Abstract
Study Objectives:
Due to a limited number of pediatric sleep centers, the aim was to test the feasibility of ambulatory polysomnography (PSG-home) in a group of French children suspected of OSA.
Methods:
Children undergoing one-night PSG-home, with the device installed at the pediatric sleep physician’s office, were prospectively included. General failure was considered when PSG-home recording captured < 5 h of artifact-free sleep or when ≥ 1 channel (nasal flow, thoraco-abdominal belts, oximetry) presented artifacts > 75% of the recording time. No-OSA was defined as an obstructive apnea-hypopnia index (OAHI) < 1 event/h and respiratory-related arousals index (RAI) < 1 event/h. OSA was defined as upper airways resistance syndrome (UARS) with OAHI < 1 event/h with RAI ≥ 1 event/h, or mild OSA (OAHI ≥ 1 event/h-5 events/h), moderate OSA (OAHI ≥ 5 events/h-10 events/h), or severe OSA (OAHI ≥ 10 events/h). Parents completed a severity hierarchy score questionnaire, Conners Parent Rating Scale, and an adapted Epworth Sleepiness Scale.
Results:
Fifty-seven children aged 3 through 16 years were included. PSG-home was technically acceptable in 46 (81%). Failure due to nasal cannula was observed in 11% (n = 6), oximetry in 7% (n = 4), and both in 2% (n = 1) of cases. No difference in feasibility was found according to age, sex, OSA severity, or comorbidities. There were 14 (25%) children categorized as no-OSA, 43 (75%) as OSA, 4 (7%) as UARS, 26 (46%) as mild, 6 (10%) as moderate, and 7 (12%) as severe OSA. Neither questionnaires nor clinical and physical examination predicted OSA diagnosis.
Conclusions:
When equipment is installed at the professional’s office and a parent monitors the child, PSG-home is feasible and technically acceptable in children aged 3 through 16 years old. The short delay and feasibility provided by PSG-home could improve the management of children suspected of OSA.
Citation:
Ioan I, Wieck D, Schweitzer C, Guyon A, Coutier L, Franco P. Feasibility of parent-attended ambulatory polysomnography in children with suspected obstructive sleep apnea. J Clin Sleep Med. 2020;16(7):1013–1019.
Keywords: obstructive sleep apnea, ambulatory polysomnography, sleep, children
BRIEF SUMMARY
Current Knowledge/Study Rationale: Polysomnography is the gold standard for diagnosis of OSA in children. Because few pediatric centers exist, ambulatory polysomnography might be of help.
Study Impact: Following device installment at the sleep pediatrician’s office, ambulatory polysomnography was found to be feasible and interpretable in a group of French children suspected of OSA, without requiring a trained nurse or technician on site.
INTRODUCTION
Serious consequences such as neurocognitive and behavioral disorders are associated with undiagnosed OSA in children. Moreover, untreated OSA may result in growth delay and cardiovascular and metabolic complications.1 OSA occurs frequently in preschool and school-aged children, with an incidence that varies from 0.7% to 4%2, while primary snoring is present in almost 10% of children. In the definition of obstructive sleep-disordered breathing (SDB) in children, there is a severity continuum that begins with primary snoring (snoring ≥ 3 nights per week without apnea, hypopnea, frequent arousals from sleep, or gas exchange abnormalities), passes through upper airway resistance syndrome (UARS; characterized by obstructive apnea-hypopnea index [OAHI] < 1 event/h of sleep but with increasing respiratory efforts, leading to sleep fragmentation expressed as respiratory-related arousals index [RAI] ≥ 1 event/h), and progresses to mild OSA (OAHI ≥ 1 event/h and < 5 events/h of sleep), moderate OSA (OAHI ≥ 5 events/h and < 10 events/h of sleep), and severe OSA (OAHI ≥ 10 events/h of sleep).3 The severity of obstructive SDB is positively associated with morbidity risk.4 Early diagnosis of OSA is important, as treatment may prevent or reverse its complications. Diagnosis is based on clinical history and physical examination that are, however, nonspecific, polymorphic, and sometimes discordant, and do not differentiate between OSA and primary snoring. Furthermore, questionnaires available in the literature are not robust enough to establish a reliable diagnosis and distinguish among OSA severity forms.5,6 A full nighttime polysomnography (PSG, type 1) in a sleep laboratory, with video surveillance and monitoring by trained pediatric nurses who watch the sensors’ signals in real time and reposition the leads whenever necessary, remains the gold standard for diagnosing OSA in children.1,7,8 The PSG, type 1 sleep study is an objective, noninvasive test to assess a child’s sleep and breathing during sleep. PSG gives an OAHI, the frequency and the duration of respiratory events to indicate OSA severity and its consequences