Abstract
Study Objectives:
To undertake a meta-analysis of literature comparing the prevalence of cardiovascular and cerebrovascular comorbidities between patients with overlap syndrome (OS) and patients with chronic obstructive pulmonary disease (COPD) or patients with obstructive sleep apnea.
Methods:
Studies about the cardiovascular and cerebrovascular disease of OS were searched for among several electronic databases from the time of database construction to June 2019. Two independent reviewers performed the process of study screening, quality assessment, and data extraction. Meta-analysis of odds ratios (ORs) was carried out by RevMan5.3 under either fixed-effects or random-effects models. Sensitivity analysis was conducted to examine the robustness of pooled outcome.
Results:
A total of 17 articles were included. Compared with COPD/obstructive sleep apnea, OS significantly increased the risk of developing hypertension (OS vs COPD: OR = 1.94, 95% confidence interval [CI] [1.49, 2.52]; OS vs obstructive sleep apnea: OR = 2.05, 95% CI [1.57, 2.68]) and pulmonary hypertension (OS vs COPD: OR = 2.96, 95% CI [1.30, 6.77]; OS vs obstructive sleep apnea: OR = 5.93, 95% CI [1.84, 18.42]). There was no significant difference in the prevalence of coronary heart disease (OR = 1.19, 95% CI [.67,2.11]) and cerebrovascular disease (OR = 2.43, 95% CI [0.81, 7.31]) between patients with COPD and patients with OS. However, the sensitivity analysis showed that the pooled outcome of the comparison of pulmonary arterial pressure between patients with OS and patients with COPD was not stable.
Conclusions:
OS significantly increased cardiovascular risk including the prevalence of hypertension and pulmonary hypertension. However, since the pooled outcome about pulmonary arterial pressure was not stable, further studies are still required.
Citation:
Xu J, Wei Z, Wang X, Li X, Wang W. The risk of cardiovascular and cerebrovascular disease in overlap syndrome: a meta-analysis. J Clin Sleep Med. 2020;16(7):1199–1207.
Keywords: chronic obstructive pulmonary disease, obstructive sleep apnea, overlap syndrome, cardiovascular disease, cerebrovascular disease, comorbidity
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) are common respiratory diseases. Although COPD and OSA are 2 independent diseases, they also interrelate with each other. They have their own characteristics, but common pathophysiological features. Investigations have shown that the prevalence of OSA in patients with COPD is 2–10 times higher than in healthy individuals.1 Soler et al2 also found that about 66% of patients with moderate to severe COPD had OSA. The combination of both diseases is called overlap syndrome (OS), which was first named by Flenley in 1985.3 Due to the different definitions of OS in each study, the prevalence of OS reported in current studies fluctuated from 1% to 41%.4
It is well-known that COPD and OSA can independently increase the prevalence of cardiovascular and cerebrovascular diseases.5,6 Cor pulmonale and arrhythmia are common comorbidities of COPD. Intermittent hypoxia and sympathetic excitation caused by OSA can induce oxidative stress, autonomic dysfunction, systemic inflammation, and endothelial dysfunction and eventually lead to cardiovascular events.7,8 Previous studies have shown that the prognosis of patients with OS was worse than that of patients with OSA or COPD alone because of more complications that were related to increased hospitalization rate, high risk of acute exacerbation and mortality, and decreased life quality.9-11 However, some studies reported that OSA-induced compensation could improve the prognosis of severe COPD.12 It is still unknown whether OSA combined with COPD can increase the occurrence of cardiovascular and cerebrovascular diseases. The aim of the present study was to undertake a meta-analysis of research, comparing the prevalence of cardiovascular and cerebrovascular comorbidities between patients with OS and patients with COPD or OSA.
METHODS
Search strategy
We searched for the relevant English articles reporting the association between OS and cardiovascular diseases using PubMed, Web of Science, Embase, and Cochrane and searched the Chinese ones using the CNKI and Wanfang data. The keywords used for search, included chronic obstructive pulmonary disease, COPD, sleep apnea, sleep-disordered breathing, OSA, overlap syndrome, OS, comorbidity, cardiovascular disease, hypertension, coronary heart disease (CHD), coronary artery disease, coronary atherosclerosis, atherosclerotic heart disease, myocardial infarction, heart failure, arrhythmia, pulmonary hypertension (PH), cerebrovascular disease (CVD), and stroke and these words in Chinese. These terms were used in different combinations. The search time of studies was from the time of database construction to June 2019. We reviewed the reference list of retrieved studies for additional studies as well. The process of study searching and screening was performed by 2 independent reviewers (J.H. Xu and Z.J. Wei). Disagreements were resolved by discussion. The study was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (http://www.prisma-statement.org/).
Inclusion and exclusion criteria
The inclusion criteria were 1) published articles on OS compared with COPD and/or OSA, 2) the articles that reported the results of cardiovascular and cerebrovascular comorbidities, including hypertension, CHD, heart failure, PH, CVD and so on, 3) the patients were older than 18 years of age and had confirmed diagnoses of OS and OSA and/or COPD. The following studies were excluded: 1) the reviews, animal studies, conference abstracts, comments, and case reports, 2) the studies without control groups, 3) the studies that enrolled patients with exacerbated conditions or OSA treatment. 4) the studies that did not report any cardiovascular and cerebrovascular comorbidity results.
Quality assessment
The included studies were evaluated by 2 independent reviewers (J.H. Xu and Z.J. Wei) using the quality assessment method, and disagreements resolved via discussion. The Agency for Healthcare Research and Quality was used to assess the quality of cross-sectional studies.13 Studies that achieved 5 or more stars were considered high quality. The case control studies were evaluated by the Newcastle-Ottawa Scale as follows: low quality = 0–3, moderate quality = 4–7, high quality = 8–11.13
Data extraction
We recorded the data from the selected studies using standard electronic sheets. The following information was extracted: the last name of the first author, the publication year, the study design, the source of population, the diagnostic criteria of disease (including OS and the comorbidities), the number of each sample size, the cardiovascular and cerebrovascular comorbidities (including the number of events), and the main adjusted confounders. This process was performed by 2 independent reviewers (J.H. Xu and Z.J. Wei).
