Abstract
Study Objectives:
OSA has been associated with increased cancer incidence and mortality. The aim of this study was to investigate cancer-related mortality, overall survival, and progression-free survival in patients with suspected OSA and lung cancer.
Methods:
This was a case series analysis of lung cancer from a sleep cohort with suspected OSA between 2009 and 2014. The AHI, hypoxia index, and survival outcome were recorded. Immunohistochemistry was used to analyze hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor expression in tumor pathology.
Results:
In the sleep cohort comprising 8,261 patients, a total of 23 patients had lung cancer. The incidence of lung cancer was significantly higher in the sleep cohort than in the entire adult population in Taiwan (crude incidence rate: 242.1 vs 51.5 per 105 persons, P < .01). The 3-year cancer-related mortality was 25% in AHI < 15 events/h, 50% in AHI 15–29 events/h, and 80% in AHI ≥ 30 events/h (χ2 test for trend, P = .03). In Kaplan-Meier survival analysis, patients with stage III–IV lung cancer and AHI < 30 events/h exhibited significantly better overall survival (P = .02) and progression-free survival (P = .02) than patients with severe OSA. Overexpression of HIF-1α and vascular endothelial growth factor was shown in 63% and 45% of lung tumor samples. Overexpression of HIF-1α was positively associated with AHI (P = .04).
Conclusions:
In this preliminary case series, severe OSA is associated with an increased risk of cancer mortality in patients with stage III–IV lung cancer. AHI was significantly associated with HIF-1α overexpression.
Citation:
Huang H-Y, Lin S-W, Chuang L-P, et al. Severe OSA associated with higher risk of mortality in stage III and IV lung cancer. J Clin Sleep Med. 2020;16(7):1091–1098.
Keywords: obstructive sleep apnea, lung cancer, mortality, hypoxia-inducible factor
BRIEF SUMMARY
Current Knowledge/Study Rationale: OSA has been reported to be associated with increased cancer incidence and mortality. This study specifically evaluated the cancer-related mortality, overall survival, and progression-free survival of patients with lung cancer from a large Asian cohort with suspected OSA.
Study Impact: The study demonstrated that severe OSA is associated with stage III and IV lung cancer mortality and that the AHI was significantly associated with the hypoxia-inducible factor-1α overexpression. The major clinical implication is that the screening of OSA in patients with lung cancer may be a valuable strategy to identify a potentially treatable risk factor.
INTRODUCTION
OSA is characterized by intermittent hypoxia, systemic inflammation, and increased risk of death from multiple comorbidities.1 In the past decade, several population studies have shown that OSA is associated with increased cancer incidence and mortality, especially in lung cancer, colorectal cancer, and prostate cancer.2–7 However, whether OSA increases cancer mortality because of higher tumor aggressiveness or simply because of a higher incidence rate is unclear.
Some clinical hypoxia variables have been associated with cancer mortality in patients with OSA. The Wisconsin cohort study reported that the risk of cancer mortality showed a dose- response trend for the AHI after adjustment for potential confounders.2 Hypoxemia index (the percentage of sleep time with oxygen saturation < 90% [Tsat90%]) was independently associated with increased cancer mortality in smoking-related cancer and in younger patients, suggesting that hypoxia may aggravate the carcinogenic effects of tobacco.2,4,6 These results corroborate the association between OSA and cancer through hypoxic effects.
Intermittent hypoxia is one of the hypoxic models that has been shown to promote angiogenesis and tumor growth, mediated mainly by the increased expression of hypoxia-inducible factor (HIF).8–11 HIF enhances cell survival through growth factor signaling and inhibition of proapoptotic pathways, and it promotes tumor neovascularization through vascular endothelial growth factor (VEGF).8,9,12 HIF correlates with poor prognosis in lung cancer,13 which is the leading cause of cancer death worldwide with high prevalence and rapid disease progression.14,15 In addition, tumor-associated macrophages exhibit increased proliferation, migration, and invasion after exposure to intermittent hypoxia.16
Most human studies to date have not yet explored the outcome of OSA in specific cancer sites.2–4 We identified patients with lung cancer in an Asian sleep cohort to evaluate the impact of OSA on the clinical outcome. The primary outcome was lung cancer–related mortality, and the secondary outcomes were overall survival and progression-free survival (PFS). Because the biomarkers of intermittent hypoxia have not been fully evaluated, we also assessed the correlation between the severity of OSA and the expression of HIF and VEGF in lung tumors of patients with OSA.