on oxygen arterial saturation, carbon dioxide pressure, and sleep fragmentation, with arousal indexes describing sleep quality. To obtain reliable results, pediatric PSG leads and sensors have to be used and the test performed during the child’s usual night-time sleep hours, without sedative medication or sleep deprivation that may aggravate the obstructive events.7 However, accessibility to PSG in a clinical setting is frequently limited due to increasing demand, nonavailability in most regions, expensive cost, and time constraints for registration and interpretation. Moreover, PSG results are dependent on the equipment used and the environment in which it is performed9, as a child’s sleep may be altered due to hospital anxiety, noise, and interventions to reposition the sensors. PSG recorded at home (PSG, type 2 sleep study) is an alternative more accessible and less expensive. It is potentially associated with better sleep quality, because the child sleeps in their own bed.10 The few studies using PSG performed with children suspected of OSA have shown similar results between ambulatory and hospital recordings.2,11,12 However, in these studies, trained technicians or nurses installed the PSG at the home.12,13
The aim of this study was to test the feasibility and technical reliability of ambulatory PSG for diagnosing OSA in a group of French children without incurring the travel expenses for trained technicians or nurses. The second aim was to determine if certain clinical characteristics, ie, age, sex, comorbidities, or presence or severity of OSA, impacted the feasibility of this testing method.
METHODS
Population
Consecutive children aged 3 through 16 years suspected of OSA and referred to a private practice sleep physician (D.W.) by pediatricians, ear, nose, and throat (ENT) physicians, or general practitioners between September 2013 and September 2015 were prospectively recruited for this single-center study. D.W. is a general practitioner in a semirural town with mixed socioeconomic levels, she is also works part time in a pediatric sleep lab (Hôpital Femme Mère Enfant, Lyon, France). In September 2013 she decided to dedicate a half-day per week of her private practice to pediatric sleep troubles. Most of the patients had insomnia complaints. All the patients with OSA suspicion agreed to do an overnight PSG-home. All expenses were assumed by French National Health Service. Only two patients, with severe behavior problems, were sent the pediatric sleep lab.
Parents’ and children’s consents were obtained in accordance with the legislation in place at the time of the study, and the study was approved by the hospital’s ethics committee and the national data protection agency (Commission Nationale de l’Informatique et des Libertés, registration number 1835988). The clinical history and physical examination were conducted by the sleep physician on the day of the study. Mallampati score, estimating the upper airway anatomy contribution to obstructive sleep breathing, and tonsils volume, graded from 1 to 4, were noted. As previously described, Mallampati score ≥ 3 and tonsils volume ≥ 3 are associated with a higher risk of OSA.14 The parents filled out questionnaires [Adapted Epworth Diurnal Sleepiness Scale,15 Spruyt and Gozal severity hierarchy score questionnaire (SHS)6 and Conners’ Parent Rating Scale] concerning their children’s sleep and scores were retained for analysis. Adapted Epworth Sleepiness Score (Adapted ESS), in which the item “falling asleep while in a car stopped in the traffic” was replaced with “falling asleep at school.”15 Adapted ESS score >10 is associated to excessive diurnal sleepiness,15 SHS score <2.75 is a very good negative predictive value for OSA6 and Conners score >15 is related to diurnal hyperactivity.16
Nocturnal PSG
PSG-home was performed with Nox-A1 polysomnography system (ResMed, France). Electroencephalogram with frontal, central, and occipital leads (FP1, FP2, C3, C4, O1, O2, A1, and A2), two electrooculograms, and one chin electromyogram were used for defining sleep stages and arousals. Nasal cannula, inductance plethysmography of chest and abdominal respiratory movements, oximetry, and electrocardiogram served for scoring the respiratory parameters, as recommended by the American Academy of Sleep Medicine (AASM).17 The device was placed on the child by the pediatric sleep specialist at her office in the late afternoon. The child and parents returned home and the recording was set to automatically start in the evening and stop in the morning. Parents were instructed to check the nasal cannula, chest and abdominal belts, and oximetry every 2 hours during the night and reposition them as needed. Parents were asked to remove the equipment in the morning and bring it to the pediatric sleep physician’s office.
