Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a chronic condition that is characterized by recurrent pauses in breathing during sleep causing intermittent hypoxia. The main factor responsible for oxygen metabolism homeostasis is hypoxia-inducible factor 1 (HIF-1), comprised of 2 subunits: α (oxygen sensitive) and β. The aim of the study was to investigate the HIF-1α serum protein level and mRNA HIF-1α expression in patients with OSA and a healthy control group and determine their evening-morning variation and association with polysomnography parameters.
Methods:
Eighty-four individuals were enrolled in the study. All patients underwent polysomnography examination and based on the results were divided into 2 groups: OSA group (n = 60) and control group (n = 24). Peripheral blood was collected in the evening before and in the morning after the polysomnography. HIF-1α expression was evaluated on protein in blood serum and mRNA level in peripheral blood leukocytes.
Results:
HIF-1α serum protein concentration was higher in patients with OSA compared with control patients in both the evening (1,490.1 vs. 727.0 pg/mL; P < .001) and the morning (1,368.9 vs. 702.1 pg/mL; P < .001) samples. There was no difference between evening and morning HIF-1α serum protein level in either group. No differences were observed in HIF-1α mRNA expression between the OSA and control group. Additionally, evening and morning HIF-1α serum protein level correlated with number of desaturations during sleep (r = .384, P < .001 and r = .433, P < .001, respectively).
Conclusions:
Observed differences in HIF-1α serum protein level between the OSA and the control groups without difference between evening and morning measurements suggest chronic increase in this protein concentration by intermittent nocturnal hypoxia in OSA.
Citation:
Gabryelska A, Szmyd B, Szemraj J, Stawski R, Sochal M, Białasiewicz P. Patients with obstructive sleep apnea present with chronic upregulation of serum HIF-1α protein. J Clin Sleep Med. 2020;16(10):1761–1768.
Keywords: OSA, HIF-1α, HIF-1α protein, HIF-1α mRNA, diagnosis, PSG, hypoxia, hypoxemia
BRIEF SUMMARY
Current Knowledge/Study Rationale: One of the typical symptoms of obstructive sleep apnea is recurrent intermittent nocturnal hypoxia. However, not much is known how it translates into mRNA and protein expression of hypoxia-inducible factor 1α (HIF-1α), a key regulator of oxygen metabolism in hypoxia states. Therefore, we analyzed HIF-1α mRNA and serum protein expression before (in the evening) and after (in the morning) polysomnography examination.
Study Impact: We found that patients with obstructive sleep apnea have increased HIF-1α protein levels at both time points compared with those in the control group. At the same time, there was no difference between evening and morning HIF-1α serum protein levels in either group. This suggests that in OSA intermittent nocturnal hypoxia leads to a chronic increase in HIF-1α concentration.
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic disorder characterized by recurrent episodes of apneas or hypopneas during sleep caused by narrowing or collapse of upper airways. The main risk factor for OSA is obesity, especially visceral obesity, which leads to an increased volume of soft tissue around the pharynx. Recent studies estimate that almost 50% of men and 24% of women have a moderate to severe form of sleep-disordered breathing.1 Furthermore, patients diagnosed with OSA present with excessive daytime sleepiness2 and more often have immunological diseases that are associated with systemic inflammation3–5 and metabolic disorders6,7 and have increased risk of cardiovascular events.8–10 The gold standard examination for OSA diagnosis is nocturnal polysomnography (PSG), which allows for assessment of disorder severity based on apnea-hypopnea index (AHI).11
One of the typical complications of sleep-disordered breathing is recurrent hypoxia, which causes modifications of gene transcription as well as posttranslational protein modification, including the ones regulating metabolism or cardiovascular system. The main factor responsible for oxygen metabolism homeostasis is the hypoxia-inducible factor (HIF). It is a heterodimeric complex, which consists of 2 subunits: α (HIFα) and β (HIFβ). Both subunits belong to helix-loop-helix Per/Arnt/Sim transcription factor family, which are constitutively produced in cells.12 Subunit α is oxygen sensitive and, during normoxia, is associated with von Hippel-Lindau protein, which is responsible for the induction of its proteasome degradation.13 Therefore, in normoxia, the half lifetime of HIFα is greatly shorter than in hypoxia conditions,14 as low pressure of oxygen is responsible for blocking the binding of von Hippel-Lindau protein and HIFα and the degradation of HIFα is inhibited.15 Subunit β is produced continuously and is not oxygen sensitive. In humans, HIF-1α has 3 izoforms: HIF-1αcoded by HIF1A, HIF-2α coded by EPAS1, and HIF-3α coded by multiple splicing variants of HIF3a.16
