Abstract
Study Objectives:
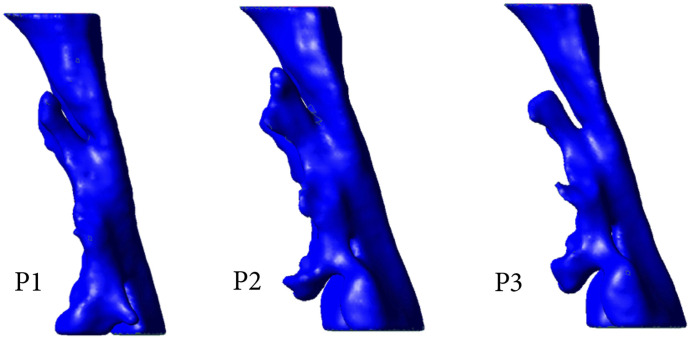
Mandibular advancement devices (MADs) constitute an alternative treatment in selected patients with OSA. A mandibular advanced position has been suggested to be beneficial, whereas its combination with an increased bite-raise may increase its adverse effects. The objective of this study was to assess upper airway (UA) volume and inspiratory pressure gradient variations in a group of 17 patients with OSA. The study was performed under 3 mandibular positions: intercuspal position (P1), MAD position in closed mouth (P2), and MAD position with an increased bite-raise (P3).
Methods:
We conducted a 3-dimensional reconstruction of the pharynx using the finite element method via a computed tomography scan and the subsequent calculation using fluid-dynamic analysis.
Results:
One hundred percent of the patients showed an increase in UA volume in both P2 and the MAD position with an increased bite-raise, P2 being the position where 76.47% of the patients showed the largest UA volume. P2/velopharynx was the position/region where the largest UA volume increase was achieved (4.73 mm3). A better gradient in P2 (mean = 0.62) in 58.82% of the patients and a better gradient in P3 (mean = 0.74) in 41.18% of patients respect P1 was observed. In 82.35% of patients, a better volume-pressure gradient match was also found.
Conclusions:
The best efficiency scores for both volume increase and better inspiratory pressure gradient were obtained in P2. This study findings suggest that in a MAD, the minimal bite opening position necessary for mandibular protrusion is more effective in increasing airway volume and inspiratory gradient compared to a larger bite-raising (15 mm).
Citation:
Barbero M, Flores-Mir C, Blanco JC, et al. Tridimensional upper airway assessment in male patients with OSA using oral advancement devices modifying their vertical dimension. J Clin Sleep Med 2020;16(10):1721–1729.
Keywords: mandibular advancement devices, computational fluid dynamics, sleep apnea, upper airway
BRIEF SUMMARY
Current Knowledge/Study Rationale: The overall goal of this article was to develop a comprehensive computational framework based on functional imaging and computational fluid dynamics tools to investigate mandibular advancement devices changes in the upper airways using realistic constitutive laws and device and upper airway geometries. Occasionally, studies about mandibular advancement devices are controverted because of the variability between devices and degree of mouth opening, but in this study we standardize both with the intention to clarify these topics.
Study Impact: Computational modeling techniques can provide unprecedented insight into the in vivo working conditions under which mandibular advancement devices operate. Our study aims to provide solutions for procedure planning and medical device performance evaluation on a patient-specific basis.
INTRODUCTION
Passive manipulation of the lower jaw position by means of intraoral mandibular advancement devices (MADs) likely involves changes in the morphology and volume of the upper airway (UA). However, although the mandibular advancement position is the most beneficial mandibular position to increase UA volume (more specifically in the oropharyngeal region),1 combining it with the bite-raised position may be of questionable benefit to increase pharyngeal volume, and it has been suggested that in some patients it can even be counterproductive.2,3
Although CPAP remains the gold standard management approach in patients with OSA, some individuals do not handle or respond well to it. CPAP is considered a highly efficacious treatment, but at the same time numerous studies confirm the beneficial use of the MAD as an alternative for certain patients with OSA as shown through polysomnographic recordings.4,5 The use of the MAD seems to generate localized pharyngeal changes in the respiratory air pressure, which could contribute to a normalization trend of UA physiological responses. The new anatomical relationship achieved via mandibular advancement may also lead to increased neurosensory stimulation as muscle tone increases and UA collapsibility is reduced.6 Different functional and structural techniques have been employed in recent years to study such a complex structure as the pharynx.7–9 The present study employs 3-dimensional (3D) reconstruction of the pharynx using the finite element method after its digital capture via a computed tomography scan (CT) and subsequent calculation using fluid-dynamic analysis.