PSG analysis
PSG data was downloaded and analyzed by the pediatric sleep physician (D.W.). Sleep stages and respiratory events were scored according to the 2012 pediatric AASM guidelines.17
Feasibility was assessed in terms of interpretable recordings using quality criteria that took into account signal artifacts and minimum recording time. General failure was considered to be when PSG-home recording captured less than 5 hours that were artifact-free or when 1 or more of the channels (nasal flow, thoraco-abdominal belts, or oximetry) showed artifacts during more than 75% of the recording time. The different indexes were computed as the number of events per hour of sleep. Obstructive apnea (OAI), hypopnea (OHI), and obstructive apnea-hypopnea (OAHI) indexes, central apnea, total arousal indexes and respiratory-related arousals index (RAI), mean and minimal oxygen saturation, and percentage of total sleep time (TST) spent with an oxygen saturation < 90% were obtained for analysis.
Statistical analysis
Statistical analysis was performed using SAS software (SAS Institute, Inc., Cary, NC). Data are expressed as median and range (minimum-maximum) or number (percentage) as appropriate. As severity of obstructive SDB is positively associated with risk of morbidity,4 children were divided in 2 groups: the no-OSA group included children with an OAHI < 1/h of sleep and RAI < 1/h of sleep; the OSA group including UARS children with snoring, OAHI < 1/h of sleep, and RAI ≥ 1/h of sleep; mild OSA if OAHI ≥ 1 and < 5/h of sleep, moderate OSA if OAHI ≥ 5 and < 10/h of sleep; and severe OSA if OAHI ≥ 10/h of sleep.
Different sleep scale scores and clinical PSG and PSG-home characteristics among groups were compared using nonparametric Mann-Whitney U test or Pearson chi-square test as needed. The correlation with age between scale scores and OSA severity or percentage of TST artifact-free for different channels (oximetry, nasal cannula, thoraco-abdominal belts) was tested using Pearson correlation or linear regression. The distribution of failed PSG-home recordings according to age, sex, OSA severity, presence of comorbidities, and neurodevelopmental delay was assessed using Pearson chi-square test. To assess the prognostic factors associated with the feasibility of PSG-home recording, a multivariate logistic regression analysis was made, the variables included in the model being age, preschooler/schooler age, sex, OSA severity, presence of comorbidities, the child’s neurodevelopmental status, and the interaction between them. A p value < 0.05 was considered as statistically significant.
RESULTS
A total of 57 children were prospectively included in the study. These children were of median (range) age 7 (3 through 16) years, including 27 (47%) preschoolers (aged < 7 years). Their characteristics are presented in Table 1. Nine (16%) children had neurological disorders (ie, West syndrome in 2 [4%], epilepsy in 2 [4%], Arnold-Chiari malformation in 1 [2%], attention deficit and hyperactivity disorder in 3 [5%], and autistic disorder in 1 [2%]), 2 (4%) craniofacial malformations (ie, craniostenosis in 1 [2%], Silver Russell syndrome in 1 [2%]), and 4 (7%) had been diagnosed with obesity. Among known risk factors for OSA, a history of prematurity was found in 2 (4%) children, a history of recurrent ENT infections in 27 (47%), and gastroesophageal reflux in 6 (5%). Furthermore, 3 (5%) patients had been diagnosed with asthma, 9 (16%) with respiratory allergies, 6 (11%) with both, and 8 (14%) children had a history of adenoidectomy, 4 (7%) tonsillectomy, and 4 (7%) adenotonsillectomy.
Table 1.
Children’s characteristics.
| Patients | |
|---|---|
| Boys, n (%) | 31 (57) |
| Age (years) | 7 (3-16) |
| Weight (kg) | 23 (11-13) |
| Height (m) | 1.20 (.90-1.67) |
| Body mass index (kg/m2) | 16 (13-48) |
| Systolic/diastolic arterial tension (mm Hg) | 100 (85-120)/60 (50-80) |
| Tonsils volume | 2 (0-4) |
| Mallampati score | 1 (1-4) |
| SHS score | 1.88 (0-3.47) |
| Adapted Epworth score | 6 (0-16) |
| Conners score | 10 (1-23) |
Data are expressed as median and range (minimum-maximum). SHS = severity hierarchy score.
The main complaint was snoring in 42 (74%) children. The other children presented symptoms suggesting OSA, such as diurnal sleepiness (1 [7%]), hyperactivity (3 [20%]), recurrent ENT infections (2 [13%]), an association of these in 2 (13%), or a risk factor or syndrome predisposing to OSA in 7 (47%). Children had a median tonsils volume of 2 (0-4) with Mallampati score of 1 (1-4), and 28 out of 57 children (49%) had tonsils volume ≥ 3 and snoring.