Since hypoxia is characteristic of quickly proliferating tissues, such as tumor tissue, a considerable number of studies on HIF-1α focused on its effect on the formation and growth of neoplasms. Increased expression of HIF-1α was observed in multiple cancers,17 and higher expression of HIF-1αcorrelated with worse prognosis.18
It is estimated that HIF-1α is responsible for the activation of over 100 different genes.19 Therefore HIF-1α is a crucial transcription factor influencing many processes in organisms involved, especially in the regulation of metabolism and cardiovascular system.19,20 Nevertheless, many signaling pathways it takes part in are still not well described. For instance, among many others, it activates genes connected with angiogenesis (for example vascular endothelial growth factor) or glucose uptake by cells (glucose transporter 1 and glucose transporter 4).18,19
The information about HIF-1α in OSA is greatly based on animal and cell models of the disorder, which show increased HIF-1α expression.21,22 According to our knowledge, a limited number of studies investigated HIF-1α in patients with OSA.23–25 One group observed increased HIF-1α mRNA expression in skin biopsies of patients with OSA with severe nocturnal desaturations (hemoglobin oxygen saturation [SpO2] under 75%) compared with those in the OSA group without desaturations during night.26 Other research showed that patients with OSA had higher HIF-1α serum protein assessed semiquantitatively through Western blot.27 Therefore, the aim of our study was to investigate the HIF-1α serum protein level and mRNA HIF-α expression in patients with OSA and those in a healthy control group to determine their plausible evening-morning fluctuation and associations with PSG variables.
METHODS
The study group consisted of 84 consecutive patients who were referred to Sleep and Respiratory Disorders Centre in Lodz (Poland) with a presumptive OSA diagnosis and underwent a standard nocturnal PSG examination. Twenty-four individuals with AHI < 5 events/h were assigned to the control group, while those in the OSA group comprised 60 patients with AHI ≥ 5 events/h. Inclusion criteria for this study were age within 18–75 years and body mass index between 20 and 45 kg/m2. Individuals with an infection 1 month prior to PSG examination, history of or active cancer, total sleep time below 4 hours during PSG examination, or those with chronic respiratory diseases (eg, bronchial asthma or chronic obstructive pulmonary disease) were excluded. The study was approved by the Ethical Committee of Medical University of Lodz (RNN/77/18/KE). All participants provided written informed consent. All experiments were performed in accordance with relevant guidelines and regulations.
Polysomnography
Patients were admitted to the sleep lab at 2100 hours (± 0.5 hour) and underwent physical examination (measurement of body mass, height, heart rate, and blood pressure). A standard nocturnal PSG was performed by recording the following channels: electroencephalography (C4\A1, C3\A2), chin muscles and anterior tibialis electromyography, electrooculography, measurements of oronasal airflow (a thermistor gauge), snoring, body position, respiratory movements of chest and abdomen (piezoelectric gauges), unipolar electrocardiogram, and SpO2 (Sleep Lab, Jaeger-Viasys, Hochberg, Germany). Sleep stages were scored according to the criteria based on 30-second epoch standard.11 Desaturation has been defined in accordance with the American Academy of Sleep Medicine11 as a ≥ 3% oxygen desaturation from pre-event baseline. The following PSG parameters were included in the analysis: total sleep time, AHI, the total number of desaturations, desaturation index, mean level of SpO2 during sleep, the mean SpO2 of desaturations during sleep, minimal SpO2 during sleep, and number of desaturations below 90%.
Assessment of HIF-1α protein and mRNA level
Peripheral blood samples were collected in the evening before and in the morning following PSG examination into collection tubes with clot activator or with EDTA.
Blood samples with clot activator were centrifuged immediately following blood draws at 4°C. Serum was collected and stored at −80°C. The serum HIF-1α protein concentration was assessed by ELISA kit (Invitrogen, Carlsbad, CA). The absorbance was measured at λ = 450 nm wavelength by GloMax-Multi Detection System (Promega, Madison, WI).