Statistical analysis
We performed a meta-analysis using the Cochrane Collaboration’s RevMan software (https://community.cochrane.org/help/tools-and-software/revman-5). OR was used to estimate the association between OS and cardiovascular and cerebrovascular comorbidity. Interstudy heterogeneity was examined by the Cochrane Q test, and chi-square tests with I2 tests. We considered I2 > 50% as high heterogeneity, in which condition the random-effects model was used; otherwise the fixed-effects model was used. Sensitivity analysis was also performed to explore the sources of high heterogeneity and the stability of the results. A value of P < .05 was considered statistically significant.
RESULTS
Results of literature search and characteristics of studies
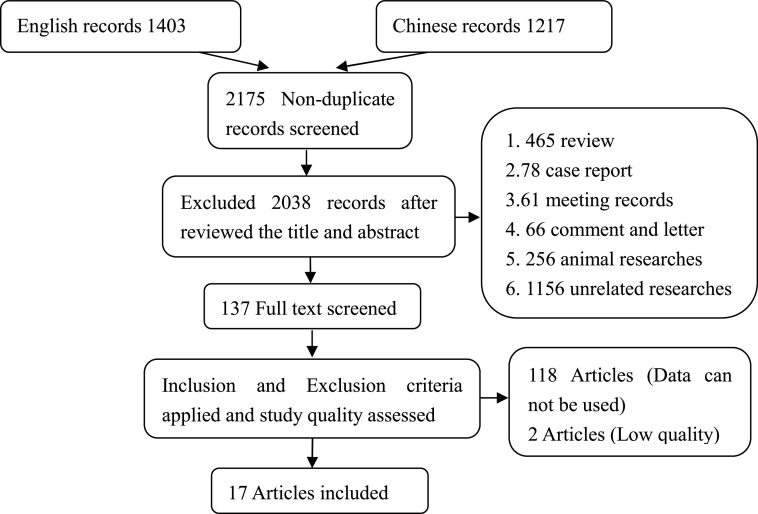
A total of 2,175 studies were identified from different sources. Finally, data were taken from 17 articles, including 5 Chinese articles and 12 English articles, which were in accordance with the inclusion and exclusion criteria after literature searching and screening. Figure 1 shows the process of literature search. The quality of these studies was high, and the characteristics of studies are summarized in Table 1. The funnel plots suggested no evidence of publication bias among studies.
Figure 1. Flow chart of the literature search, study screening, and selection process.
Table 1.
Characteristics of included studies.
| Study | Study Design | Diagnosis Criteria of OS | Population | Adjustment | Outcome | Quality* |
|---|---|---|---|---|---|---|
| Bai, 201714 | Case-control study | COPD: criteria established by the Chinese Medical Association (2011) | COPD: 27, OSA: 21 | Sex | Hypertension | 6 |
| OSA: criteria established by the Chinese Medical Association (2013) (PSG) | OS: 22 | |||||
| Chaouat et al, 199515 | Cross-sectional study | COPD: FEV1/FVC ratio ≤ 60% | OSA: 194 | No | PH | 7 |
| OSA: AHI > 20 (PSG) | OS: 26 | |||||
| Du et al, 201816 | Cross-sectional study | COPD: answered yes to the question “Has a doctor or other health professional ever told you that you had chronic bronchitis or emphysema?” | COPD: 695, OSA: 366 | No | Hypertension | 8 |
| OSA: answered yes to the question “Have you ever been told by a doctor or other health professional that you have a sleep disorder?” and reported sleep apnea to “What was the sleep disorder?” | OS:90 | |||||
| Steveling et al, 20144 | Cross-sectional study | COPD: GOLD (2013) | COPD: 144 | Age | Hypertension | 8 |
| OSA: AHI > 10 events/h (Apnealink) | OS: 33 | Smoking | ||||
| Greenberg-Dotan et al, 20131 | Case-control study | COPD: International Classification of Diseases, Ninth Revision | COPD: 41 | Age, sex | Hypertension | 8 |
| OSA: AHI ≥ 5 events/h (PSG) | OS: 57 | Smoking | PH | |||
| Gunduz et al, 201817 | Cross-sectional study | COPD: GOLD (2014) | COPD:19 | Age, sex | PH | 7 |
| OSA: RDI ≥ 15(Watch-PAT) | OS: 26 | Smoking | ||||
| Han et al, 201318 | Cross-sectional study | COPD: GOLD (2008) | COPD: 36, OSA: 96 | Age, sex | Hypertension | 6 |
| OSA: criteria established by the Chinese Medical Association (2011) (PSG) | OS: 32 | |||||
| Hang et al, 201611 | Cross-sectional study | COPD: 5 questions about COPD and respiratory symptoms to identify diagnosed COPD or related respiratory symptoms of 3 months duration | COPD: 206 | Age, sex | Stroke | 8 |
| OSA: Berlin Questionnaire | OS: 86 | Smoking | ||||