Although passive mandibular advancement seems to be the most effective mechanism in OSA treatments employing intraoral devices, a number of studies 3,10–12 have reported that increases in the vertical dimension with these devices is not recommended because they do not improve UA functional conditions and could also reduce MAD acceptance rates.
The aim of this study was to assess the UA volume and inspiratory pressure gradient variations in a group of 17 patients with OSA, developing a comprehensive computational framework based on functional imaging and computational fluid dynamics (CFD) tools. The study was performed under 3 mandibular positions: intercuspal position without the use of any oral device (P1), MAD position in closed mouth (an advanced closed mandible held by intermaxillary elastics; P2), and an MAD position with increased bite-raise (P3).
METHODS
The current study was a pre- and posttrial comparison study of patients with OSA who were unwilling to wear CPAP but who agreed to wear an MAD. This study was reviewed and approved by the ethics committee of the Research of the Principado de Asturias, Spain. Patients were referred from the pulmonary department at Cruces Hospital, Barakaldo (Vizcaya), Spain.
Participants were assessed for eligibility and asked to participate in the study. Inclusion criteria were moderate or severe OSA (AHI ≥ 15 events/h) and CPAP intolerance. Exclusion criteria were neuromuscular or psychiatric disease, obesity (BMI > 35), Class III malocclusion, grade 3 or 4 on the Friedman scale, ≤ 8 mm protrusive capacity, insufficient retention for the MAD, and active periodontal disease.
A total of 17 male patients were finally included in the study. Their average age was 50 years ± 16, their average body mass index was 26.1 ± 3.0 kg/m2, and their average AHI was 30.15 events/h.
In the present study, a titratable MAD (Narval, ResMed SAS, Saint-Priest Cedex, France) was used. Measurements were performed under 3 vertical mandible positions: P1, P2, and P3. Initially the MAD was set at 60% of the maximum mandibular protrusion position. The mandible was then advanced to at least 70%–75% of the maximum comfortable protrusion position. The optimal advancement level per individual was based on a weighted compromise between clinical improvement (patient self-reported impression plus Epworth Sleepiness Scale) and absence of adverse effects.
The advancement was the same in both the P2 and P3 positions. The increase of bite-raising for P3 was 15 mm.
A CT in each position (P1, P2, and P3) was taken for each patient. Thus, 51 different CTs were obtained. The scanned data were sent directly to a personal computer and stored in the digital imaging and communications in medicine format. The region of interest was segmented using the 3D computer-aided design system DIPPO software (Biomedical Engineering Department, International Center for Numerical Methods in Engineering, Barcelona, Spain). 13
Using CFD in combination with functional imaging, this study focused on understanding how 3 different 3D positions of the mandible potentially resulted in changes in UA dimensions and airflow characteristics. To do so, rigid geometric models were obtained from 3D CT image data converted into a 3D computational model that allowed the measurement of anatomical structures/regions and the use of CFD to investigate the potential influence of altered geometry on airway flow dynamics (Figure 1).
Figure 1. 3D computational model allowing the measurement of anatomical structures/regions and the use of CFD.
CFD = computational fluid dynamics; P1 = intercuspal position without the use of any oral device; P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise.
The CT studies were conducted on a Toshiba Aquilion 64 system (Toshiba Medical, Otawara, Japan). Two locator views were performed in anteroposterior and lateral projections to plan the acquisition of a volume using a spiral scan. The acquisition parameters were 120 kV tube power, tube current with a dose modulation system with milliamp automatic dose reduction correction, 64 × 0.5 mm collimation, 0.5 mm slice thickness, 0.3 mm reconstruction interval, 0.5 second tube rotation speed, pitch factor of 0.828 and helical pitch of 53, and average duration of 6 seconds.
The study included the entire pharynx from the nasopharynx. The segmented regions started at the pharynx (first cervical vertebra) and extended down to the larynx (fourth cervical vertebra) with 3 series of CTs taken at P1, P2, and P3. All the series were taken in the supine position. During the CTs, patients were asked to breathe gently through their nose, not to swallow, and not to move their tongue. Steps were taken to avoid including corneas and thyroid glands in the CTs, because these are the most sensitive organs to ionizing radiation in this anatomical area.
Acquired data were stored in digital imaging and communications in medicine format. They were processed first on a Vitrea 2 Work Station (version 3.9.0.1, Vital Images, Minnetonka, MN) and an Advanced Work Station (version 4.5, General Electric Healthcare, Madrid, Spain) to calculate the volume of the UA using 3D reconstructions. The axial, sagittal, and coronal planes were studied, and 3D volume rendering reconstructions of the facial skeleton and the UA were performed via automatic segmentation using values in Hounsfield units. The region of interest was segmented using the DIPPO 3D computer-aided design system.