The questionnaires showed normal SHS and no pathological scores on both Conners scale and adapted ESS.
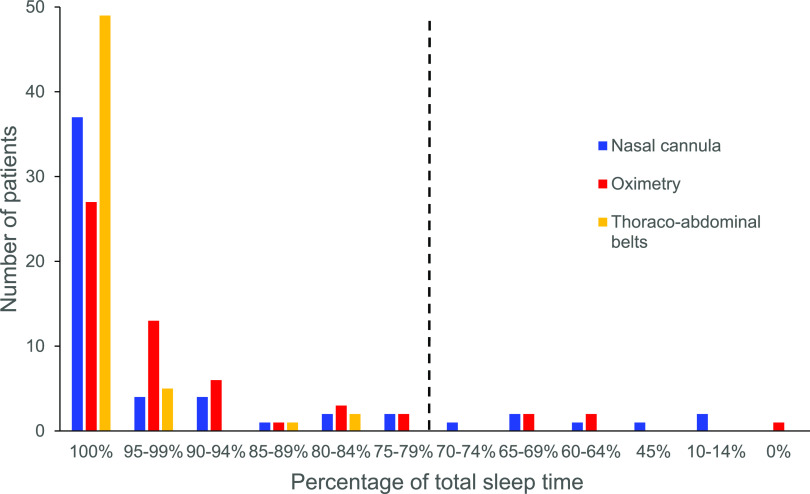
PSG-home median total recording time was 732 min (507-747), TST 547 min (331-673), and an overall sleep efficiency of 94% (52-98) (Table 2). PSG recording was interpretable in all children, except one in whom the oximetry signal was off all night, and was technically acceptable in 46 (81%) children. The proportion of failures related to nasal cannula was 11% (n = 6), oximetry 7% (n = 4), and both 2% (n = 1) The oximeter signal was present in 99% (0-100) of TST, flow signal in 100% (11-100), and thoraco-abdominal belt signals in 100% (81-100) of TST. Among the 11 children with nonfeasible PSG-home (< 75% of TST with artifact-free signal), the oximetry signal was off over the whole recording in 1 (2%), only present for 63% to 64% of TST in 2 (4%), and for 68% in 1 (2%); the nasal cannula signal was only present for 10-14% of TST in 2 (4%), for 45% in 1 (2%), for 66-70% in 2 (4%), and for 61% in 1 (2%); the oximetry signal was on for 67% and the nasal cannula for 69% of TST in 1 (2%) child (Figure 1).
Table 2.
At home polysomnography results.
| Patients | |
|---|---|
| Total recording time (min) | 732 (507-747) |
| Total sleep time (min) | 547 (331-673) |
| Sleep efficiency (%) | 94 (52-98) |
| NREM stage 1 (min/% of total sleep time) | 51 (18-140)/9 (3-24) |
| NREM stage 2 (min/% of total sleep time) | 245 (135-322)/46 (26-57) |
| NREM stage 3 (min/% of total sleep time) | 113 (74-156)/21 (13-33) |
| REM (min / % of total sleep time) | 131 (45-197)/24 (13-34) |
| Arousal index (events/h of sleep) | 7.0 (2-20) |
| Respiratory-related arousals index (events/h of sleep) | 1.5 (0-18) |
| Obstructive apnea index (events/h of sleep) | 1.0 (0-49) |
| Obstructive hypopnea index (events/h of sleep) | 1.1 (0-27) |
| Obstructive apnea-hypopnea index (events/h of sleep) | 2.6 (0-76) |
| Central apnea index (events/h of sleep) | 0.2 (0-1.3) |
| Central hypopnea index (events/h of sleep) | 0 |
| Central apnea-hypopnea index (events/h of sleep) | 0.2 (0-1.3) |
| Mean oxygen saturation (%) | 97 (93-99) |
| Minimal oxygen saturation (%) | 89 (72-94) |
| Time with oxygen saturation < 90% (% of total sleep time) | 0 (0-10) |
Data are expressed as median (minimum-maximum).
Figure 1. The number of children according to percentages of total sleep time with artifact-free oximetry, nasal cannula, and thoraco-abdominal belts signals. The interrupted line represents the 75% limit.
No correlation was found between age and the percentage of TST with artifact-free oximetry recording (r = .10, P = .47), nasal cannula (r = .19, P = .16), thoraco-abdominal belts (r = .09, P = .49), or the 4 channels (r = .21, P = .12).