METHODS
Study population
This was a retrospective case-series analysis of lung cancer from a sleep cohort at Chang Gung Memorial Hospital. Patients were assessed for suspected OSA between 2009 and 2014. We excluded patients aged < 18 years and those whose chief complaint was insomnia before the sleep study. Some patients with insomnia did receive sleep study after poor response to the treatment of insomnia. All patients underwent an overnight in-laboratory polysomnography (Somnologica Studio 3.0, Medcare, Reykjavik, Iceland) using a standard protocol.17 Apnea, hypopnea, oxygen desaturation index (ODI), and AHI were defined according to the standard guideline.18 Tsat90% was defined as the percentage of sleep time with oxygen saturation < 90%.
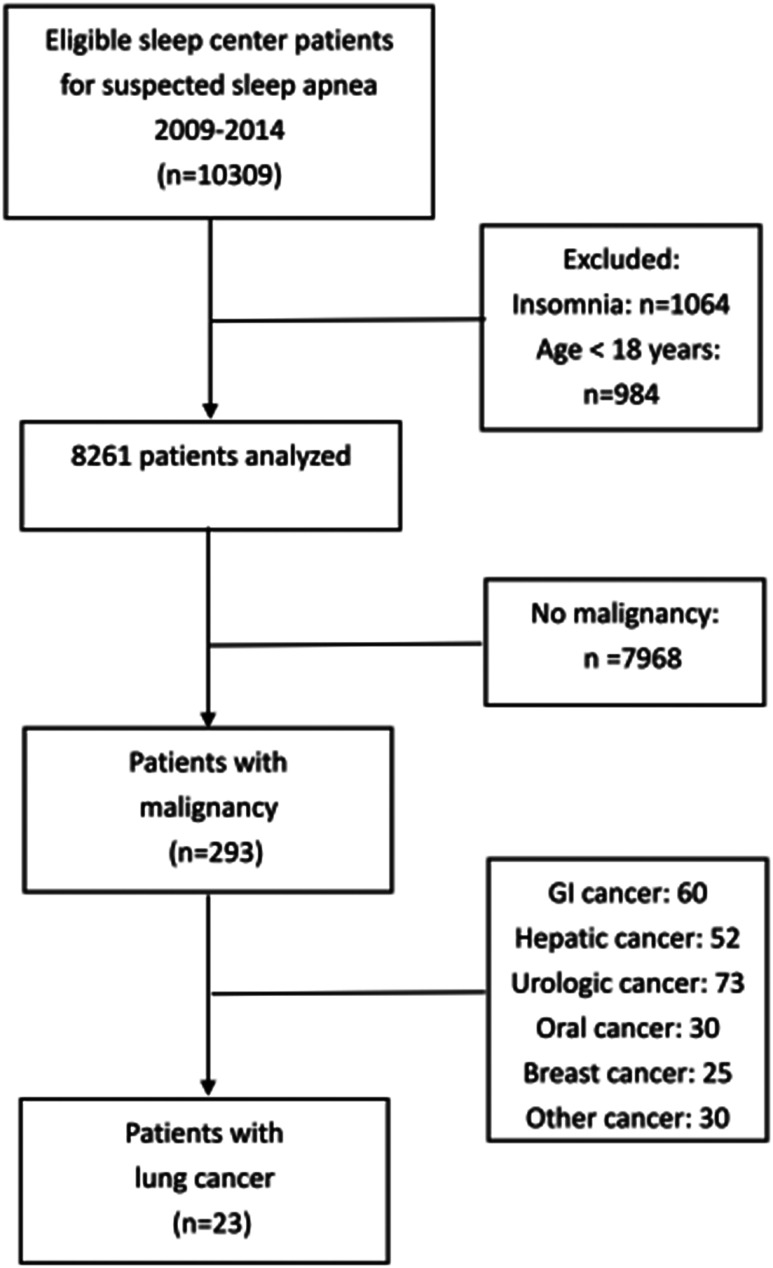
Overall, 293 patients with malignancy were found in the sleep cohort (Figure 1). We identified patients with histologically proven lung cancer and used the hospital information system and medical records to collect data on patient characteristics, comorbidities (chronic obstructive pulmonary disease, ischemic heart disease, diabetes mellitus, and hypertension), pathologic diagnosis, epidermal growth factor receptor gene mutation study, tumor stage, cancer therapy, and PFS. Tumor stage was evaluated according to the guidelines of the American Joint Committee on Cancer classification system (seventh edition).19 We used the national cancer registry of Taiwan to confirm survival status. Cancer-related mortality was the main endpoint, and April 1, 2018, was the census date for overall survival analysis. Secondary outcomes were overall survival and PFS of lung cancer. The Ethics Committee of Chang Gung Memorial Hospital approved the study (IRB Nos. 201601392B0 and 201800559B0).