The UA images were segmented from CT digital imaging and communications in medicine images combining 2 different segmentation procedures: thresholding and the level set method (based on snakes). After UA segmentation, a 3D volume image that could be used to create a 3D computational model to analyze flow behavior inside the UA using CFD was obtained.
To generate a properly finite element mesh from the 3D segmentation image, an isosurface stuffing procedure was used in the personal pre- and postprocessor (GiD; International Center for Numerical Methods in Engineering, Barcelona, Spain). Mesh sensibility analysis was performed to ensure the accuracy of the simulations, obtaining 3D volume meshes consisting of 1,000,000–1,200,000 tetrahedral elements depending on the complexity of the UA model. Using the isostuffing algorithm, a smooth element and an aspect radius for the entire mesh > 0.9 (ideal ratio = 1 for an equilateral triangle) were obtained. The same medical image protocol, image processing, and volume mesh technique were used for all CT scans.
CFD solver
CFD analysis was performed using a fluid dynamics and multiphysics simulation environment based on the stabilized finite element method, which solves the Navier-Stokes equations (Biodyn; International Center for Numerical Methods in Engineering, Barcelona, Spain). A velocity profile was defined at the inlet (upper soft palate) and a pressure profile at the outlet (larynx) of the model. The input flow was replicated by means of a sine wave (U=Uisin [2π·f·t]), which was used to simulate the inlet profile, corresponding to the transient respiration mode in a breathing period at the inlet of the upper airway. A frequency of 0.2667 Hz was adopted (period 3.75 seconds), in line with the usual frequency in humans. The velocity, U, was calculated to obtain the same total tidal volume of 500 mL for the entire breathing cycle of inspiration/expiration for each patient and position.
To characterize the fluid flow in the UA accurately, a Reynolds number was calculated for all the positions. Because the Reynolds numbers in the inlet is low (< 1,000), we decided to use a CFD solver for laminar flow, considering the airflow to be steady, homogeneous, incompressible, adiabatic, and Newtonian. However, 3D flow features such as flow separation and recirculation may trigger a transition to turbulence at lower Reynolds numbers.14,15 The Navier-Stokes equation was also used.
The spatial discretization of the Navier-Stokes equation was carried out by means of the finite element method and an iterative algorithm that can be considered an implicit 2-step fractional method was used for time discretization. The gravitational effect, heat source, heat transfer, phase change, and chemical reactions were all ignored.
A no-slip condition (rigid model pharynx wall) was imposed on the surface of the UA. This choice was motivated by the fact that the physiological parameters characterizing the mechanical behavior (muscular tone) of the UA wall are not well known. This approach also considerably reduces the discretization effort, especially boundary layer gridding and computational cost. However, other approaches consider fluid structure interaction models.
The outlet boundary condition was set at 0 atm pressure in the larynx. The total CPU time on a Microsoft Windows XP 32-bit personal computer with 4 GB-RAM and a dual-core 2.83 GHz CPU was between 4 and 5 hours depending on the patient.
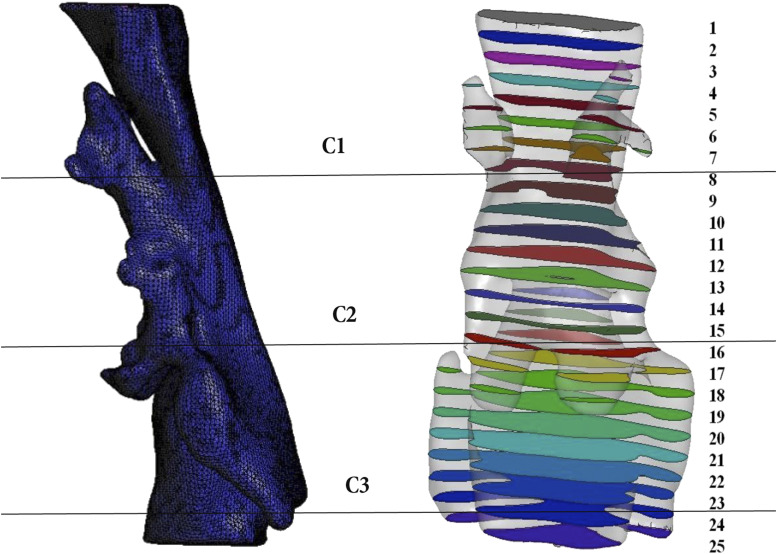
To determine the flow characteristics into the domain of each patient/mandible position, the domain was divided into 25 cross-sectional sagittal planes (Figure 2), obtaining the value of area and pressure at each point of the model.
Figure 2. UA studied regions.