| Hawrylkiewicz et al, 200419 | Cross-sectional study | COPD: Optimal assessment and management of chronic obstructive pulmonary disease (COPD) (1995) | OSA: 67 | No | PH | 6 |
| OSA: ATS recommendations (PSG) | OS: 17 | |||||
| Lacedonia et al, 201820 | Cross-sectional study | COPD: postbronchodilator FEV1/FVC ratio of < .7 | OSA: 721 | No | Hypertension | 6 |
| OSA: AASM criteria (cardio-respiratory overnight monitoring) | OS: 123 | |||||
| Taranto- Montemurro et al, 201621 | Cross-sectional study | COPD: GOLD (2013) | COPD: 16, OSA: 24 | No | Hypertension | 7 |
| OSA: AASM (2012) (cardio-respiratory polysomnography) | OS: 14 | |||||
| Niu et al, 201022 | Cross-sectional study | COPD: criteria established by the Chinese Medical Association (2007) | COPD: 53, OSA: 50 | Not mentioned | PH | 6 |
| OSA: criteria established by the Chinese Medical Association (2002) (PSG) | OS: 25 | |||||
| Ou et al, 201823 | Cross-sectional study | COPD: GOLD (2011) | COPD: 55 | BMI | PH | 6 |
| OSA: criteria established by the Chinese Medical Association (2011) (PSG) | OS: 32 | |||||
| Silva Junior et al, 201824 | Cross-sectional study | COPD: postbronchodilator FEV1/FVC ratio < 70 | COPD: 25 | Age, sex | Hypertension | 8 |
| OSA: AHI ≥ 15 events/h (PSG) | OS: 14 | Smoking,BMI | PH | |||
| Sun, 201525 | Cross-sectional study | COPD: criteria established by the Chinese Medical Association (2011) | COPD: 54 | Age, sex | Hypertension | 8 |
| OSA: AHI ≥ 5 events/h (Apnealink) | OS: 28 | Smoking | CHD, PH | |||
| Sun et al, 201926 | Cross-sectional study | COPD: GOLD (2017) | COPD: 50 | Age, sex | Hypertension | 9 |
| OSA: AHI ≥ 10 events/h (Apnealink) | OS: 56 | Smoking,BMI | CHD, PH, CVD | |||
| Xie et al, 201927 | Cross-sectional study | COPD: GOLD (2007) | COPD: 62, OSA: 735 | No | Hypertension | 8 |
| OSA: AHI ≥ 15 events/h (PSG) | OS: 49 | CHD, CVD |
The cross-sectional study was measured by the Agency for Healthcare Research and Quality; the case-control study was evaluated by the Newcastle-Ottawa Scale. AASM = American Academy of Sleep Medicine, AHI = apnea-hypopnea index, BMI = body mass index, CHD = coronary heart disease, COPD = chronic obstructive pulmonary disease, CVD = cerebrovascular disease, GOLD = global initiative for obstructive lung disease, OS = overlap syndrome, OSA = obstructive sleep apnea, PH = pulmonary hypertension, PSG = polysomnography, RDI = respiratory disturbance index.
Hypertension
Comparison of hypertension prevalence between patients with OS and patients with COPD
A total of 395 patients with OS and 1,150 patients with COPD from 10 studies were included. There was little heterogeneity among them (I2 = 30%, P = .17). Fixed-effects meta-analysis showed that OS significantly increased the risk of developing hypertension (Figure 2, OR = 1.94, 95% CI [1.49, 2.52]).
Figure 2. Forest plot showing the comparison of hypertension prevalence between patients with OS and patients with COPD.
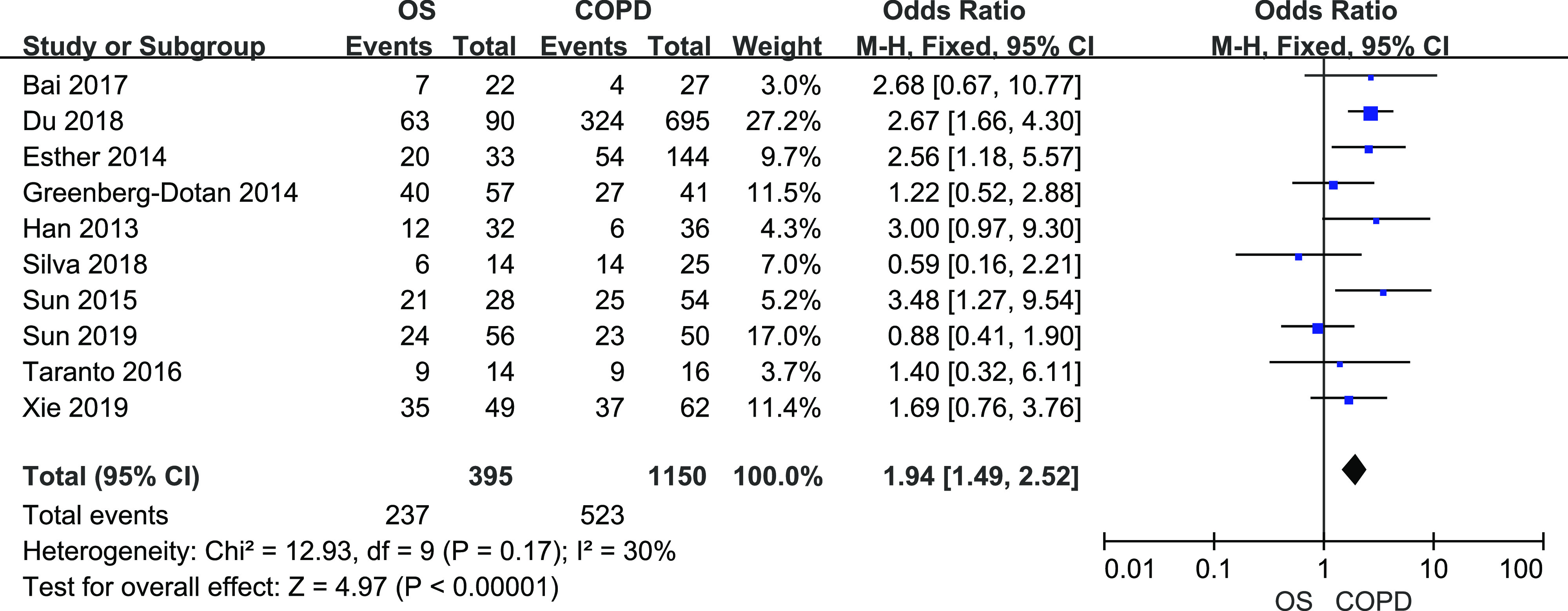
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome.
Comparison of hypertension prevalence between patients with OS and patients with OSA
A total of 330 patients with OS and 1,963 patients with OSA from 6 studies were included. There was little heterogeneity among studies (I2 = 36%, P = .17). Fixed-effects meta-analysis showed that OS significantly increased the risk of developing hypertension (Figure 3, OR = 2.05, 95% CI [1.57, 2.68]).
Figure 3. Forest plot showing the comparison of hypertension prevalence between patients with OS and patients with OSA.