RNA was isolated from peripheral blood leukocytes (PBL) (EDTA collection tubes) with the TRI Reagent Solution (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA) according to the standard acid guanidinium-phenol-chloroform procedure.28 The quality of the isolated RNA was evaluated with spectrometry at 260 nm using a Nanodrop ND-1000 analyzer (Thermo Fisher Scientific, Inc., Waltham, MA). Then, 1 μg RNA was reversely transcribed using the ImProm-II™ Reverse Transcription system (Promega Corporation, Madison, WI) according to the manufacturer's protocol. Reaction consisted of 3 steps, where annealing of assays was performed at 60°C in 60 seconds. Each quantitative real-time polymerase chain reaction mixture consisted of nuclease-free water, Master Mix TaqMan Universal, TaqMan HIF-1α assay (ID: Hs00153153_m1) (or reference gene, TaqMan glyceraldehyde phosphate dehydrogenase [GAPDH] assay) and cDNA. Reactions were carried out in triplicate for each sample for both HIF-1α and the reference GAPDH gene. For each sample, a threshold cycle (Ct) was calculated using Mx-Pro v4.10 software (Agilent Technologies, Inc.), and the mean value of 3 measurements was obtained. Then, ΔCt was calculated and used in mRNA expression analysis in accordance with the following equation 2−ΔCt.29
Statistical analysis
Shapiro-Wilk test was used to test data distribution. Data with normal distribution were presented as a mean and standard deviation, otherwise a median with interquartile range was used. Two independent groups were compared using an unpaired t test (normal distribution) or, otherwise, Mann–Whitney U test. Dependent groups were compared with a paired t test or Wilcoxon test, respectively. Correlations were determined using Spearman's rank correlation. The statistical analysis was performed using Statistica 13.1 (StatSoft, Tulsa, OK). P values < .05 were considered significant.
RESULTS
Characteristics
The baseline characteristics of the study groups are shown in Table 1. Control and OSA groups did not differ regarding sex and total sleep time. Body mass index was higher in the OSA group, while all SpO2-related parameters recorded during the night with PSG examination differed between the study groups.
Table 1.
Baseline characteristics of study groups.
| Parameter | Control group (n = 24) | OSA group (n = 60) | P Value | |
|---|---|---|---|---|
| Sex | Women | 7 (29.2%) | 7 (11.7%) | .103 |
| Men | 17 (70.8%) | 53 (88.3%) | ||
| Age | 50.5 (41.3–59.0) | 56.5 (44.8–63.0) | .139 | |
| BMI | 27.4 ± 4.1 | 31.7 ± 4.8 | < .001 | |
| Total sleep time (h) | 6.0 ± 0.7 | 6.0 ± 0.9 | .879 | |
| AHI, events/h | 2.4 (1.2–4.0) | 24.3 (14.3–50.1) | < .001 | |
| Total number of desaturations | 14.0 (9.0–32.5) | 122.0 (80.5–287.5) | < .001 | |
| Desaturation index | 3.0 (1.8 – 5.5) | 27.0 (15.0–51.1) | < .001 | |
| Mean level of SpO2 during sleep (%) | 93.8 (92.5–94.7) | 92.0 (90.2–93.3) | < .001 | |
| Mean SpO2 of desaturations during sleep (%) | 90.7 (90.0–92.3) | 87.0 (84.9–89.0) | < .001 | |
| Minimal SpO2 during sleep (%) | 88.2 (85.7–90.9) | 76.2 (71.3–82.3) | < .001 | |
| Number of desaturations below 90% | 5.0 (0–20.5) | 98.0 (52.5–159.5) | < .001 | |
Variables with normal distribution are presented as mean ± standard deviation; variables with a nonnormal distribution are shown as median interquartile range. AHI = apnea-hypopnea index, BMI = body mass index, OSA = obstructive sleep apnea, SpO2 = hemoglobin oxygen saturation.
HIF-1α mRNA expression level
No differences were observed in HIF-1α mRNA expression level between OSA and control groups, neither in the evening, 0.11 (0.03–0.77) vs 0.25 (0.10 – 0.49); P = .381, or in the morning, 0.22 (0.05–0.87) vs 0.15 (0.07–0.29); P = .414, respectively (Figure 1). Moreover, no differences were observed in HIF-1α mRNA expression between evening and morning samples within both groups (P > .05).
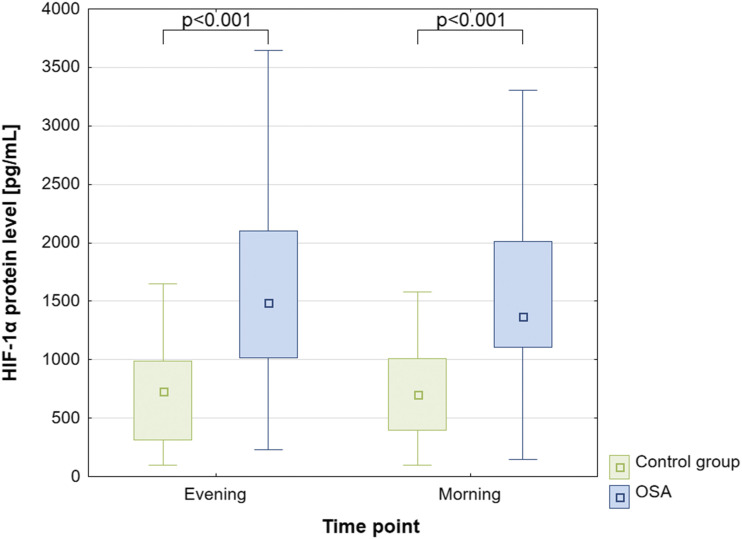
Figure 1. HIF-1α protein concentration in OSA and control groups.