Velopharynx: C1; oropharynx: C2; hypopharynx: C3. UA = upper airway.
The UA was divided into 3 regions (Figure 2):
Region 1—velopharynx: from the hard palate to the tip of the uvula
Region 2—oropharynx: from the tip of the uvula to the free edge of the epiglottis
Region 3—hypopharynx: from the free edge of the epiglottis to the flow edge of the aryepiglottic folds
By analyzing the different patients in all 3 positions (P1, P2, and P3), it was possible to estimate the gains in volume and opening achieved in the pharynx through the use of the MAD.
Statistical analysis
Descriptive measurements, including the mean, median, or standard deviation, were performed. The Student or Wilcoxon t test was applied for paired samples to study the differences after treatment, depending on whether or not the normality hypothesis was verified.
Absolute and relative frequency distributions for the qualitative variables were provided. The association between qualitative variables was studied through the use of contingency tables and the Pearson chi-square test. The level of significance considered was .05.
The statistical analysis was carried out through the R (R Development Core Team) program, version 3.2.0.18.16
RESULTS
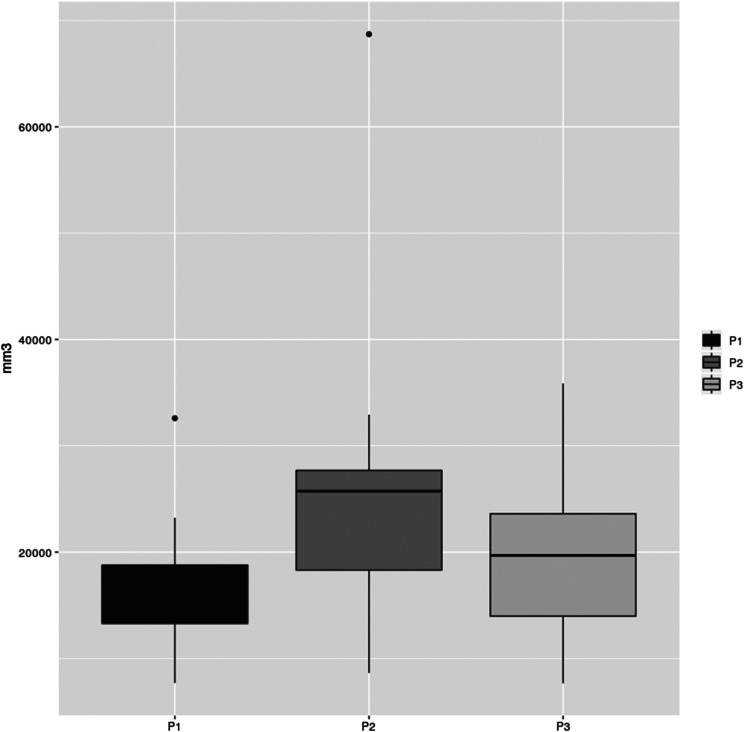
An increase in UA volume for P2 and P3 compared with P1 (Figure 3) was noticed in 100% of patients. We found that 76.47% of the patients had the largest UA volume increase with the smallest areas of stenosis in P2 (P < .001). These measurements are described in Table 1.
Figure 3. UA volume measurement graphic.
P1 = intercuspal position without the use of any oral device; P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise; UA = upper airway.
Table 1.
UA volume comparisons.
| P2−P1 | P2/P1 | P3−P1 | P3/P1 | P2−P3 | P2/P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DM (mm3) | SD | P value | DM (mm3) | DM (mm3) | SD | P value | DM (mm3) | DM (mm3) | SD | P value | DM (mm3) |
| 9,162.93 | 11,094.29 | <.001 | 1.66 | 3,610.70 | 7,562.39 | .07 | 1.38 | 5,552.23 | 9,555.32 | <.001 | 1.35 |
DM = difference in means; P1 = intercuspal position without the use of any oral device; P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise; SD = standard deviation; UA = upper airway.