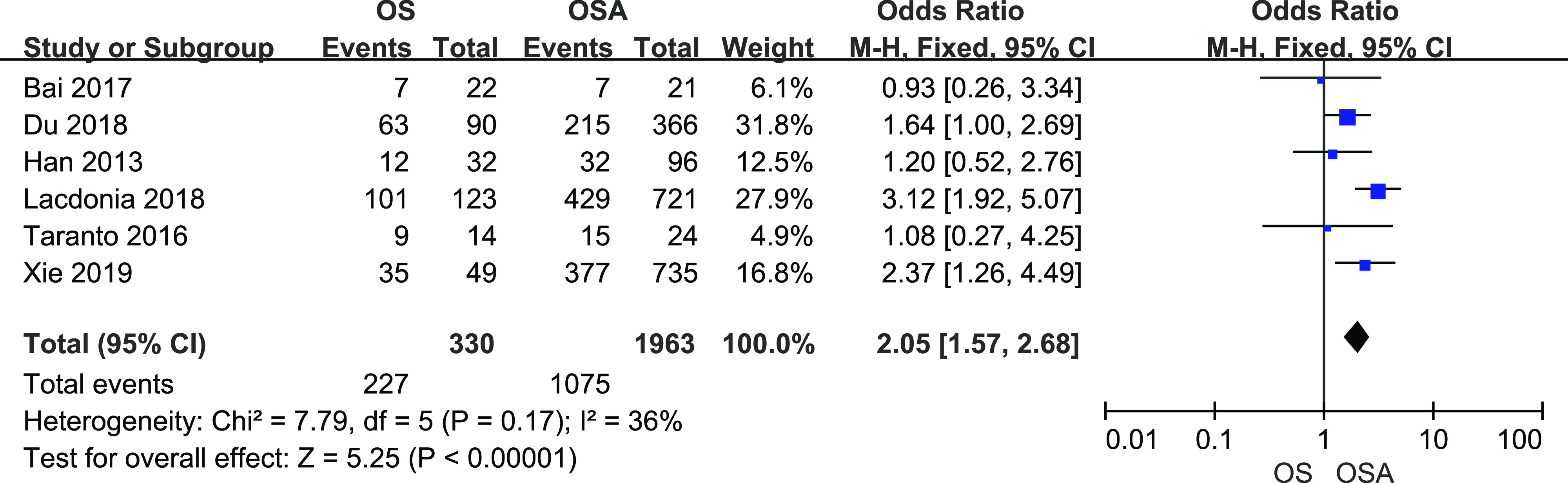
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome.
Coronary heart disease
A total of 133 patients OS and 166 COPD patients from 3 studies were included. There was mild heterogeneity among them (I2 = 50%, P = .14). Fixed-effects meta-analysis showed that there was no significant difference in CHD prevalence between patients with COPD and patients with OS (Figure 4, OR = 1.19, 95% CI [0.67, 2.11]).
Figure 4. Forest plot showing the comparison of CHD prevalence between patients with OS and patients with COPD.
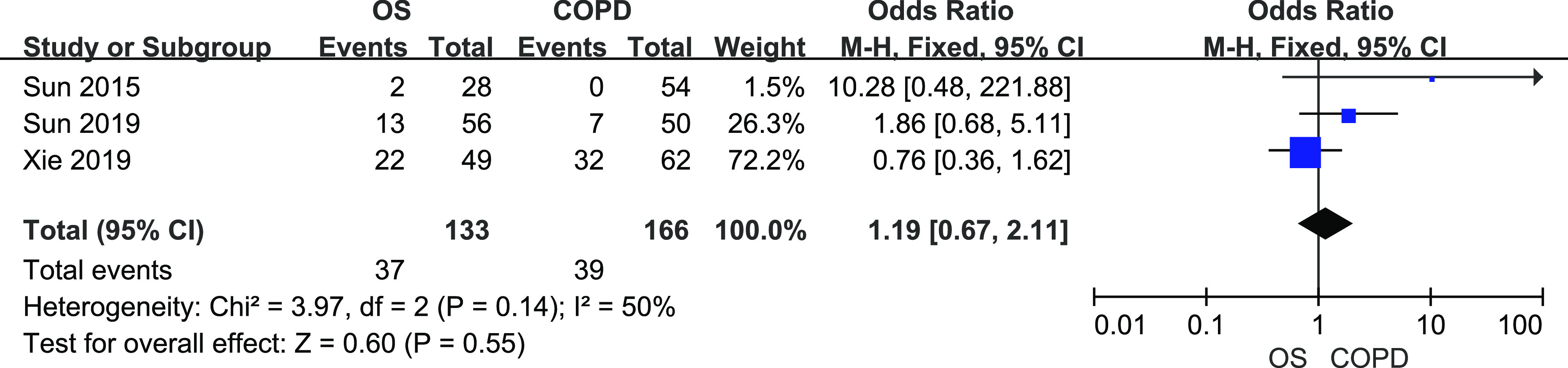
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CHD = coronary heart disease, CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome.
Pulmonary hypertension
Comparison of PH prevalence between patients with OS and patients with COPD
A total of 184 patients with OS and 224 patients with COPD from 5 studies were included. There was mild heterogeneity among studies (I2 = 52%, P = .08). Figure 5 shows the results from random-effects meta-analysis. Overall OS significantly increased the risk of developing PH (OR = 2.96, 95% CI [1.30, 6.77]).
Figure 5. Forest plot showing the comparison of PH prevalence between patients with OS and patients with COPD.