The median number of times when parents checked sensor positioning during the night was 5 [5; 6] for both nasal canula and oximetry, and the median number of instances of repositioning was 1 [1; 3] for nasal cannula and 1 [1; 2] for oximetry. No parents complained about the burden of the PSG home study. They were relieved to have quickly obtained a PSG recording for their child.
Median OAHI was 2.6 events/h (0-76) of sleep with an OAI of 1.0 event/h (0-49) of sleep, an OHI of 1.1 events/h (0-27) of sleep, and a RAI of 1.5 events/h (0-18) of sleep. Median oxygen saturation was 97% (93-99) with a minimum of 89% (72-94) (Table 2), and 1 (2%) patient had an oxygen saturation < 90% for > 5% of TTS.
Fourteen (25%) children were categorized in the no-OSA group. All of these children had primary snoring except 4 who were referred for PSG for repetitive tonsil infections, asthma, TSA, or ADHD. Forty-three (75%) children were in the OSA group: 4 (7%) had UARS, 26 (46%) mild OSA, 6 (10%) moderate OSA, and 7 (12%) severe OSA.
No difference was found for age and body mass index between OSA and no-OSA groups. Compared to no-OSA children, the OSA group had significantly larger tonsils volume (P = .01) with tonsils volume ≥ 3 being more frequent (21% vs 58%, P = .02). There was no significant difference for Mallampati, SHS, adapted ESS, or Conners scores between groups.
Among the 28 (49%) children with tonsils volume ≥ 3 and snoring, 4 (14%) had an OAHI < 1 events/h, 14 (50%) had a mild OSA, and 10 (36%) had a moderate-to-severe OSA.
The feasibility according to age, sex, OSA severity, the presence of comorbidities or neurodevelopmental delay is presented in Table 3. More school-aged children had satisfactory recordings than did preschoolers, 33 (58%) schoolers vs 13 (23%) preschoolers, but without statistical significance (P = .27). No difference was found for feasibility according to sex, 24 (42%) boys and 22 (39%) girls (P = .49). More children with mild OSA had interpretable recordings (20 [35%]) than did no-OSA (14 [25%]), or moderate (5 [9%)] or severe OSA (7 [12%]) (P = .56), and the feasibility was better in children without comorbidities (35 [61%]) than in those with comorbidities (11 [19%]), but with no statistical significance (P = .40). The number of successful PSG-home recordings was higher in children with normal neurodevelopmental status (38 [67%]) than in children with neurodevelopmental delay (8 [14%]), with no statistical difference (P = .95). The multivariate analysis for the predictive factors for the PSG-home feasibility showed that none of the variables entered in the model (age, sex, school age, absence of comorbidities, absence of OSA, and normal neurodevelopmental status) was found to be a significant prognostic factor (Table 4).
Table 3.
Distribution of failed and non-failed ambulatory polysomnography according to age, sex, OSA severity, presence of comorbidities, and neurodevelopmental status.
| No Failure | Failure | Total | P | |
|---|---|---|---|---|
| Age | ||||
| Preschooler (age < 7 years) | 21 (37%) | 6 (11%) | 27 (47%) | P = .60 |
| Schooler (age ≥ 7 years) | 25 (44%) | 5 (9%) | 30 (53%) | |
| Sex | ||||
| Female | 22 (39%) | 4 (7%) | 26 (46%) | P = .49 |
| Male | 24 (42%) | 7 (12%) | 31 (54%) | |
| OSA severity | ||||
| No-OSA | 14 (25%) | 4 (7%) | 18 (32%) | P = .56 |
| Mild OSA | 20 (35%) | 6 (11%) | 26 (46%) | |
| Moderate OSA | 5 (9%) | 1 (2%) | 6 (11%) | |
| Severe OSA | 7 (12%) | 0 | 7 (12%) | |
| UARS | 4 (7%) | 0 | 4 (7%) | |
| Comorbidities | ||||
| No comorbidity | 35 (61%) | 7 (12%) | 42 (74%) | P =.40 |
| Comorbidity | 11 (19%) | 4 (7%) | 15 (26%) | |
| Neurodevelopmental status | ||||
| Normal | 38 (67%) | 9 (16%) | 47 (82%) | P = .95 |
| Delayed | 8 (14%) | 2 (4%) | 10 (18%) |
Table 4.
The multivariate analysis for the predictive factors (DF) for the feasibility of PSG-home.
| Effect | PF | Score Chi-Square | P |
|---|---|---|---|
| Age | 1 | .497 | .48 |
| Schooler | 1 | .282 | .59 |
| Sex | 1 | .470 | .49 |
| No-OSA | 2 | 1.775 | .41 |
| No comorbidity | 1 | .719 | .39 |
| Normal development | 1 | .004 | .95 |
No additional effects met the .2 significance level for entry into the model. PF = predictive factors.