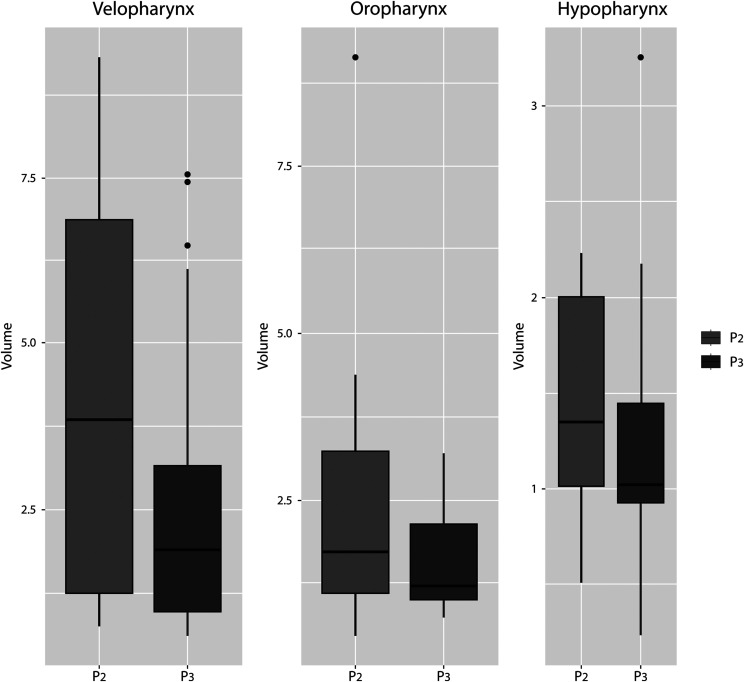
Figure 4 shows the differences in UA volume between P2 and P3 in all 3 studied regions (velopharynx, oropharynx, and hypopharynx). Region comparisons are described in Table 2. A significant difference was observed in P2-P3 in both the velopharynx (P < .001) and oropharynx (P = .02) but not in the hypopharynx (P = .30). The velopharynx was the region where the largest UA volume in all studied positions was obtained, followed by the oropharynx and hypopharynx. Note that P2/velopharynx was the position/region where the largest UA volume increase was achieved (4.73 mm3), although it also had the greatest variability between patients (standard deviation = 3.28).
Figure 4. Differences in UA volume between P2 and P3 in all 3 studied regions (velopharynx, oropharynx, and hypopharynx).
P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise; UA = upper airway.
Table 2.
UA volume region comparisons: velopharynx, oropharynx, and hypopharynx.
| Velopharynx P2-P3 | Oropharynx P2-P3 | Hypopharynx P2-P3 | ||||||
|---|---|---|---|---|---|---|---|---|
| DM (mm3) | SD | P value | DM (mm3) | SD | P value | DM (mm3) | SD | P value |
| 1.63 | 1.71 | <.001 | 0.45 | 0.4 | .02 | 0.13 | 0.53 | .3 |
DM = difference in means; P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise; SD = standard deviation; UA = upper airway.
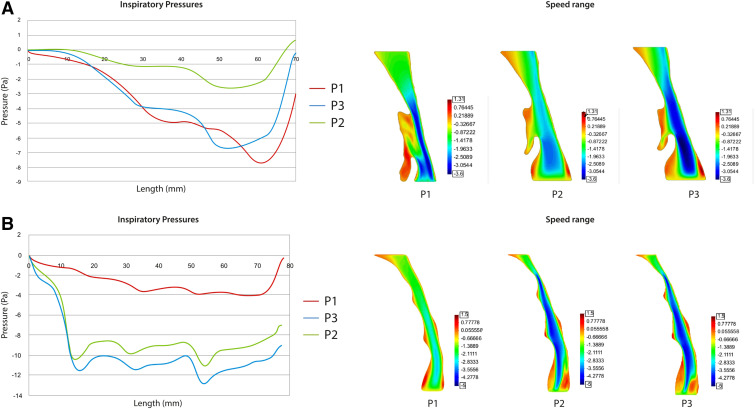
The authors observed a better inspiratory pressure gradient in P2 in 58.82% of patients (mean = 0.62) and in 41.18% of patients in P3 (mean = 0.74) respect P1. An example of a patient with better scores in P2 than in P3 and P1 is shown in Figure 5. Notably, in 82.35% of patients a match between a larger increase in volume and better inspiratory pressure gradient was found in both P3 and P2.
Figure 5. Inspiratory pressure gradient and flow velocity of 1 patient in the study.
P1 = intercuspal position without the use of any oral device; P2 = mandibular advancement device position in closed mouth; P3 = mandibular advancement device position with increased bite-raise.
DISCUSSION
The permeability of the UA depends on a complex system that combines mechanical factors (muscular action) and the balance of airflow pressures that interact to maintain a suitable pharyngeal caliber. The balance between the associated muscles may be disturbed because the permeability of the UA depends on the opposing action between the extra luminal pressure exerted by muscles such as the diaphragm and the intercostal muscles and the action of the dilator muscles that are regulated by neurosensory stimuli.