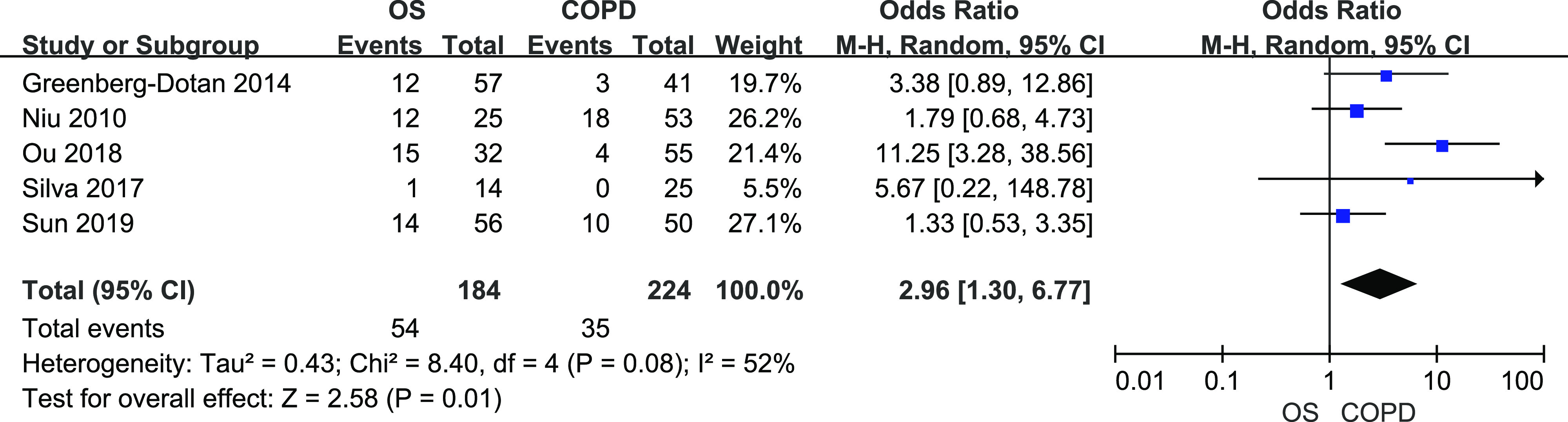
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome, PH = pulmonary or hypertension.
Comparison of pulmonary artery pressure (PAP) level between patients with OS and patients with COPD
A total of 142 patients with OS and 178 patients with COPD from 4 studies were included. There was heterogeneity among studies (I2 = 89%, P < .01). Figure 6 shows the results from random-effects meta-analysis. Patients with OS had higher PAP than patients with COPD (mean difference (MD) = 5.93, 95% CI [0.87,10.99]).
Figure 6. Forest plot showing the comparison of PAP level between patients with OS and patients with COPD.
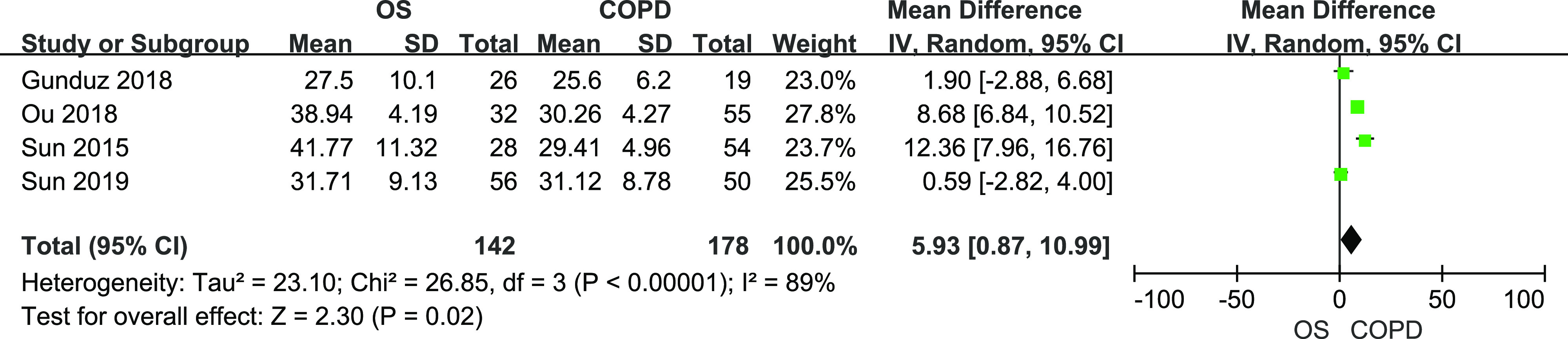
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome, PAP = pulmonary artery pressure.
Comparison of PH prevalence between patients with OS and patients with OSA
A total of 68 patients with OS and 311 patients with OSA from 3 studies were included. There was heterogeneity among studies (I2 = 71%, P = .03). Figure 7 shows the results from random-effects meta-analysis. Overall OS significantly increased the risk of developing PH (OR = 5.83, 95% CI [1.84, 18.42]).
Figure 7. Forest plot showing the comparison of PH prevalence between patients with OS and patients with OSA.
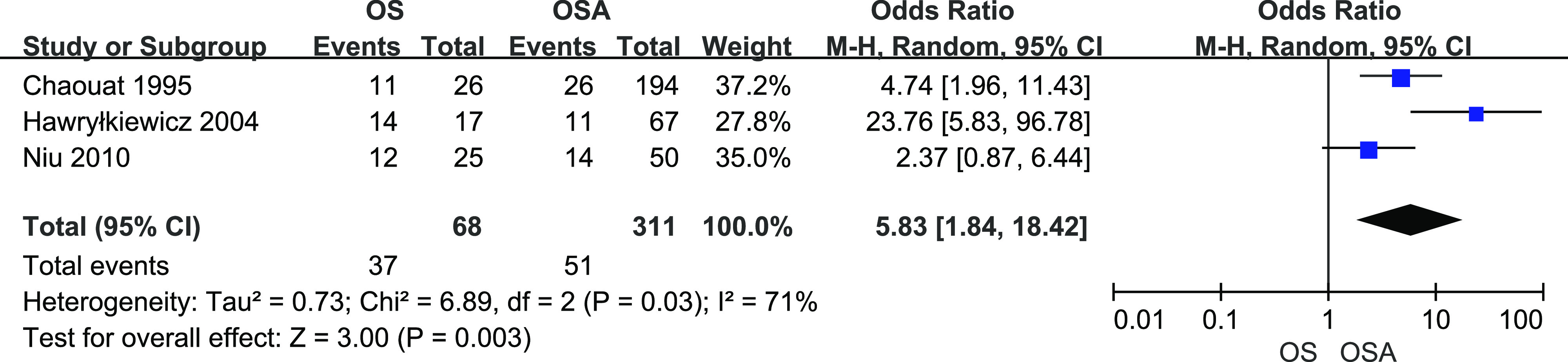
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, OS = overlap syndrome, PH = pulmonary hypertension.