Figure 1. Flowchart of the study.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections (5 μm thick) by using a bond polymer detection system and a bond-automated machine with a polymer refine kit. The polyclonal rabbit anti-HIF-1α antibody (20960-1-AP, Proteintech, USA) at a dilution of 1:100 and a monoclonal rabbit anti-VEGF antibody (SC-7269, Santa Cruz, USA) at a dilution of 1:300 were used. After deparaffinization, antigen retrieval was performed using an Epitope Retrieval 2 buffer (Leica Biosystems, USA). Sections were incubated with primary antibodies at room temperature for 30 minutes, followed with a polyhorseradish peroxidase antimouse or antirabbit immunoglobulin G reagent to localize the primary antibody, and diaminobenzidine was used to visualize the complex. The sections were then counterstained with hematoxylin, dehydrated, cleared, and mounted.
Immunohistochemical scoring
The interpretation of immunohistochemistry results for HIF-1α and VEGF was performed with blinding to patients’ outcomes. The immunoreactivity of HIF-1α and VEGF was graded from 0–3+ (0, no nuclear staining; 1+, 1%–25% nuclear staining; 2+, 26%–50% nuclear staining; 3+, 50% nuclear staining) according to nuclear expression and only 3+ (50% nuclear staining) was considered a positive immunohistochemistry result.
Statistical analysis
The Mann-Whitney U test and the Fisher exact test were used for determining single-factor signify`cance. The correlation analysis of clinicopathologic and immunohistochemical parameters was performed using the Spearman test corrected for multiple testing. Kaplan-Meier curves were calculated for the cumulative survival rate and cumulative survival time. Univariate and multivariate survival analyses were performed using the Cox regression model. P values < .05 were considered significant. Two-sided tests were used throughout the study. Statistical calculations were performed using SPSS 17.0 software (IBM, USA).
RESULTS
The cohort included 8,261 adult patients with clinical diagnosed OSA and with a median follow-up of 5 years. A total of 23 patients were diagnosed with lung cancer, and 16 (70%) had stage III or IV lung cancer. The crude incidence rate of lung cancer was significantly higher in the sleep cohort than in the general adult population in Taiwan, 2009∼2014 (242.1 vs 51.5 per 105 persons, P < .001). Table 1 shows the baseline characteristics of patients with lung cancer in this cohort. All patients with stage I–II lung cancer received surgical removal of the lung tumor (s). As expected, the 5-year mortality of patients with stage I–II lung cancer was lower than that in patients with stage III–IV lung cancer (14.2% vs 93.7%, P < .01). Only 1 patient with stage II cancer developed tumor recurrence during follow-up. The proportion of stage III and IV lung cancer was comparable in the AHI < 15 events/h (3/4 (3 of 4 patients had stage III and IV lung cancer), 75%), moderate (2/4, 50%), and severe OSA (11/15, 73%) groups (P = .804). The 3-year cancer-related mortality was 25% in patients with AHI < 15 events/h, 50% in patients with AHI 15–29 events/h, and 80% in patients with AHI ≥ 30 events/h (χ2 test for trend, P = .03). In the Kaplan-Meier survival analysis, the trend of better survival in groups with AHI < 15 events/h and 15 ≤ AHI ≤ 29 events/h was not significant (P = .275, Figure S1 in the supplemental material).
Table 1.
Clinical characteristics of sleep cohort with lung cancer.