The use of an MAD leads to an increased stiffness and widening of the space between the anterior and posterior pillars of the pharynx. It has also been shown via MRI7,17 that a significant reduction in the thickness of the lateral pharyngeal walls is achieved that is even greater than the anteroposterior luminal increase.1
Airflow in curved sections is much more complex than in straight sections because of the induced secondary currents that arise. Tsuiki et al18 argued that the increase of the pharyngeal radius produced by MAD placement may have been 1 of the factors responsible for the reduction in resistance and the pressure drop in the UA, all of which created a significant improvement in dynamic airflow in their study. This study considered P1 as the baseline, comparing it with the other two positions (P2 and P3) for nominal analysis in the different sections of the pharynx. In line with the work of other authors,19 the velopharynx region constituted the segment of the pharynx where most changes were observed. Increased pharyngeal volume likely enables a considerable improvement in inspiratory pressure gradient. This hypothesis was validated for 82.35% of the sample. In the present study, the average improvements in inspiratory pressure gradient were more noticeable in P2 (58.82%). Regarding the pressure distribution in P2, there was also a considerable theoretical reduction in pressure drop with respect to P1. P3 also led to a reduction in pressure drop with respect to P1, although it was less prevalent than in P2 (41.18%). Another difference that could be appreciated with respect to P1 compared to P2 and P3 was that negative pressures appeared during inspiration in P1, this trend being reversed in the 2 positions employing an MAD.
MADs aim to standardize the pressure along the pharynx, avoiding the sudden changes in pressure that can cause obstructive phenomena. These devices generally improve the pressure gradient along the central axis of the pharynx. In P2 and P3, the MAD generated a fairly significant stable pressure gradient, which seemed to match with the change in volume generated by the MAD by raising the pharynx volume and making it theoretically less collapsible.
We found that 76.47% of the patients in P2 showed the largest UA caliber with the smallest areas of stenosis, which meant that the pressure inside the UA was the most homogeneous, with a flatter curvature. Consequently, the velocities would likely be more homogeneous throughout the UA and have less turbulence.
The present study corroborates other CFD studies20,21 concerning pharynx morphology improvement and airway conditions improving after the use of the MAD. However, although Zhao et al21 considered that geometrical changes alone did not correspond with treatment response, the present study argues that such changes could help predict the areas of UA collapse to more efficiently choose which treatment approach should be used because they can provide a visual idea about the increase or decrease of those critical areas after a patient undergoes different therapies.
P1 presented the smallest UA volume with areas of smaller caliber in 100% of patients, especially in the velopharynx, where the velocity of airflow increased and hence the pressure decreased, with a greater tendency to collapse. P3 presented a larger caliber than P1 but a smaller one than P2. However, P3 showed highly heterogeneous pressures and a pressure curve with more negative values, closer to those of P1.
In this study, as other authors have concluded,6 although UA volume was increased in P3 compared to P1, the morphology that developed in the UA led to increased turbulence in the theoretically dynamic airflow. In some studies, the increase in UA volume because of the use of an MAD is usually associated with an increased AHI reduction.17 An increase in airflow will decrease pharyngeal collapsibility by lowering its critical closing pressure. However, sometimes patients without significant pharyngeal widening show high AHI reductions, and vice versa. Airway patency is reduced during sleep in patients with OSA, but response to the MAD differs between patients because the UA collapse sites are quite heterogeneous.22 Other reasons for this wide variability may include pathophysiologic causes (ie, OSA phenotypes),23 likely because of the multifactorial nature of OSA.
For this particular investigation, polysomnography tests were not performed because other studies have done so previously. For example, Pitsis et al,24 who concluded that even though the difference between 2 different vertical MAD positions was not statistically significant, found that it was possible that a clinical small difference between the 2 appliances did exist (which could represent a type II error). However, although Pitsis et al24 and the present study used bite-raising devices (14 mm vs 15 mm), the target measurements differed. Whereas Pitsis et al focused on measuring AHI and patient acceptance, this study analyzed the morphology of the airway. The present results also showed that although P2 and P3 maintained the same advancement degree, analysis in P3 showed a decreased airflow and an increase of the inspiratory pressure gradient. It is possible that in some patients, when their jaw is open, soft tissues are further relocated backward, resulting in a narrowing of the pharynx.25 According to Mayoral et al,26 as the vertical dimension increases, the range of mandibular advancement is reduced because the mandible rotates posteriorly and places itself in a more retrusive location.
Other possible reasons for the variability between several studies in the literature may be explained by methodological differences, not just physical variations.24 These could include noncollaboration with CPAP, different degree of OSA severity, body mass index, individual anatomical conditions, or the specific design of each type of intraoral device.
Note that UA morphology alone is not always a good predictor of success or failure of an MAD, but it can help identify the most likely location of UA collapse in every patient. CFD can help with the individual assessment and best treatment approach for each patient. It also provides a visual idea about the increase or decrease of those critical areas of the UA after patients undergo different therapies. CFD studies may offer perspective about the widening space of the pharynx. Increased pharyngeal volume likely enables a considerable improvement in the inspiratory flow.