Cerebrovascular disease
A total of 191 patients with OS and 318 with patients COPD from 3 studies were included. There was no heterogeneity among them (I2 = 0%, P = .80). The fixed-effects meta-analysis showed that there was no difference in CVD prevalence between patients with OS and patients with COPD (Figure 8, OR = 2.43, 95% CI [0.81, 7.31]).
Figure 8. Forest plot showing the comparison of CVD prevalence between patients with OS and patients with COPD.
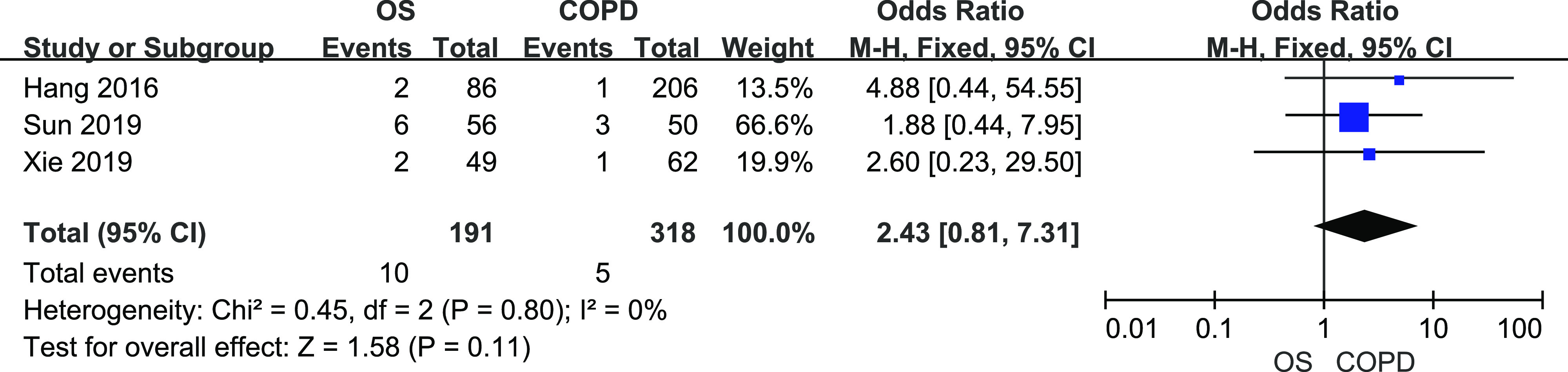
The horizontal line represents the confidence interval of the study, and the small square in middle represents the odds ratio of this study. The prismatic symbol at the bottom of the figure represents the pooled results. CI = confidence interval, COPD = chronic obstructive pulmonary disease, CVD = cardiovascular disease, OS = overlap syndrome.
Sensitivity analysis
Table 2 shows the results of sensitivity analysis. No matter whether we restricted the studies to the use of polysomnography or portable sleep monitor for OSA diagnosis and to the use of spirometry for COPD diagnosis or we restricted the enrolled population or instrument for measuring pulmonary artery pressure, the results of the prevalence of PH did not change among studies. Additionally, the heterogeneity among the studies that compared the PH prevalence between OS and COPD significantly decreased after excluding studies that enrolled only older people, as did the heterogeneity among the studies about the comparison of PH prevalence between OS and OSA after excluding studies that diagnosed COPD by medical history. However, the combined result of PAP in patients with OS and patients with COPD was not stable. When we excluded studies that included only older people, the combined result showed that the PAP value was similar in patients with OS and patients with COPD.
Table 2.
Sensitivity analysis of studies about PH.
| Inclusion Criteria | PH (OS vs COPD) | PH (OS vs OSA) | PAP (OS vs COPD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | I2 | OR (95% CI) | n | I2 | OR (95% CI) | n | I2 | MD (95% CI) | |
| Total | 5 | 52% | 2.96 (1.30, 6.77) | 3 | 71% | 5.83 (1.84, 18.42) | 4 | 89% | 5.93 (0.87, 10.99) |
| Population | 4 | 0% | 1.91 (1.07, 3.41) | - | - | - | 3 | 89% | 4.90 (−2.40, 12.20) |
| Criteria of OS | 3 | 62% | 3.91 (1.30, 11.74) | 2 | 5% | 3.94 (1.78, 6.78) | 2 | 56% | 9.16 (6.52, 13.39) |
| PAP detection | 4 | 64% | 2.96 (1.03, 8.35) | 2 | 73% | 9.65 (1.84, 46.91) | - | - | - |
CI = confidence interval, COPD, chronic obstructive pulmonary disease, MD, mean difference, OR = odds ratio, OS = overlap syndrome, PAP = pulmonary arterial pressure, PH = pulmonary hypertension.
DISCUSSION
It is a hot issue whether patients with OS had more risk of hypertension and PH than patients with COPD or OSA alone. Some small-sample observational studies reported that OS increased the risk of cardiovascular disease, but others showed that the prevalence of cardiovascular and cerebrovascular disease was similar in patients OS and patients with COPD/OSA.4,14 Due to the lack of prospective studies, especially the large-sample ones, the exact association between OS and cardiovascular/cerebrovascular disease was still unclear. For the first time, we made a meta-analysis to compare the prevalence of cardiovascular and cerebrovascular disease between OS and COPD/OSA and found that different types of cardiovascular diseases, such as hypertension and PH, showed different prevalence between patients with OS and patients with COPD/OSA. However, the prevalence of CHD and CVD were similar between patients with OS and those with COPD.