| Total (n = 23) | Non-OSA (n = 2) | |
|---|---|---|
| Age, y | 62.4 ± 11.6 | 67.5 ± 10.6 |
| Sex, male (%) | 22 (95) | 2 (100) |
| BMI | 26.6 ± 4.8 | 27.5 ± .7 |
| Smoker, PKY | 37.4 ± 37.2 | 20 ± 14.0 |
| ESS score | 11.6 ± 4.9 | 13.5 ± 2.1 |
| Polysomnography | ||
| AHI | 41.3 ± 27.0 | 3.0 ± .0 |
| ODI | 31.6 ± 25.0 | 4.0 ± 1.4 |
| Lowest SpO2 | 80.7 ± 8.8 | 84.0 ± 2.8 |
| Tsat90% | 17.1 ± 26.7 | 4.0 ± 2.5 |
| Tsat90 mins | 32.5 ± 63.8 | 8.5 ± 6.3 |
| OSA severity (%) | ||
| Mild | 2 (9) | 0 (0) |
| Moderate | 4 (17) | 0 (0) |
| Severe | 15 (65) | 0 (0) |
| ECOG status | .7 ± .7 | .5 ± .7 |
| Cancer stage (%) | ||
| Stage I | 6 (26) | 0 (0) |
| Stage II | 1 (4) | 1 (50) |
| Stage III | 4 (17) | 1 (50) |
| Stage IV | 12 (53) | 0 (0) |
| Pathology (%) | ||
| Adenocarcinoma | 14 (61) | 1 (50) |
| Squamous cell | 6 (26) | 1 (50) |
| Other | 3 (13) | 0 (0) |
| 3-y mortality (%) | 65% | 0% |
| 5-y mortality (%) | 83% | 50% |
Data presented as mean ± SD or number (percentage). BMI = body mass index, ECOG = Eastern Cooperative Oncology Group, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, PKY = packs per year, SpO2 = oxygen saturation, Tsat90% = percentage of sleep time with oxygen saturation < 90%, Tsat90 mins = total sleep time (minutes) with oxygen saturation < 90%.
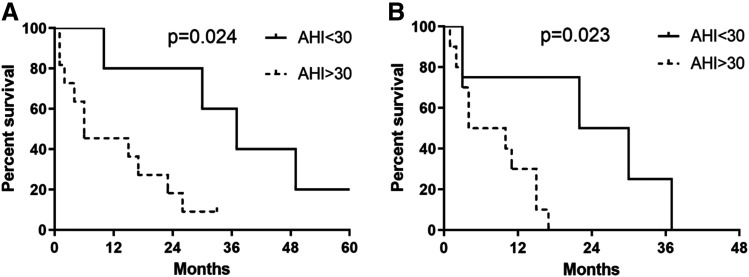
In patients with stage III and IV lung cancer, the prognosis of those with severe OSA was significantly poor. Table 2 shows the baseline characteristics between the stage III and stage IV lung cancer groups divided by AHI. The age, body mass index, performance status, tumor pathology, and epidermal growth factor receptor mutation were similar in the 2 groups, but AHI and ODI were significantly higher in patients with severe OSA. The cancer treatment and survival outcomes of patients with stage III and IV lung cancer are shown in Table 2. Figure 2 shows the Kaplan-Meier curves for survival after diagnosis of lung cancer in all groups. Patients with stage III and IV lung cancer and AHI < 30 events/h had a significantly better overall survival (median survival 37 vs 6 months, P = .024) and PFS (median survival 26 vs 7 months, P = .023) than did patients with severe OSA. In a univariate analysis of overall survival in patients with stage III and IV lung cancer, AHI (hazard ratio [HR] = 5.21, 95% confidence interval [CI], 1.08–25.09, P = .04), Tsat90% (HR = 1.04, 95% CI, 1.01–1.07, P = .016), and Eastern Cooperative Oncology Group status (HR = 5.20, 95% CI, 1.22–22.19, P = .026) were significant risk factors; ODI was nonsignificant (HR = 1.01, 95% CI, 0.99–1.04, P = .289) (Table S1 in the supplemental material). In a univariate analysis of PFS in patients with stage III and IV lung cancer, AHI (HR = 11.19, 95% CI, 1.37–91.55, P = .024) and Tsat90% (HR = 1.03, 95% CI, 1.01–1.06, P = .020) were significant risk factors; ODI was nonsignificant (HR = 1.01, 95% CI, 0.99–1.03, P = .301) (Table S1). In a multivariate analysis of overall survival, 3 variables (AHI, Tsat90%, and Eastern Cooperative Oncology Group status) were introduced in the stepwise logistic regression, but all 3 variables were nonsignificant (Table S2 in the supplemental material). Other models of multivariate analysis were used to explore the association of AHI and survival, and AHI was observed to be a significant risk factor compared with age and body mass index separately in overall survival and was also a significant risk factor compared with age, body mass index, and ODI separately in PFS (Table S2).
Table 2.
