory of Personality Questionnaire (RST-PQ): development and validation. Psychol Assess. 2016;28(11):1427–1440. 10.1037/pas0000273 [DOI] [PubMed] [Google Scholar]
- 30.Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory: STAI. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 31.Erdmann G, Janke W. Stressverarbeitungsfragebogen (SVF). Stress, Stressverarbeitung und ihre Erfassung durch ein mehrdimensionales Testsystem. Göttingen, Germany: Hogrefe; 2008. [Google Scholar]
- 32.Ising M, Weyers P, Reuter M, Janke W. Comparing two approaches for the assessment of coping: part II. Differences in stability in time. J Individ Differ. 2006;27(1):15–19. 10.1027/1614-0001.27.1.15 [DOI] [Google Scholar]
- 33.Corr PJ. Reinforcement Sensitivity Theory (RST): Introduction. In: PJ Corr, ed. The Reinforcement Sensitivity Theory of Personality. Cambridge, UK: Cambridge University Press; 2008:1-43. [Google Scholar]
- 34.Krebs RM, Schott BH, Düzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry. 2009;65(2):103–110. 10.1016/j.biopsych.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 35.Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychol Assess. 2004;16(3):244–254. 10.1037/1040-3590.16.3.244 [DOI] [PubMed] [Google Scholar]
- 36.Muris P, Merckelbach H, Schmidt H, Gadet BB, Bogie N. Anxiety and depression as correlates of self-reported behavioural inhibition in normal adolescents. Behav Res Ther. 2001;39(9):1051–1061. 10.1016/S0005-7967(00)00081-4 [DOI] [PubMed] [Google Scholar]
- 37.Lopez R, Jaussent I, Scholz S, Bayard S, Montplaisir J, Dauvilliers Y. Functional impairment in adult sleepwalkers: a case-control study. Sleep. 2013;36(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labelle MA, Desautels A, Montplaisir J, Zadra A. Psychopathologic correlates of adult sleepwalking. Sleep Med. 2013;14(12):1348–1355. 10.1016/j.sleep.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 39.Kramer U. The role of coping change in borderline personality disorder: a process-outcome analysis on dialectical-behaviour skills training. Clin Psychol Psychother. 2017;24(2):302–311. 10.1002/cpp.2017 [DOI] [PubMed] [Google Scholar]
- 40.Eldar S, Yankelevitch R, Lamy D, Bar-Haim Y. Enhanced neural reactivity and selective attention to threat in anxiety. Biol Psychol. 2010;85(2):252–257. 10.1016/j.biopsycho.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 41.Ulke C, Tenke CE, Kayser J, et al. Resting EEG measures of brain arousal in a multisite study of major depression. Clin EEG Neurosci. 2019;50(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zadra A, Desautels A, Petit D, Montplaisir J. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285–294. 10.1016/S1474-4422(12)70322-8 [DOI] [PubMed] [Google Scholar]
- 43.Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci Regul Ed. 2012;16(2):122–128. 10.1016/j.tics.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 44.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. [DOI] [PubMed] [Google Scholar]
- 45.Schredl M, Hofmann F. Continuity between waking activities and dream activities. Conscious. Cogn. 2003;12(2):298–308. 10.1016/S1053-8100(02)00072-7 [DOI] [PubMed] [Google Scholar]
- 46.Giuliani NR, Drabant EM, Gross JJ. Anterior cingulate cortex volume and emotion regulation: is bigger better? Biol. Psychol. 2011;86(3):379–382. 10.1016/j.biopsycho.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLaren ME, Szymkowicz SM, O’Shea A, Woods AJ, Anton SD, Dotson VM. Dimensions of depressive symptoms and cingulate volumes in older adults. Transl Psychiatry. 2016;6(4):e788. 10.1038/tp.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]