HIF = hypoxia-inducible factor, OSA = obstructive sleep apnea.
HIF-1α serum protein level
Serum HIF-1α protein level in the OSA group was significantly higher than in the control group, both in the evening, 1,490.1 (1,021.6–2,095.7) vs 727.8 (328.0–968.9) pg/mL; P < .001, and in the morning, 1,368.9 (1,122.2–2,010.6) vs 702.1 (396.8–1,008.1) pg/mL; P < .001, respectively (Figure 1). Furthermore, no differences were observed in serum HIF-1α protein levels between evening and morning samples within both groups (P > .05).
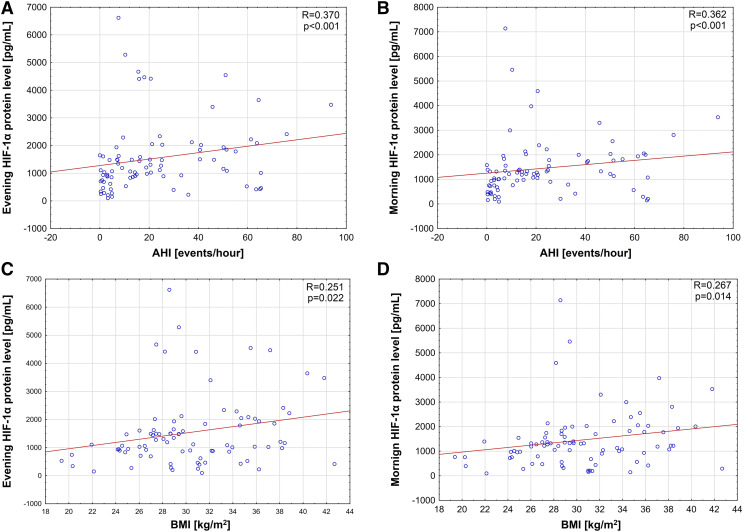
Evening and morning HIF-1α protein levels correlated positively with AHI (R = .370, P < .001 and R = .362, P < .001, respectively; Figure 2, A and B). Moreover, body mass index disclosed positive correlations with both evening and morning HIF-1α protein levels (R = .251, P = .022 and R = .267, P = .014, respectively; Figure 2, C and D).
Figure 2. Correlations between both evening and morning HIF-1α protein level and AHI and BMI.
(A) correlation between evening hypoxia inducible factor (HIF)-1α protein level and apnea-hypopnea index (AHI; events/h). (B) Level between morning HIF-1α protein level and AHI (events/h). (C) Correlation between evening HIF-1α protein level and body mass index (BMI). (D) Correlation between morning HIF-1α protein level and BMI.
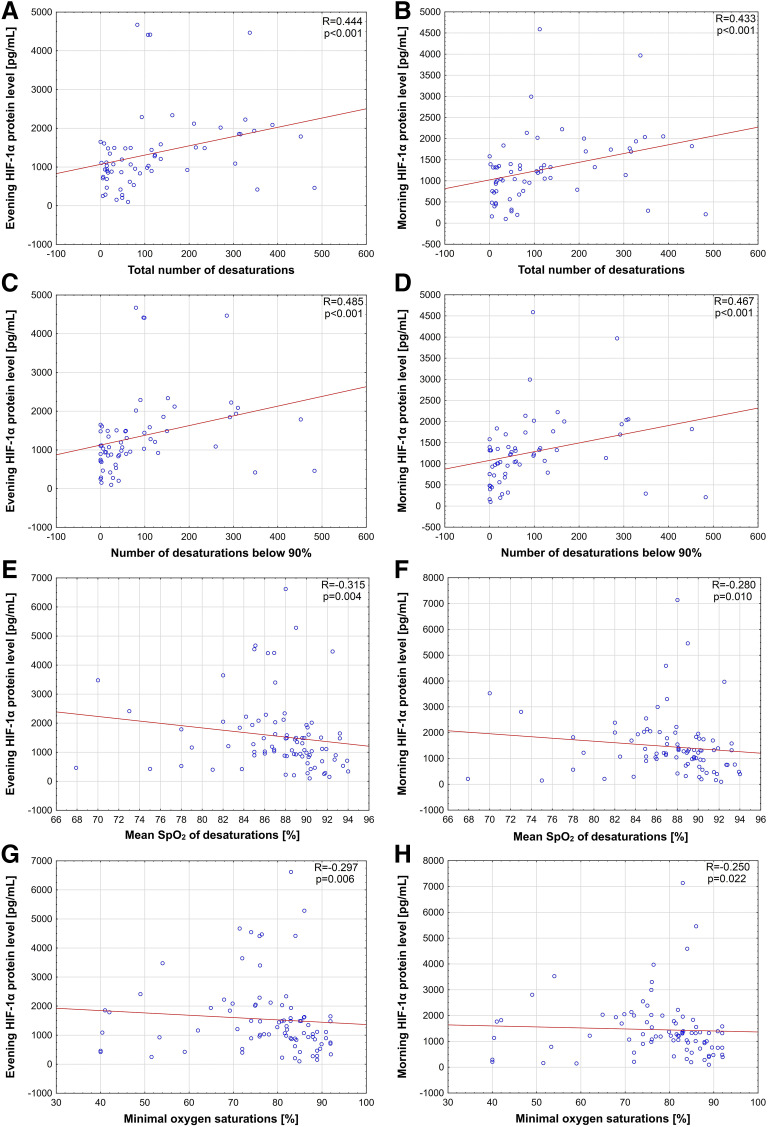
The total number of desaturations correlated positively with HIF-1α protein concentrations both in the evening (R = .444, P < .001; Figure 3A) and in the morning (R = .433, P < .001; Figure 3B). Similar positive correlations were observed for number of desaturations below 90% (R = .485, P < .001 and R = 0.467, P < .001, respectively; Figure 3, C and D). Furthermore, evening and morning HIF-1α protein level negatively correlated with the mean SpO2 of desaturations during the night (R = −.315, P = .004 and R = −.280, P = .010, respectively; Figure 3, E and F) as well as minimal saturation (R = −.297, P = .006 and R = −.250, P = .022, respectively, Figure 3, G and H). No significant correlations were found for evening and morning HIF-1α protein levels and total sleep time as well as the mean SpO2 during sleep.
Figure 3. Correlations between both evening and morning HIF-1α protein level and oxygen saturation parameters.
(A) Correlation between evening hypoxia inducible factor (HIF)-1α protein level and total number of desaturations. (B) Correlation between morning HIF-1α protein level and total number of desaturations. (C) Correlation between evening HIF-1α protein level and number of desaturations below 90%. (D) Correlation between morning HIF-1α protein level and number of desaturations below 90%. (E) Correlation between evening HIF-1α protein level and mean hemoglobin oxygen saturation (SpO2) of desaturation during sleep. (F) Correlation between morning HIF-1α protein level and mean SpO2 of desaturations during sleep. (G) Correlation between evening HIF-1α protein level and minimal SpO2. (H) Correlation between morning HIF-1α protein level and minimal SpO2.
DISCUSSION
To the best of our knowledge, this is the first work that shows absolute HIF-1α protein concentration in peripheral blood samples of patients with OSA. Previously it was evaluated only once using a semiquantitative Western blot method, in which HIF-1α level was calculated in comparison to an endogenous control (GAPDH).27 Similarly to our study, Lu et al27 observed significantly higher serum HIF-1α protein concentration in patients with OSA vs the control group.