Clinical characteristics of stage III and IV lung cancer.
| Total (n = 16) | AHI < 30 events/h | AHI ≥ 30 events/h | P | |
|---|---|---|---|---|
| (n = 5) | (n = 11) | |||
| Age, y | 62.4 ± 11.5 | 64.2 ± 10.9 | 63.3 ± 11.4 | > .99 |
| Sex, male | 15 | 4 | 11 | .31 |
| BMI | 26.8 ± 4.2 | 25.8 ± 4.3 | 27.2 ± 4.3 | .46 |
| Smoker, PKY | 43.1 ± 40.5 | 20.0 ± 15.8 | 53.6 ± 44.3 | .09 |
| ECOG status | 1 ±.6 | .8 ± .4 | 1.1 ± .7 | .43 |
| Polysomnography | ||||
| AHI | 43.3 ± 29.4 | 10.8 ± 8.9 | 59.5 ± 20.8 | < .01* |
| ODI | 28.2 ± 25.8 | 7.8 ± 4.0 | 41.0 ± 25.5 | < .01* |
| Lowest SpO2 | 81.8 ± 6.9 | 84.0 ± 5.3 | 80.7 ± 7.5 | .50 |
| Tsat90% | 19.1 ± 27.1 | 2.5 ± 2.1 | 29.5 ± 31.5 | .05 |
| Tsat90 mins | 29.8 ± 56.6 | 5.2 ± 5.0 | 41.0 ± 66.0 | .16 |
| PFS, months | 18.4 ± 29.2 | 42.4 ± 45.2 | 7.5 ± 6.3 | .03* |
| 3-y mortalitya (%) | 81.3 | 40 | 100 | .08 |
| 5-y mortalitya (%) | 93.7 | 80 | 100 | .31 |
| Cancer stage (%) | .55 | |||
| Stage III | 4 (25) | 2 (40) | 2 (18) | |
| Stage IV | 12 (75) | 3 (60) | 9 (82) | |
| Pathology (%) | .59 | |||
| Adenocarcinoma | 10 (66) | 4 (80) | 6 (55) | |
| Squamous cell | 3 (18) | 1 (20) | 2 (18) | |
| Other | 3 (18) | 0 (0) | 3 (27) | |
| EGFR mutation | 7 | 3 | 4 | > .99 |
| No mutation | 3 | 1 | 2 | |
| First-line cancer treatment | .36 | |||
| Target therapy | 4 | 3 | 4 | |
| CCRT | 3 | 2 | 1 | |
| Chemotherapy | 4 | 0 | 4 | |
| Radiotherapy | 1 | 0 | 1 | |
| Supportive care | 1 | 0 | 1 | |
| Comorbid disease | ||||
| COPD | 1 | 0 | 1 | > .99 |
| Ischemic heart disease | 4 | 1 | 3 | > .99 |
| Diabetes mellitus | 5 | 1 | 4 | > .99 |
| Hypertension | 8 | 2 | 6 | > .99 |
Data presented as mean ± SD or number (percentage). *Cancer-related mortality. BMI = body mass index, CCRT = concurrent chemoradiotherapy, COPD = chronic obstructive pulmonary disease, ECOG = Eastern Cooperative Oncology Group, EGFR = epidermal growth factor receptor, ODI = oxygen desaturation index, PFS = progression-free survival, PKY = packs per year, SpO2 = oxygen saturation, Tsat90% = percentage of sleep time with oxygen saturation < 90%, Tsat90 mins = total sleep time (minutes) with oxygen saturation < 90%.
Figure 2. Kaplan-Meier survival analysis in a sleep cohort with lung cancer.
Survival analysis in a cohort with lung cancer with (A) overall survival of stage III–IV lung cancer and (B) PFS of stage III and IV lung cancer. PFS = progression-free survival.
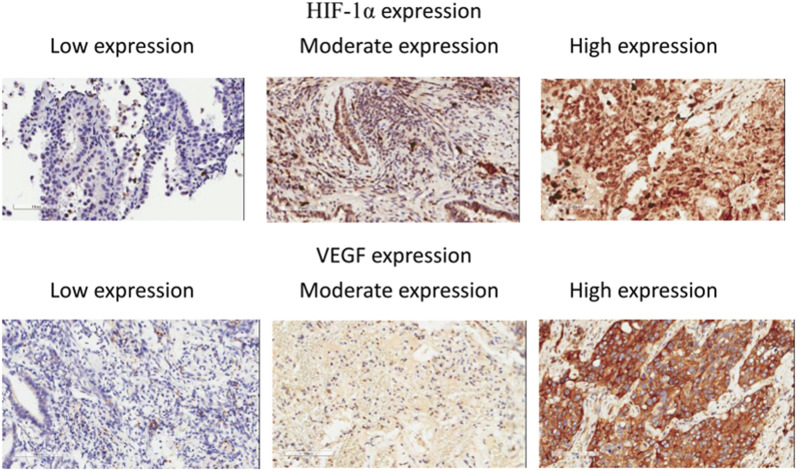
Immunohistochemical analysis of HIF-1α and VEGF expression was performed in 22 sets of lung cancer samples. Representative immunohistochemical staining of both markers is shown in Figure 3. Overexpression of HIF-1α and VEGF was exhibited in 63% and 45% of lung tumor samples, respectively. Those patients with high HIF-1α expression exhibited significantly higher AHI values (odds ratio = 1.05, 95% CI, 1.0–1.11, P = .04) (Table 3). The 3-year mortality in patients with high HIF-1α expression and non-high HIF-1α expression was 73% and 50%, respectively. The Kaplan-Meier survival analysis of HIF-1α expression was nonsignificant (Figure S2). The patients with high and non-high VEGF expression had similar clinical characteristics (Table S3).