DISCUSSION
This single-center prospective study confirms the feasibility and technical reliability of unsupervised ambulatory PSG, without home settling by sleep technicians, in a population of French children suspected of OSA, even in those with comorbidities. General failure reported herein was relatively low and it was mainly due to loss of the nasal cannula or oximetry signals. The device was installed by the pediatric sleep physician in her office in the late afternoon, and parents were trained to check their child’s sensors every 2 hours, mostly nasal cannula, thoraco-abdominal belts, and oximetry, to increase chances of success.
Compared to two other studies evaluating PSG-home in children,12,18 the proportion of patients with artifact-free signal was slightly lower in the present population (91% and 92% vs 81%), probably due to the more restrictive feasibility criteria applied regarding interpretability. Herein, PSG was considered noninterpretable if the recording time was less than 5 hours (vs < 4 h18), or if 1 or more of the main respiratory signals had artifacts > 75% of the recording time (vs 2 or more of the respiratory signals with artifacts12). Despite the fact that the device was not installed by trained technicians at the child’s home and that nearly a half of the cohort included preschoolers, satisfactory nasal flow signal was obtained in more patients in the present study compared to the other 2 studies, because the parents performed the nurse’s role by checking the sensors every 2 hours during the night. Importantly, in all 3 studies, children slept very well during PSG-home, with a long total sleep time, high sleep efficiency, and low arousal index.
Ambulatory polygraphy is another way to evaluate child’s breathing for OSA diagnosis. In this technique, only the breathing parameters are recorded. Previous studies with these devices have shown a very good positive predictive value, a sensitivity of 88%, and a specificity of 98% for AHI > 5 events/h.2,19 However, contrarily to PSG-home, polygraphy does not assess whether a child sleeps nor in which sleep stage the sleep is. Moreover, arousals related to respiratory events are not recorded. This results in an underestimation of AHI and of OSA severity.7,11
Installment of the device by a technician or medical provider as well as continual monitoring are crucial to successful recordings whether at home or in the laboratory.20 Indeed, it has been shown that when ambulatory polygraphy was installed at home by a layperson after receiving instructions by a technician or medical provider, successful recordings were much lower. The nasal cannula and oximetry are the signals most frequently lost in ambulatory polygraphy, in almost half of the tests.21 The interpretability may increase to 94% if the device is installed by an experienced nurse and a technician is on-call.19 Interestingly, the present results showed that installment at the pediatric sleep physician’s office coupled with continual monitoring by the parents enabled satisfactory results in terms of feasibility. Moreover, parents were very satisfied that the children slept in their own beds and that the delay in obtaining a recording was shorter compared to scheduling a night in the sleep lab.
Herein, no physiological factor was found to influence PSG-home feasibility as no significant difference was seen in general failure according to age, sex, OSA severity, comorbidities, or neurodevelopmental status. Indeed, more school-aged children and more children with mild OSA, with no comorbidities or with normal neurodevelopmental status, had successful recordings, but with no statistical significance, and multivariate analysis found that either status is a prognostic factor for feasibility. The lack of significance could be due to the low number of patients. Accordingly, a report comparing home vs hospital polygraphy concluded that neither age, sex, AHI, nor place of performance (home or hospital) were significantly associated with the interpretability of recordings. However, age and sex were found to be associated with greater error in ambulatory polygraphy vs in-lab PSG, male patients having less error in AHI values from the home test and younger children, aged < 5 years, showing much greater difference in AHI values than older children.20
In recent years, a number of studies in pediatric OSA have evaluated questionnaires for their OSA diagnostic utility, such as the SHS, the pediatric sleep questionnaire,22 or a sleep clinical record.23 The six-item SHS questionnaire, validated in a French population of OSA children as a screening tool to prioritize sleep tests, found that a score ≥ 2.75 is associated with large sensitivity and specificity, and very high negative predictive value for detecting an AHI of ≥ 5s events/h of sleep.6 In the current study, no significant difference was found concerning SHS between OSA and no-OSA children. Nevertheless, more OSA patients presented with SHS ≥ 2.75 vs the no-OSA patients, although without statistical significance, suggesting, as previously noted, the predictive value of SHS for selecting the more severely affected children urgently in need of PSG.