We observed this difference in both the evening and the morning HIF-1α protein concentrations between the study groups. At the same time, no differences were observed between the evening and morning HIF-1α protein concentration. This suggests that a single night with the presence of apneas and, consequently, a decrease in oxygen saturation of hemoglobin does not directly affect the concentration of this protein, but rather a chronic effect is observed for this protein upregulation in OSA patients. There is no data in the available literature on the daily variation in the concentration of HIF-1α protein. Furthermore, our study showed direct correlations between the concentration of HIF-1α protein and hypoxia variables during sleep, including the total number of desaturations, desaturation index, mean oxygen saturation, mean SpO2 of desaturations during sleep, minimal oxygen saturation, and number of desaturations below 90%. These results are in line with the ones of Lu et al27, who demonstrated an association between the relative concentration of HIF-1α protein in blood serum and the average level of saturation during sleep and the minimum oxygen saturation during sleep. Additionally, we obtained a positive correlation between the concentration of HIF-1α protein and the severity of OSA. Lu et al showed a difference in the concentration of HIF-1α protein between severe OSA and a group consisting of individuals with moderate and mild severity of the disorder.27
Similar differences in the concentration of HIF-1α protein were also observed in animal models simulating OSA. It has been shown that in rats the severity of recurrent hypoxia stimulated by a decrease in oxygen concentration in the ambient air caused a gradual increase in the concentration of HIF-1α protein as well as HIF-1α mRNA and glucose transporter 1 protein.21 Another study in the rat OSA model also showed an increased concentration of HIF-1α protein in rats exposed to chronic intermittent hypoxia (CIH), but it did not correlate with the exposure time to CIH.30 An increase in the concentration of HIF-1α protein in rats following CIH has also been shown in other organs, eg, brain22 and liver.31 The value of animal models in which OSA is simulated only by a temporary reduction of oxygen concentration in the ambient air has significant limitations. Other factors, such as intrathoracic pressure swings, chronic inflammation, or obesity present in patients with OSA, often lasting for years, may initiate compensatory mechanisms that are absent in the short-term animal model of OSA. Furthermore, the difference in the concentration of HIF-1α protein between the control group and OSA individuals in both evening and morning measurements suggests a chronic effect. This is also supported by the lack of difference between evening and morning HIF-1α protein levels. To the best of our knowledge, there are no studies assessing HIF-1α protein levels at different time points of the day, neither in patients with OSA nor in OSA animal models. This suggests the participation of compensatory mechanisms that redirect oxygen metabolism into the HIF-1-dependent pathway12,32 in patients with OSA causing chronic HIF-1α protein level upregulation. According to Lu et al, this increase returned to the level observed in the control group following 2 months of continuous positive airway pressure therapy. Possible engagement of posttranslational rather than transcriptional processes may explain the lack of correlation between HIF-1α expression at mRNA level and HIF-1α protein concentration in blood serum. However, this is in contrast to the lack of differences between mRNA HIF-1α expression between patients with OSA and those in the control group and results obtained by Kaczmarek et al.26 In their study, individuals with OSA with AHI ≥ 10 events/h were divided into 2 groups based on the minimal hemoglobin oxygen saturation during PSG examination over or under 