Figure 3. Immunohistochemistry pictures of HIF-1α expression and VEGF expression.
HIF = hypoxia-inducible factor, VEGF = vascular endothelial growth factor.
Table 3.
Comparisons between groups with high and low expression of HIF-1α.
| HIF-1α High Expression |
HIF-1α Low Expression |
Odds Ratio | P | |
|---|---|---|---|---|
| (n = 14) | (n = 8) | |||
| Age, y | 62.3 ± 10.8 | 62.3 ± 14.2 | 1 (.93–1.08) | .99 |
| Sex, male | 14 | 7 | > .99 | |
| Polysomnography | ||||
| AHI | 46.5 ± 21.8 | 22.7 ± 22.7 | 1.05 (1.0–1.11) | .04* |
| ODI | 32.8 ± 21.6 | 21.4 ± 21.8 | 1.03 (.98–1.08) | .28 |
| Lowest SpO2 | 82.3 ± 5.6 | 82.7 ± 5.4 | 0.99 (.83–1.17) | .88 |
| Tsat90% | 14.3 ± 26.2 | 16.4 ± 27.1 | 1.0 (.96–1.03) | .86 |
| Cancer stage (%) | ||||
| Stage I | 5 (35) | 1 (12) | > .99 | |
| Stage II | 0 (0) | 1 (12) | ||
| Stage III | 3 (21) | 1 (12) | 0.60 (.03–13.5) | .75 |
| Stage IV | 6 (43) | 5 (63) | 0.24 (.02–2.79) | .25 |
| Pathology (%) | ||||
| Adenocarcinoma | 8 (57) | 5 (63) | > .99 | |
| Squamous cell | 4 (29) | 2 (25) | 1.25 (.16–9.54) | .83 |
| Other | 2 (14) | 1 (12) | 1.25 (.09–17.6) | .87 |
Data presented as mean ± SD or number (percentage). HIF = hypoxia-inducible factor, ODI = oxygen desaturation index, SpO2 = oxygen saturation, Tsat90% = percentage of sleep time with oxygen saturation < 90%.
DISCUSSION
This study revealed that among patients with stage III and IV lung cancer, those with severe OSA had higher cancer-related mortality than did those with mild-moderate OSA. AHI and Tsat90% were positively associated with higher overall cancer mortality and shorter overall survival and PFS in stage III and IV lung cancer. The pathological staining provided evidence that AHI was significantly associated with HIF-1α overexpression.
The incidence of specific cancer types in patients with OSA may be increased, decreased, or unaffected.7 The most frequent cancer incidences in the OSA population in Spain and Canada, for example, are colorectal cancer and prostate cancer, respectively.3,4 In the United States, the incidences of melanoma and renal tumors in patients with reportedly increased, whereas those of colorectal cancer and lung cancer decreased.7 Although lung cancer was not the most common types of cancer in our sleep cohort, the crude incidence rate of lung cancer was higher in patients with OSA compared with the general population.20 One Asian study investigated the incidence and mortality of lung cancer in the patients admitted to a University hospital (Henan, China) from 2013 to 2014 and found that the incidence of lung cancer was similar among patients with OSA and other patients in the hospital.21 The major limitation of that study was that the lung cancer incidence was compared with that in a single hospital group rather than with the general population.21 In contrast, the current study compared the incidence of lung cancer in the sleep cohort with that in the entire population of Taiwan during the study period, providing a more informative and less biased report.