To summarize, differences in terms of volume and inspiratory pressure gradient throughout the pharynx between the 3 analyzed positions can be suggested. In P2, a benefit concerning inspiratory pressure gradient and volume compared with P1 and P3 in all 3 regions of the pharynx was obtained. However, pharyngeal critical pressure was difficult to calculate because of the great variability between patients.
Because the reason for the variability of response in each patient remains unknown, one could expect that future 3D models may help clinicians have a better understanding of UA using MADs to make the necessary changes to obtain a significant increased success rate in clinical practice.
The overall goal of this article was to develop a comprehensive computational framework based on functional imaging and CFD tools to investigate the MAD changes in the UA using realistic constitutive laws, devices, and UA geometries. Understanding the factors governing the position of the MAD is a key element toward improving the long-term behavior of these factors. Computational modeling techniques can provide unprecedented insight into the in vivo working conditions under which MADs operate.
These computational modeling techniques together with recent progress in the areas of diagnostic medical imaging, image processing, and computer hardware hold the promise of providing solutions for procedural planning and medical device performance evaluation on a patient-specific basis.
Limitations
This was a single-center observational study with a small number of patients. This study has some risk of bias because all patients were treated with a single oral appliance design.
The current study excluded patients with known relevant diseases because these would affect the dynamic flow study. As a consequence of this, the results of the present study cannot be applied to patients with OSAS and coexistent pulmonary disorder. Finally, the sample of patients consisted of all male patients, so the results may not be generalized to female patients with OSA.
Note also that the findings of this study were performed under patients’ awake conditions because of the technical complexity of obtaining 3D images under sleeping conditions.
CONCLUSIONS
Based on the parameters set for these models, the velopharynx was the UA region with the largest increase in volume using MADs. In MADs, the closed-mouth position had the best efficiency scores for both volume increase and inspiratory pressure gradient. These findings suggest that in an MAD, lower bite-raising is more effective in increasing airway volume and inspiratory gradient compared with a larger bite-raising.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Work for this study was performed at Baracaldo Cruces University Hospital, Barakaldo Spain and Unit of Radiology Hospital Central of Asturias Oviedo, Spain. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge support from the Statistical Consulting Unit of Scientific and Technological Resources of University of Oviedo, Spain, and from the Center for Numerical Methods in Engineering, Biomedical Engineering Department, Universidad Politécnica de Cataluña, Barcelona, Spain.
ABBREVIATIONS
- CFD
computational fluid dynamics
- CT
computed tomography scan
- MAD
mandibular advancement device
- P1
intercuspal position without the use of any oral device
- P2
MAD position in closed mouth
- P3
MAD position with increased bite-raise
- 3D
3-dimensional
- UA
upper airway
REFERENCES
- 1.Choi JK, Hur YK, Lee JM, Clark GT. Effects of mandibular advancement on upper airway dimension and collapsibility in patients with obstructive sleep apnea using dynamic upper airway imaging during sleep. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):712–719. 10.1016/j.tripleo.2009.11.034 [DOI] [PubMed] [Google Scholar]
- 2.Inoko Y, Morita O. Relationship between mandibular position and oropharyngeal space in obstructive sleep apnea syndrome patients with dental oral appliance. Prosthodont Res Pract. 2007;6:194–199. 10.2186/prp.6.194 [DOI] [Google Scholar]
- 3.Inazawa T, Ayuse T, Kurata S, et al. Effect of mandibular position on upper airway collapsibility and resistance. J Dent Res. 2005;84(6):554–558. 10.1177/154405910508400613 [DOI] [PubMed] [Google Scholar]
- 4.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215–227. 10.5664/jcsm.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000–1028. 10.1183/09031936.00099710 [DOI] [PubMed] [Google Scholar]
- 6.Chan AS, Sutherland K, Schwab RJ, et al. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65(8):726–732. 10.1136/thx.2009.131094 [DOI] [PubMed] [Google Scholar]