Previous research has shown that the major comorbidities in patients with OS include hypertension, cardiovascular disease, and diabetes, among which hypertension was the most common.20 Bai14 and Taranto-Montemurro et al21 did not find the statistical difference of the prevalence of hypertension between patients with OS and those with COPD/OSA. This negative result might be due to the criteria they used to diagnose OSA (apnea-hypopnea index [AHI] > 5 events/h). However, the studies of Xie et al27 and Steveling et al4, in which the definitions of OS were AHI ≥ 15 events/h and > 10 events/h respectively, found that patients with OS had higher hypertension prevalence than patients with COPD/OSA. In these studies, patients with OS had lower levels of nocturnal mean arterial oxygen saturation than patients with COPD/OSA and longer time of nocturnal arterial oxygen saturation < 90% than patients with COPD. In contrast, the study of Silva Junior et al, in which the criteria of OS was AHI ≥ 15 events/h as well, showed that there was no difference of hypertension prevalence between patients with OS and COPD when they only enrolled the patients with mild hypoxia. These results suggested that hypoxia might play an important role in the occurrence of hypertension. Considering that the above studies were small-sample, we made this meta-analysis and confirmed that the prevalence of hypertension in patients with OS was higher than that in those with COPD or OSA alone. As we all know, patients with OSA had sympathetic hyperactivity that was closely related to hypertension. When combined with COPD, patients with OS usually had more severe hypoxia than patients with OSA alone. This could further enhance sympathetic activity and damage the endothelial function.28 Wang and his colleagues29 found that the endothelial injury was more severe in patients with OS than those with COPD/OSA, and this was in line with the incidence of hypertension. These findings could explain why OS had increased risk of hypertension.
In recent years, the occurrence of PH in patients with OS has received increasing attention. The studies conducted by Greenberg-Dotan et al1 and Niu et al22, with AHI > 5 events/h as the diagnostic criteria, found that patients with OS were more likely to develop PH than patients with COPD alone. Sun et al26 further found that the increased risk for PH in patients with OS was associated with the increased severity of OSA. They found that compared with patients with COPD, only patients with OS with AHI ≥ 30 events/h, but not patients with OS with AHI ≥ 10 events/h, showed higher prevalence of PH. It is well known that chronic intermittent hypoxia in patients with OSA could induce high level of reactive oxygen species and increase intracellular Ca2+ concentration in pulmonary arterial smooth muscle cells, which is a key process in vascular remodeling and PH development.30 In addition, hypoxia could increase endothelin-1 level in lung, which contributes to the development of PH.28 It has been reported that the endothelin-1 level was higher in patients with OS than those with COPD/OSA, especially in patients with OS and PH.22 In this study, the oxygen levels of patients with OS did decrease compared with patients with COPD/OSA. However, the sensitivity analysis showed that the combined result of comparison of PAP between OS and COPD was not stable. There were just 4 articles included, and all of them measured the PAP by ultrasound. As the catheter is the gold standard for PAP measurement, studies to compare PAP measured by catheter with larger sample size are needed.
The hypoxic model of OS was chronic intermittent desaturation during sleep from a baseline hypoxemia. Hence, OS should theoretically induce more CHD and CVD than COPD/OSA. However, in terms of CHD and CVD prevalence, we did not find a difference between patients with OS and those with COPD. It might be related to the similar level of hypoxia between them. Our results were consistent with research conducted by Hang et al,11 Sun et al,26 and Xie et al27. In addition, OSA may present a potential cardioprotective effect because of the compensation to intermittent episodes of apneas via preconditioning,31 especially in those with low hypoxia burden. Since the patients in the OS group were not further divided according to the severity of OSA, the protective effect of mild OSA may explain the negative results.12,32,33
This study had some limitations as well. First, the enrolled studies were observational ones, which meant that some unmeasured variables might not be fully ruled out. Second, the statistical heterogeneity between the studies was high in some comparisons such as prevalence of PH and PAP level. Although the result of comparison of PAP between OS and COPD patients was unstable, the sensitivity analysis showed that the combined results were stable in most of the comparisons. Further studies about PAP in patients with OS and those with COPD are needed. Finally, the current study was not registered and there might be small offsets, but we strictly followed the steps of the systematic review.
CONCLUSIONS
In summary, this meta-analysis provided further evidence that coexistence of COPD and OSA significantly increased the prevalence of hypertension and PH. The severity of OSA and hypoxemia should be considered when evaluating the comorbidities of OS. Further prospective cohort studies and randomized controlled trials about the management of OS are still required for better understanding the association between OS and cardio- and cerebrovascular disease.