In the Wisconsin study, lung cancer had the highest cancer mortality rate.2 Whether OSA increases lung cancer incidence or accelerates cancer progression, leading to higher mortality, is unclear. In this study, severe OSA was associated with an increased risk of death and significantly reduced PFS in patients with lung cancer, which suggested that severe OSA may cause tumor progression during treatment. In the Wisconsin cohort, severe OSA was an independent predictor of cancer mortality, and there was a trend that patients with higher AHI had increased cancer mortality.2 Intermittent hypoxia appears to be the key element in the relationship between OSA and cancer. This study found that the negative effect of AHI on mortality was most pronounced in stage III and IV lung cancer rather than in stage I and II lung cancer. One study reported that OSA severity affects the prognosis of lung cancer in all stages.21 However, in that study, most of the patients with stage I or II lung cancer did not receive surgical treatment (58% in mild OSA and 75% in moderate OSA).21 Currently, surgical resection is acknowledged to be the recommended treatment for early-stage lung cancer.22 In our study, all patients with stage I or II lung cancer underwent surgical resection, and only 1 patient with stage II lung cancer experienced recurrence. Therefore, the severity of OSA does not appear to affect patients with stage I–II lung cancer after surgical resection but may nonetheless cause poor prognosis in those unable to receive surgery. Additional research is needed to investigate whether patients with stage II lung cancer and severe OSA have a higher postoperative recurrence rate.
Our study found that Tsat90% as a hypoxia marker, in addition to AHI, is also a prognostic factor, consistent with previous studies.2,6 Tsat90% represents the extent of hypoxia during sleep, but overall hypoxia status may not fully represent the unique intermittent hypoxia pattern of OSA. Few studies provide information on ODI, and ODI was not a significant prognostic factor in this study. Future study may be needed to confirm the role of ODI in patients with cancer.
Intermittent hypoxia is known to increase resistance to radiation and migration in lung cancer cells.23 In animal studies, intermittent hypoxia has also enhanced lung tumor progression and altered phenotypes of tumor-associated macrophage, causing adverse cancer outcomes.11,16 HIF is the major pathway of the hypoxia effect on cancer cells.12,24 The role of HIF-1 signaling in cancer progression, metastasis, and resistance to therapy has been validated in both hypoxia and intermittent hypoxia conditions.8–10,23 HIF-1α has also been reported to be a prognosis marker of lung cancer.13 This study showed that the expression of HIF-1α was associated with the severity of sleep-disordered breathing in patients with OSA and lung cancer. The prevalence (49%) of sleep-disordered breathing in patients with lung cancer was higher than previously expected, and moderate to severe OSA was up to 17% in one cohort.25 Additional research is required to investigate if high expression of HIF-1α in patients with lung cancer and OSA is associated with poor survival. Although circulating levels of VEGF are reportedly increased in OSA,26 this study did not show the association of VEGF expression with the severity of OSA.
The main strength of the study was the large sleep cohort population, with enough patients with cancer for analysis of a specific cancer type. Previous studies have typically analyzed the pooled mortality of all cancer types and may not include patients with cancer in early stages. Another advantage of this study is the precision of the diagnosis. All enrolled patients underwent overnight standard polysomnography in a sleep laboratory, and the diagnosis of lung cancer was based on pathological reports. In addition, the medical record system had detailed staging, cancer treatment, and follow-up records; thus, we could assess the impact of AHI on the prognosis of patients with stage I–IV lung cancer receiving different treatments. Another advantage of this study was that we used pathological staining to explore the effects of intermittent hypoxia on human lung cancer tissues and clinical prognosis. No other studies have provided relevant data.
This study has some limitations. First, the sleep cohort of the present study may not represent patients with OSA in the general population because the sleep cohort was from a tertiary medical center. To investigate the incidence or prevalence of cancer in OSA, a study on the general population should be conducted by administering sleep studies and identifying OSA and then correlating with the diagnosis of cancer. Second, this was a pilot study to examine the relationship between OSA and lung cancer mortality, but the number of patients with lung cancer was relatively small. Therefore, the multivariate analysis may be underpowered to examine significant factors associated with survival. Future clinical trials designed to assess cancer-related mortality and survival are required to provide more conclusive evidence. However, this is currently one of the largest OSA population studies to date, focusing on lung cancer analysis, and we collected valuable information on cancer parameters such as performance status, cancer stages. and cancer treatment, which were unavailable in other studies. Finally, patients with OSA in this study were not treated with CPAP treatment. Although CPAP is the standard treatment for OSA, the national health insurance in Taiwan does not cover the cost of the treatment and these patients were reluctant to receive CPAP at their own expense. Therefore, we cannot evaluate the effect of CPAP treatment on the prognosis of lung cancer in patients with OSA.