75%. The selection of such study groups might be one of the causes of observed differences in mRNA HIF-1α expression, which were not present in our study. Another factor that may affect this disparity is the fact that in our study expression was evaluated in PBLs, while Kaczmarek et al used skin biopsies for the measurements. Skin specimens are characterized by more limited oxygen availability in comparison to PBL due to the character of hypoxia: recurrent in PBL and chronic in skin samples. This may promote a shift of oxygen homeostasis to HIF-1-dependent via nuclear factor kappa-light-chain-enhancer of activated B cells pathway.32 Another factor that might affect the lack of correlation between HIF-1α protein concentration and mRNA expression is the fact that in our study mRNA was only evaluated in PBLs, while other cells, eg, vascular endothelium, can also be the source of the protein in the blood serum.
The participation of HIF-1 in the etiopathogenesis of other chronic respiratory diseases, such as chronic obstructive respiratory disease (COPD), has been better understood than in OSA, in which only a few papers have been published so far.26,27 Several studies have shown an increased level of HIF-1α serum protein in patients with COPD compared with a healthy control group,33,34 which is in line with our results. Moreover, in patients with COPD, HIF-1α protein concentration negatively correlated with functional lung test results and severity of the disorder assessed by Global Initiative for Chronic Obstructive Lung Disease standards.33,34 Despite the different characteristics of hypoxia present in COPD and OSA, ie, continuous vs intermittent, respectively, its effect on the concentration of HIF-1α protein seems to trigger the similar changes on the molecular level.
Increased HIF-1α protein levels in patients with OSA might have direct clinical implications. In epidemiological studies, one of the most common comorbidities in patients with OSA are metabolic disorders, such as insulin resistance35,36 and type 2 diabetes.37 The involvement of HIF-1α in disorders of glucose metabolism has been partially understood as it increases the expression of glucose transporters and leptin,15,32 suggesting a possible etiopathogenetic association.38 He et al21 demonstrated a significantly higher concentration of both protein and mRNA for HIF-1α and glucose transporter 1 under hypoxia in the adipocyte cell model. These changes might be reversible as shown by Shin et al39 in mice with diet-induced obesity. Shin et al39 inhibited the action of HIF-1α by administering missense oligonucleotides, thereby significantly reducing the expression of HIF-1α in liver and fat cells. As a result, they observed a significant reduction in fasting glucose and plasma insulin, without changes in the amount of food consumed and physical activity.
Cardiovascular disease, in particular arterial hypertension, is another complication often found in patients with OSA where HIF-1α may play a role.10,40 Atherosclerosis, which can be induced by increased endothelin-1 expression, seems to have a significant impact on the development of arterial hypertension in patients with OSA.37 Gras et al41 demonstrated that HIF-1 transcription is necessary to cause systemic and vascular inflammation under the influence of CIH. Importantly, the severity of inflammatory changes and an increase in the thickness of the inner aorta layer was not observed in the group of mice with HIF-1 deficit, as well as in the group of mice with no deficiency of this factor, which was given an endothelin receptor antagonist, presenting a key pathway in which HIF-1 and endothelin-1 are involved for the development of CIH-induced cardiovascular complications.