DISCLOSURE STATEMENT
All authors have reviewed and approved the manuscript. This study was supported by the National Key Research and Development Program of China (No. 2018YFC1313600) and the National Natural Science Foundation of China (81670085). The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
Author contributions: JH Xu: study design, data analysis, manuscript drafting, and final approval of the version to be published. ZJ Wei: in charge of the process of study searching and assessment and data extraction. XJ Wang and XM Li: providing language help, writing assistance. W Wang: study design, data interpretation, critical manuscript revision, and final approval of the version to be published.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CVD
cerebrovascular disease
- GOLD
global initiative for obstructive lung disease
- MD
mean difference
- OR
odds ratio
- OS
overlap syndrome
- OSA
obstructive sleep apnea
- PAP
pulmonary artery pressure
- PH
pulmonary hypertension
- PSG
polysomnography
REFERENCES
- 1.Greenberg-Dotan S, Reuveni H, Tal A, et al. Increased prevalence of obstructive lung disease in patients with obstructive sleep apnea. Sleep Breath. 2014;18(1):69–75. 10.1007/s11325-013-0850-3 [DOI] [PubMed] [Google Scholar]
- 2.Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(8):1219–1225. 10.1513/AnnalsATS.201407-336OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6:651–661. [PubMed] [Google Scholar]
- 4.Steveling EH, Clarenbach CF, Miedinger D, et al. Predictors of the overlap syndrome and its association with comorbidities in patients with chronic obstructive pulmonary disease. Respiration. 2014;88(6):451–457. 10.1159/000368615 [DOI] [PubMed] [Google Scholar]
- 5.Yin HL, Yin SQ, Lin QY, Xu Y, Xu HW, Liu T. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: A meta-analysis. Medicine (Baltimore). 2017;96(19):e6836. 10.1097/MD.0000000000006836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis. 2013;229(2):489–495. 10.1016/j.atherosclerosis.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 7.McNicholas WT, Bonsignore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. 10.1183/09031936.00027406 [DOI] [PubMed] [Google Scholar]
- 8.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. 10.1038/nrcardio.2010.145 [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. 10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 10.Kendzerska T, Leung RS, Aaron SD, Ayas N, Sandoz JS, Gershon AS. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am Thorac Soc. 2019;16(1):71–81. 10.1513/AnnalsATS.201802-136OC [DOI] [PubMed] [Google Scholar]
- 11.Hang LW, Hsu JY, Chang CJ, et al. Predictive factors warrant screening for obstructive sleep apnea in COPD: a Taiwan National Survey. Int J Chron Obstruct Pulmon Dis. 2016;11:665–673. 10.2147/COPD.S96504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He BT, Lu G, Xiao SC, et al. Coexistence of OSA may compensate for sleep related reduction in neural respiratory drive in patients with COPD. Thorax. 2017;72(3):256–262. 10.1136/thoraxjnl-2016-208467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng XT, Liu H, Chen X, Leng WD. The fourth series of meta analysis: quality assessment tools for observational research. Chin J Evid Based Cardiovasc Med. 2012;4(4):297–299. [Google Scholar]
- 14. Bai XY. The clinical characteristics of chronic obstructive pulmonary disease-obstructive sleep apnea syndrome overlap syndrome (Master dissertation). Changchun Jilin, China: Jilin University; 2017. [Google Scholar]
- 15.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151(1):82–86. 10.1164/ajrccm.151.1.7812577 [DOI] [PubMed] [Google Scholar]
- 16.Du W, Liu J, Zhou JL, Ye D, OuYang Y, Deng QD. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005-2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2018;13:665–674. 10.2147/COPD.S148735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunduz C, Basoglu OK, Tasbakan MS. Prevalence of overlap syndrome in chronic obstructive pulmonary disease patients without sleep apnea symptoms. Clin Respir J. 2018;12(1):105–112. 10.1111/crj.12493 [DOI] [PubMed] [Google Scholar]
- 18.Han R, Yang W, Ma LN, Liu C, Zhang YH. Analysis of clinical characteristics of chronic obstructive pulmonary disease obstructive sleep apnea hypopnea syndrome overlap syndrome. Chin J Diffic Compl Cas. 2013;12(9):673–675. [Google Scholar]
- 19.Hawryłkiewicz I, Sliwiński P, Górecka D, Pływaczewski R, Zieliński J. Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis. 2004;61(3):148–152. 10.4081/monaldi.2004.693 [DOI] [PubMed] [Google Scholar]
- 20.Lacedonia D, Carpagnano GE, Patricelli G, et al. Prevalence of comorbidities in patients with obstructive sleep apnea syndrome, overlap syndrome and obesity hypoventilation syndrome. Clin Respir J. 2018;12(5):1905–1911. 10.1111/crj.12754 [DOI] [PubMed] [Google Scholar]
- 21.Taranto-Montemurro L, Messineo L, Perger E, et al. Cardiac sympathetic hyperactivity in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2016;13(6):706–711. 10.1080/15412555.2016.1199668 [DOI] [PubMed] [Google Scholar]
- 22.Niu ZC, Ping F, Li X, et al. Relationship of endothelin-1 and nitro oxide to pulmonary arterial pressure in COPD patients with OSAHS. Chin Gen Pract. 2010;13(8c):2691–2693. [Google Scholar]
- 23.Ou M, Zhang C, Fang TZ, Han WJ. Clinical study in elderly patients with chronic obstructive pulmonary disease and obstructive sleep apnea hypopnea syndrome. Chin J Health Care Med. 2018;20(1):19–21. [Google Scholar]
- 24.Silva Junior JLR, Conde MB, Corrêa KS, Rabahi H, Rocha AA, Rabahi MF. Sleep-disordered breathing in patients with COPD and mild hypoxemia: prevalence and predictive variables. J Bra Pneumol. 2017;43(3):176–182. 10.1590/s1806-37562016000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun L. Overlap syndrome: Additive effects of OSA on the cardiovascular damages in patients with COPD. Suzhou Jiangsu, China: Suzhou University; 2015.
- 26.Sun WL, Wang JL, Jia GH, et al. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chin Med J (Engl). 2019;132(11):1272–1282. 10.1097/CM9.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J, Li F, Wu XF, Hou WW. Prevalence of pulmonary embolism in patients with obstructive sleep apnea and chronic obstructive pulmonary disease: The overlap syndrome. Heart Lung. 2019;48(3):261–265. 10.1016/j.hrtlng.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 28.Iturriaga R, Castillo-Galán S. Potential contribution of carotid body-induced sympathetic and renin-angiotensin system overflow to pulmonary hypertension in intermittent hypoxia. Curr Hypertens Rep. 2019;21(11):89. 10.1007/s11906-019-0995-y [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Li X, Hou WJ, Dong LX, Cao J. Endothelial function and T-lymphocyte subsets in patients with overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea. Chin Med J (Engl). 2019;132(14):1654–1659. 10.1097/CM9.0000000000000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao J, Wang P, Liu A, Du X, Bai J, Chen M. Punicalagin prevents hypoxic pulmonary hypertension via antioxidant effects in rats. Am J Chin Med. 2016;44(4):785–801. 10.1142/S0192415X16500439 [DOI] [PubMed] [Google Scholar]
- 31.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17(2):819–826. 10.1007/s11325-012-0770-7 [DOI] [PubMed] [Google Scholar]
- 32.Wang W. Obstructive sleep apnea and chronic airway disease. Chin J Tuberc Respir Dis. 2019;42(8):563–565. 10.3760/cma.j.issn.1001-0939.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 33.Wang W. Sleep and sleep related breathing disorders in chronic obstructive pulmonary disease. Natl Med J China. 2019;99(6):411–413. 10.3760/cma.j.issn.0376-2491.2019.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.