Reported symptoms such as sleepiness or hyperactivity are frequent in OSA children24, due to nighttime sleep fragmentation from arousals and desaturations associated with respiratory events. Scales such as adapted ESS and Conners hyperactivity may help clinicians to determine OSA’s impact on a child’s everyday life. No significant difference was found for adapted ESS or Conners scores in the OSA patients of the present cohort compared to no-OSA and less than a quarter presented an adapted ESS score > 10 or a Conners score > 15. However, it was previously acknowledged that clinical symptoms have high specificity but very low sensitivity, and that questionnaires are poor predictors for an OSA diagnosis in children.25 Thus, PSG remains the gold standard for OSA diagnosis.26,27
According to French health recommendations, a tonsillectomy without a previous PSG recording has to be directly performed in children with tonsils volume ≥ 3 and snoring or other OSA clinical symptoms.28 These criteria applied to almost half of the present population studied. Nevertheless, some of these children may present a simple snoring associated with an OAHI < 1 events/h of sleep, as did 14% in the present cohort. The need for a surgical intervention in this particular subset of patients should be questioned. Such an intervention is performed under general anesthesia and shows a non-negligible morbidity risk, such as for hemorrhage, dehydration, subglottic stenosis, respiratory decompensation, and mortality risk, with 1-2 deaths reported annually per 30,000 surgeries.7,29 Moreover, it has been shown that a non-negligible number (about half) of children with a mild OSA may experience spontaneous recovery.30 Overall, almost two-thirds of the children herein with tonsils volume ≥ 3 would have undergone a surgery that might have been avoided if PSG was systematically performed before the intervention.
The limitations of the present study are the population size and the rather restrictive selection criteria comprising children aged > 3 years old without severe behavior disorders. Moreover, parents had to be reliable and fulfill the nurse’s role to check the right positioning of the sensors every 2 hours. All of these factors may have increased the success rate. Although PSG-home appears feasible, the lead and sensor prices are non-negligible and installation and reading times are long and expensive. In future studies, other simpler and more economical devices such as polygraphy may be useful if their sensitivity is improved. This could be achieved using supplementary tools such as video-monitoring, pulse transit time, peripheral arterial tonometry, mandibular movements, or telemetry.10
CONCLUSION
PSG-home is feasible, less expensive, and represents a good alternative to hospital PSG. In selected patients, it may be proposed as it could prevent unnecessary treatments.
DISCLOSURE STATEMENT
Each author listed on the manuscript has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. The authors report they have no potential conflicts of interest and no financial relationships relevant to this article.
ACKNOWLEDGMENTS
The authors thank Véréna Landel for proofreading the manuscript. Authorship contributions: Conception and study design—Diane Weick, Aurore Guyon, Patricia Franco; patient data collection—Diane Weick, Aurore Guyon; polysomnographic analysis—Diane Weick; statistical analysis—Iulia Ioan, Cyril Schweitzer; interpretation of results—Iulia Ioan, Cyril Schweitzer, Laurianne Coutier, Patricia Franco; manuscript preparation: Iulia Ioan, Diane Weick, Laurianne Coutier, Patricia Franco; final revision—Iulia Ioan, Cyril Schweitzer, Laurianne Coutier, Patricia Franco.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ENT
ear, nose, and throat
- ESS
Epworth Sleepiness Scale
- OAHI
obstructive apnea-hypopnea index
- OAI
obstructive apnea
- OHI
hypopnea
- PSG
polysomnography
- PSG-home
ambulatory polysomnography
- RAI
respiratory-related arousals index
- SDB
sleep-disordered breathing
- SHS
severity hierarchy score
- TST
total sleep time
- UARS
upper airways resistance syndromes
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. 10.1542/peds.2012-1672 [DOI] [PubMed] [Google Scholar]
- 2.Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013;77(12):1960–1964. 10.1016/j.ijporl.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 3.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 4.Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. ERS statement on obstructive sleep disordered breathing in 1- to 23-month-old children. Eur Respir J. 2017;50(6):1700985. 10.1183/13993003.00985-2017 [DOI] [PubMed] [Google Scholar]