The upregulation of HIF-1α protein and involvement of this factor in common OSA complications suggests its consideration as a possible therapeutic target, as nowadays great attention is paid to more personalized treatment of OSA comorbidities.42 One of these drugs targeting HIF-1 is an antisense oligonucleotide, which caused downregulation of genes regulated by HIF-1α.43 Furthermore MiR-210 molecule has been described as a prognostic factor and therapeutic agent for both myocardial infarction and pulmonary hypertension.44 It is responsible for HIF-1α stabilization by suppression of GAPDH and cullin-2. Therefore MiR-210 antagonists might be considered as therapeutic agents for OSA comorbidities. These drugs might be considered beneficial among OSA patients, especially in control of comorbidities;45,46 however, this first requires verification on animal models and in clinical trials.
In conclusion, patients with OSA present with increased HIF-1α serum protein concentration compared with healthy individuals, which even more interestingly did not reveal morning to evening variability as could have been expected. This suggests HIF-1α protein might be one of the mediators involved in multiple comorbidities present in this group of patients. However, further studies are needed to broaden our understanding of this possible pathogenic pathway involving a larger group and long-term observation.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was funded by founded by Polish Ministry of Science and Higher Education no. 0067/DIA/2018/47 and Nacional Science Centre, Poland Grant no. 2018/31/N/NZ5/03931 to AG and Medical University of Lodz Science Grant no. 503/1-079-06/503-11-001-19-00. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
AG and PB created the concept of the study. AG collected biological material and generated the database. AG performed biochemical and molecular analysis. BS was responsible for statistical analysis.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CIH
chronic intermittent hypoxia
- COPD
chronic obstructive respiratory disease
- Ct
cycle threshold
- GAPDH
glyceraldehyde phosphate dehydrogenase
- SpO2
hemoglobin oxygen saturation
- HIF
hypoxia inducible factor
- OSA
obstructive sleep apnea
- PBL
peripheral blood leucocytes
- PSG
polysomnography
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabryelska A, Białasiewicz P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci Rep. 2020;10(1):34–36. 10.1038/s41598-019-56478-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med. 2009;5(1):71–78. 10.5664/jcsm.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabryelska A, Sochal M, Wasik B, Białasiewicz P. Patients with obstructive sleep apnea are over four times more likely to suffer from psoriasis than the general population. J Clin Sleep Med. 2018;14(1):153. 10.5664/jcsm.6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabryelska A, Kuna P, Antczak A, Białasiewicz P, Panek M. IL-33 mediated inflammation in chronic respiratory diseases-understanding the role of the member of IL-1 superfamily. Front Immunol. 2019;10:692. 10.3389/fimmu.2019.00692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian Y, Xu H, Wang Y, Yi H, Guan J, Yin S. Obstructive sleep apnea predicts risk of metabolic syndrome independently of obesity: a meta-analysis. Arch Med Sci. 2016;12(5):1077–1087. 10.5114/aoms.2016.61914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iftikhar IH, Hoyos CM, Phillips CL, Magalang UJ. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11(4):475–485. 10.5664/jcsm.4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140(2):534–542. 10.1378/chest.10-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 2014;11(2):e1001599. 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabryelska A, Łukasik ZM, Makowska JS, Białasiewicz P. Obstructive sleep apnea: from intermittent hypoxia to cardiovascular complications via blood platelets. Front Neurol. 2018;9:635. 10.3389/fneur.2018.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. 10.1016/j.cell.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. 10.1016/j.molcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Berra E, Roux D, Richard DE, Pouysségur J. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O(2)-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep. 2001;2(7):615–620. 10.1093/embo-reports/kve130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- 16.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469–1480. 10.1124/mol.106.027029 [DOI] [PubMed] [Google Scholar]
- 17.Cao D, Hou M, Guan Y-S, Jiang M, Yang Y, Gou H-F. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9(1):432–439. 10.1186/1471-2407-9-432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–627. 10.1038/cdd.2008.12 [DOI] [PubMed] [Google Scholar]
- 19.Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105(2):659–669. 10.1182/blood-2004-07-2958 [DOI] [PubMed] [Google Scholar]