- 7.Oh KM, Hong JS, Kim YJ, Cevidanes LS, Park YH. Three-dimensional analysis of pharyngeal airway form in children with anteroposterior facial patterns. Angle Orthod. 2011;81(6):1075–1082. 10.2319/010711-8.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan ASL, Lee RWW, Srinivasan VK, Darendeliler MA, Cistulli PA. Use of flow-volume curves to predict oral appliance treatment outcome in obstructive sleep apnea: a prospective validation study. Sleep Breath. 2011;15(2):157–162. 10.1007/s11325-010-0395-7 [DOI] [PubMed] [Google Scholar]
- 9.Johal A, Sheriteh Z, Battagel J, Marshall C. The use of videofluoroscopy in the assessment of the pharyngeal airway in obstructive sleep apnoea. Eur J Orthod. 2011;33(2):212–219. 10.1093/ejo/cjq058 [DOI] [PubMed] [Google Scholar]
- 10.Ayuse T, Inazawa T, Kurata S, et al. Mouth-opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res. 2004;83(9):718–722. 10.1177/154405910408300912 [DOI] [PubMed] [Google Scholar]
- 11.Meurice JC, Marc I, Carrier G, Sériès F. Effects of mouth opening on upper airway collapsibility in normal sleeping subjects. Am J Respir Crit Care Med. 1996;153(1):255–259. 10.1164/ajrccm.153.1.8542125 [DOI] [PubMed] [Google Scholar]
- 12.Morikawa S, Safar P, Decarlo J. Influence of the headjaw position upon upper airway patency. Anesthesiology. 1961;22(2):265–270. 10.1097/00000542-196103000-00016 [DOI] [PubMed] [Google Scholar]
- 13.Soudah E, Pennecot J, Pérez JS, Bordone M, Oñate E. Medical-GiD: From Medical Images to Simulations, 4D MRI Flow Analysis. In: Tavares JMRS, Natal Jorge RM, eds. Computational Vision and Medical Image Processing: Recent Trends. Dordrecht, the Netherlands: Springer; 2011:145–160. [Google Scholar]
- 14.Lucey AD, King AJC, Tetlow GA, et al. Measurement, reconstruction, and flow-field computation of the human pharynx with application to sleep apnea. IEEE Trans Biomed Eng. 2010;57(10):2535–2548. 10.1109/TBME.2010.2052808 [DOI] [PubMed] [Google Scholar]
- 15.Mylavarapu G, Murugappan S, Mihaescu M, Kalra M, Khosla S, Gutmark E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J Biomech. 2009;42(10):1553–1559. 10.1016/j.jbiomech.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 16.The R project for for statistical computing . Vienna, Austria: R Foundation for Statistical Computing R Core Team; 2016. Available from: https://www.R-project.org/. Accessed July 13, 2020.
- 17.Ciscar MA, Juan G, Martínez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17(1):79–86. 10.1183/09031936.01.17100790 [DOI] [PubMed] [Google Scholar]
- 18.Tsuiki S, Lowe AA, Almeida FR, Kawahata N, Fleetham JA. Effects of mandibular advancement on airway curvature and obstructive sleep apnoea severity. Eur Respir J. 2004;23(2):263–268. 10.1183/09031936.04.00094304 [DOI] [PubMed] [Google Scholar]
- 19.Pirilä-Parkkinen K, Pirttiniemi P, Pääkkö E, Tolonen U, Nieminen P, Löppönen H. Pharyngeal airway in children with sleep-disordered breathing in relation to head posture. Sleep Breath. 2012;16(3):737–746. 10.1007/s11325-011-0569-y [DOI] [PubMed] [Google Scholar]
- 20.De Backer JW, Vanderveken OM, Vos WG, et al. Functional imaging using computational fluid dynamics to predict treatment success of mandibular advancement devices in sleep-disordered breathing. J Biomech 2007;40(16):3708–3714. 10.1016/j.jbiomech.2007.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Zhao M, Barber T, Cistulli PA, Sutherland K, Rosengarten G. Simulation of upper airway occlusion without and with mandibular advancement in obstructive sleep apnea using fluid-structure interaction. J Biomech. 2013;46(15):2586–2592. 10.1016/j.jbiomech.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 22.Op de Beeck S, Dieltjens M, Verbruggen AE, et al. Phenotypic labelling using drug-induced sleep endoscopy improves patient selection for mandibular advancement device outcome: a prospective study. J Clin Sleep Med. 2019;15(8):1089–1099. 10.5664/jcsm.7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(6):860–864. 10.1164/rccm.200204-342OC [DOI] [PubMed] [Google Scholar]
- 25.Aarab G, Lobbezoo F, Hamburger HL, Naeije M. Effects of an oral appliance with different mandibular protrusion positions at a constant vertical dimension on obstructive sleep apnea. Clin Oral Investig. 2010;14(3):339–345. 10.1007/s00784-009-0298-9 [DOI] [PubMed] [Google Scholar]
- 26.Mayoral P, Lagravère MO, Míguez-Contreras M, Garcia M. Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health. 2019;19(1):85. 10.1186/s12903-019-0783-8 [DOI] [PMC free article] [PubMed] [Google Scholar]