- 5.Certal V, Camacho M, Winck JC, Capasso R, Azevedo I, Costa-Pereira A. Unattended sleep studies in pediatric OSA: a systematic review and meta-analysis. Laryngoscope. 2015;125(1):255–262. 10.1002/lary.24662 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen XL, Levy P, Beydon N, Gozal D, Fleury B. Performance characteristics of the French version of the severity hierarchy score for paediatric sleep apnoea screening in clinical settings. Sleep Med. 2017;30:24–28. 10.1016/j.sleep.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 7.Franco P, Bourdin H, Braun F, Briffod J, Pin I, Challamel M-J. Diagnostic du syndrome d’apnée obstructive du sommeil chez l’enfant (2–18 ans): place de la polysomnographie et de la polygraphie ventilatoire. Med Sommeil. 2017;14(2):77–88. 10.1016/j.msom.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 8.Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child. 2006;91(3):233–237. 10.1136/adc.2005.085365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patriquin MA, Mellman TA, Glaze DG, Alfano CA. Polysomnographic sleep characteristics of generally-anxious and healthy children assessed in the home environment. J Affect Disord. 2014;161:79–83. 10.1016/j.jad.2014.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirk V, Baughn J, D’Andrea L, et al. American Academy of Sleep Medicine position paper for the use of a home sleep apnea test for the diagnosis of OSA in children. J Clin Sleep Med. 2017;13(10):1199–1203. 10.5664/jcsm.6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan HL, Kheirandish-Gozal L, Gozal D. Pediatric home sleep apnea testing: slowly getting there!. Chest. 2015;148(6):1382–1395. 10.1378/chest.15-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus CL, Traylor J, Biggs SN, et al. Feasibility of comprehensive, unattended ambulatory polysomnography in school-aged children. J Clin Sleep Med. 2014;10(8):913–918. 10.5664/jcsm.3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin JL, Enright PL, Kaemingk KL, et al. Feasibility of using unattended polysomnography in children for research–report of the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). Sleep. 2001;24(8):937–944. 10.1093/sleep/24.8.937 [DOI] [PubMed] [Google Scholar]
- 14.Liistro G, Rombaux P, Belge C, Dury M, Aubert G, Rodenstein D. High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J. 2003;21(2):248–252. 10.1183/09031936.03.00292403 [DOI] [PubMed] [Google Scholar]
- 15.Inocente CO, Gustin MP, Lavault S, et al. Quality of life in children with narcolepsy. CNS Neurosci Ther. 2014;20(8):763–771. 10.1111/cns.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melendres CS, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768–775. 10.1542/peds.2004-0730 [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin JL, Kaemingk KL, Mulvaney SA, Morgan WJ, Quan SF. Clinical screening of school children for polysomnography to detect sleep-disordered breathing–the Tucson Children’s Assessment of Sleep Apnea study (TuCASA). J Clin Sleep Med. 2005;1(3):247–254. 10.5664/jcsm.26338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142(4):383–389. 10.1067/mpd.2003.28 [DOI] [PubMed] [Google Scholar]
- 20.Scalzitti N, Hansen S, Maturo S, Lospinoso J, O’Connor P. Comparison of home sleep apnea testing versus laboratory polysomnography for the diagnosis of obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2017;100:44–51. 10.1016/j.ijporl.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 21.Moss D, Urschitz MS, von Bodman A, et al. Reference values for nocturnal home polysomnography in primary schoolchildren. Pediatr Res. 2005;58(5):958–965. 10.1203/01.PDR.0000181372.34213.13 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell RB, Garetz S, Moore RH, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(2):130–136. 10.1001/jamaoto.2014.3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villa MP, Paolino MC, Castaldo R, et al. Sleep clinical record: an aid to rapid and accurate diagnosis of paediatric sleep disordered breathing. Eur Respir J. 2013;41(6):1355–1361. 10.1183/09031936.00215411 [DOI] [PubMed] [Google Scholar]
- 24.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769–e778. 10.1542/peds.2005-1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Certal V, Catumbela E, Winck JC, Azevedo I, Teixeira‐Pinto A, Costa‐Pereira A. Clinical assessment of pediatric obstructive sleep apnea: A systematic review and meta‐analysis. Laryngoscope. 2012;122(9):2105–2114. 10.1002/lary.23465 [DOI] [PubMed] [Google Scholar]
- 26.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389–398. 10.1093/sleep/34.3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388. 10.1093/sleep/34.3.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Place et conditions de réalisation de la polysomnographie et de la polygraphie respiratoire dans les troubles du sommeil. Haute Autorité de Santé, SEAP-SEESP. Accessed May 2012. Saint-Denis La Plaine CEDEX, France. https://www.has-sante.fr/plugins/ModuleXitiKLEE/types/FileDocument/doXiti.jsp?id=c_1250993.
- 29.Konstantinopoulou S, Gallagher P, Elden L, et al. Complications of adenotonsillectomy for obstructive sleep apnea in school-aged children. Int J Pediatr Otorhinolaryngol. 2015;79(2):240–245. 10.1016/j.ijporl.2014.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]