CONCLUSIONS
In this preliminary case series, OSA severity was associated with an increased risk of death in stage III and IV lung cancer in a sleep cohort. The pathological staining provided evidence that AHI was significantly associated with HIF-1α overexpression. This study provides a preliminary reference for future research on how OSA affects the risk of lung cancer death.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Chang Gung Memorial Hospital, Taoyuan, Taiwan. This study was funded by a grant from the Chang Gung Medical Foundation (CORPG3F0861 and CORPG1F0071), a grant from the Research Services Center for Health Information at Chang Gung Memorial Hospital for statistical analysis (Grant CIRPD1D0032), and the support of the Maintenance Project of the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital (Grant CLRPG3D00441). The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank Yu-Jr Lin and the Research Services Center for Health Information at Chang Gung Memorial Hospital for statistical analysis. The authors thank May Lu for her assistance in editing this manuscript.
ABBREVIATIONS
- COPD
chronic obstructive pulmonary disease
- EGFR
epidermal growth factor receptor
- HIF
hypoxia-inducible factor
- ODI
oxygen desaturation index
- PFS
progression-free survival
- Tsat90%
the percentage of sleep time with oxygen saturation < 90%
- VEGF
vascular endothelial growth factor
REFERENCES
- 1.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266–274. 10.1378/chest.14-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186(2):190–194. 10.1164/rccm.201201-0130OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187(1):99–105. 10.1164/rccm.201209-1671OC [DOI] [PubMed] [Google Scholar]
- 4.Kendzerska T, Leung RS, Hawker G, Tomlinson G, Gershon AS. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ. 2014;186(13):985–992. 10.1503/cmaj.140238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. 10.5664/jcsm.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-García MA, Campos-Rodriguez F, Durán-Cantolla J, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15(7):742–748. 10.1016/j.sleep.2014.01.020 [DOI] [PubMed] [Google Scholar]
- 7.Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep. 2016;39(8):1493–1500. 10.5665/sleep.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- 9.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15(4):678–685. 10.1038/cdd.2008.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275(12):2991–3002. 10.1111/j.1742-4658.2008.06454.x [DOI] [PubMed] [Google Scholar]
- 11.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39(1):215–217. 10.1183/09031936.00185110 [DOI] [PubMed] [Google Scholar]
- 12.Challapalli A, Carroll L, Aboagye EO. Molecular mechanisms of hypoxia in cancer. Clin Transl Imaging. 2017;5(3):225–253. 10.1007/s40336-017-0231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ. Prognostic significance of hypoxia-inducible factor-1α, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64(12):1082–1089. 10.1136/thx.2009.115691 [DOI] [PubMed] [Google Scholar]
- 14.Chiang CJ, Chen YC, Chen CJ, You SL, Lai MSTaiwan Cancer Registry Task Force . . Cancer trends in Taiwan. Jpn J Clin Oncol. 2010;40(10):897–904. 10.1093/jjco/hyq057 [DOI] [PubMed] [Google Scholar]
- 15.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almendros I, Wang Y, Becker L, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189(5):593–601. 10.1164/rccm.201310-1830OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magalang UJ, Chen NH, Cistulli PA, et al. Agreement in the scoring of respiratory events and sleep among international sleep centers. Sleep. 2013;36(4):591–596. 10.5665/sleep.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 20. Taiwan Cancer Registry. Statistics web site. http://tcr.cph.ntu.edu.tw/main.php?Page=A5B2. Accessed October 18, 2019.
- 21.Li L, Lu J, Xue W, et al. Target of obstructive sleep apnea syndrome merge lung cancer: based on big data platform. Oncotarget. 2017;8(13):21567–21578. 10.18632/oncotarget.15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Videtic GM, Chang JY, Chetty IJ, et al. ACR appropriateness Criteria® early-stage non-small-cell lung cancer. Am J Clin Oncol. 2014;37(2):201–207. 10.1097/COC.0000000000000013 [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Song X, Wang X, et al. Effect of chronic intermittent hypoxia on biological behavior and hypoxia-associated gene expression in lung cancer cells. J Cell Biochem. 2010;111(3):554–563. 10.1002/jcb.22739 [DOI] [PubMed] [Google Scholar]
- 24.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl.). 2015;3:83–92. 10.2147/HP.S93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreher M, Kruger S, Schulze-Olden S, et al. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm Med. 2018;18(1):72. 10.1186/s12890-018-0645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165(1):67–70. 10.1164/ajrccm.165.1.2101062 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.