- 20.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24(5):843–851. 10.1016/j.beem.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Q, Yang Q-C, Zhou Q, et al. Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS One. 2014;9(1):e86326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacramento JF, Ribeiro MJ, Rodrigues T, et al. Insulin resistance is associated with tissue-specific regulation of HIF-1α and HIF-2α during mild chronic intermittent hypoxia. Respir Physiol Neurobiol. 2016;228:30–38. 10.1016/j.resp.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 23.Gabryelska A, Szmyd B, Panek M, Szemraj J, Kuna P, Białasiewicz P. Serum hypoxia-inducible factor-1α protein level as a diagnostic marker of obstructive sleep apnea. Pol Arch Intern Med. 2020;130(2):158–160. [DOI] [PubMed] [Google Scholar]
- 24.Gabryelska A, Stawski R, Sochal M, Szmyd B, Białasiewicz P. Influence of one-night CPAP therapy on the changes of HIF-1α protein in OSA patients: A pilot study. J Sleep Res. 2020:e12995. [DOI] [PubMed] [Google Scholar]
- 25.Gabryelska A, Sochal M, Turkiewicz S, Białasiewicz P. Relationship between HIF-1 and circadian clock proteins in obstructive sleep apnea patients-preliminary study. J Clin Med. 2020;9(5):1599. 10.3390/jcm9051599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaczmarek E, Bakker JP, Clarke DN, et al. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One. 2013;8(7):e70559. 10.1371/journal.pone.0070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu D, Li N, Yao X, Zhou L. Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn J Basic Med Sci. 2017;17(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006;1(2):581–585. 10.1038/nprot.2006.83 [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Li P, Wu X, Chen W. Chronic intermittent hypoxia decreases pain sensitivity and increases the expression of HIF1α and opioid receptors in experimental rats. Sleep Breath. 2015;19(2):561–568. 10.1007/s11325-014-1047-0 [DOI] [PubMed] [Google Scholar]
- 31.Wall AM, Corcoran AE, O’Halloran KD, O’Connor JJ. Effects of prolyl-hydroxylase inhibition and chronic intermittent hypoxia on synaptic transmission and plasticity in the rat CA1 and dentate gyrus. Neurobiol Dis. 2014;62:8–17. 10.1016/j.nbd.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365(6):537–547. 10.1056/NEJMra1011165 [DOI] [PubMed] [Google Scholar]
- 33.Rong B, Liu Y, Li M, Fu T, Gao W, Liu H. Correlation of serum levels of HIF-1α and IL-19 with the disease progression of COPD: a retrospective study. Int J Chron Obstruct Pulmon Dis. 2018;13:3791–3803. 10.2147/COPD.S177034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu X, Zhang F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp Ther Med. 2018;16(6):4553–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seetho IW, Wilding JPH. Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chron Respir Dis. 2014;11(4):257–275. 10.1177/1479972314552806 [DOI] [PubMed] [Google Scholar]
- 36.Morgenstern M, Wang J, Beatty N, Batemarco T, Sica AL, Greenberg H. Obstructive sleep apnea: an unexpected cause of insulin resistance and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):187–204. 10.1016/j.ecl.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 37.Ivey ME, Osman N, Little PJ. Endothelin-1 signalling in vascular smooth muscle: pathways controlling cellular functions associated with atherosclerosis. Atherosclerosis. 2008;199(2):237–247. 10.1016/j.atherosclerosis.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 38.Girgis CM, Cheng K, Scott CH, Gunton JE. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol Metab. 2012;23(8):372–380. 10.1016/j.tem.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 39.Shin M-K, Drager LF, Yao Q, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One. 2012;7(10):e46562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 41.Gras E, Belaidi E, Briançon-Marjollet A, Pépin J-L, Arnaud C, Godin-Ribuot D. Endothelin-1 mediates intermittent hypoxia-induced inflammatory vascular remodeling through HIF-1 activation. J Appl Physiol. 2016;120(4):437–443. 10.1152/japplphysiol.00641.2015 [DOI] [PubMed] [Google Scholar]
- 42.Serinel Y, Yee BJ, Grunstein RR, Wong KH, Cistulli PA, Arima H, Phillips CL. Chronotherapy for hypertension in obstructive sleep apnoea (CHOSA): a randomised, double-blind, placebo-controlled crossover trial. Thorax. 2017;72(6):550–558. 10.1136/thoraxjnl-2016-209504 [DOI] [PubMed] [Google Scholar]
- 43.Jeong W, Rapisarda A, Park SR, et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014;73(2):343–348. 10.1007/s00280-013-2362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cottrill KA, Chan SY, Loscalzo J. Hypoxamirs and mitochondrial metabolism. Antioxid Redox Signal. 2014;21(8):1189–1201. 10.1089/ars.2013.5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis CK, Jain SA, Bae O-N, Majid A, Rajanikant GK. Hypoxia mimetic agents for ischemic stroke. Front Cell Dev Biol. 2019;6:175. 10.3389/fcell.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen R, Forsyth N. Editorial: the development of new classes of hypoxia mimetic agents for clinical use. Front Cell Dev Biol. 2019;7:120. 10.3389/fcell.2019.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]