Abstract
Study Objectives:
Low serum vitamin D levels are known to be associated with working conditions and poor sleep, but precedent studies on this issue were limited by the absence of objective sleep measurements or clear distinction between daytime and night shift work. Hence, we aimed to examine serum vitamin D levels and sleep in daytime and night-shift workers using actigraphy.
Methods:
A total of 412 night-shift and 432 daytime workers at Seoul National University Bundang Hospital was recruited. All participants completed questionnaires regarding demographic and clinical characteristics. They underwent blood tests for serum vitamin D levels. Objective sleep data were obtained from 150 night-shift workers and 203 daytime workers using actigraphy.
Results:
There was no significant difference in serum vitamin D levels between night-shift and daytime workers after controlling for possible confounders. In daytime workers, vitamin D deficiency was closely related to shorter duration of total sleep time (odds ratio [OR]: 3.07, 95% confidence interval [CI]: 1.51–6.26, P = .002) and higher risk of excessive daytime sleepiness (OR: 2.20, 95% CI: 1.30–3.74, P = .003). Deficient vitamin D was also associated with life quality impairment regarding psychological health (OR: 1.83, 95% CI: 1.07–3.29, P = .028) and social relationship (OR: 1.78, 95% CI: 1.10–2.88, P = .020). However, in night-shift workers, no significant association was observed between serum vitamin D level and sleep parameters, depressive/anxiety symptoms, or quality of life.
Conclusions:
The modest adverse impact of poor vitamin D status on sleep could be attenuated by substantial shift work-related sleep disturbances in night-shift workers. Further studies might be needed to clarify the beneficial effect of vitamin D supplementation for improving sleep and daytime sleepiness in workers with various working conditions.
Citation:
Lee HJ, Choi H, Yoon I-Y. Impacts of serum vitamin D levels on sleep and daytime sleepiness according to working conditions. J Clin Sleep Med. 2020;16(7):1045–1054.
Keywords: actigraphy, excessive daytime sleepiness, night shift work, sleep, vitamin D
BRIEF SUMMARY
Current Knowledge/Study Rationale: Vitamin D deficiency can cause sleep disturbances, and working conditions such as night shift work may influence serum vitamin D levels. However, the relationship among vitamin D, sleep, and working conditions is not yet clearly established.
Study Impact: Night-shift work was not associated with serum vitamin D level. Vitamin D deficiency was directly related to short sleep duration and excessive daytime sleepiness in daytime workers, but not in night-shift workers. Our findings suggest that vitamin D optimization could be recommended for better control of sleep problems among workers without night-shift work and that modification of shift work schedules might need to be considered for night-shift workers.
INTRODUCTION
Vitamin D is photosynthesized in skin via exposure to ultraviolet B (UV-B) in sunlight. It acts as a fat-soluble hormone with numerous physiological functions. Inadequate vitamin D is associated with various health problems including musculoskeletal disorders,1 cardiovascular diseases,2 depression,3 and even cancers.4 Vitamin D also plays a crucial role in sleep regulation. Several studies have demonstrated that vitamin D receptors are expressed in brain regions related to the sleep-wake cycle.5,6 Vitamin D deficiency has been linked to sleep disorders.7 Gominak et al8 have suggested that the world epidemic of sleep disorders could be related to insufficient vitamin D. Moreover, a couple of studies have shown that vitamin D administration can improve sleep quality,9,10 supporting a causal relationship between low vitamin D and sleep disturbances.
Poor sleep has been reported to cause a substantial health and economic burden at workplace, including work injury11 and decreased productivity.12 Working conditions including work schedules could be associated factors for work-related sleep problems. They might also exert an influence on serum vitamin D levels, thereby affecting sleep of the working population. In particular, shift work with night shifts can be a risk factor for vitamin D deficiency, because nighttime workers might have less opportunity for sunlight exposure compared with daytime workers. However, the association between night-shift work and vitamin D status has not been clearly established yet. Several studies have reported that serum vitamin D levels of night-shift workers are lower than those of daytime workers.13–15 However, these studies did not consider confounding factors that might affect serum vitamin D levels, such as age, sex, seasonal variation, or other blood parameters related to vitamin D metabolism.
The direct relationship between vitamin D status and sleep may also be affected by working conditions such as night-shift work. To the best of our knowledge, only one study has evaluated the triadic link among shift work, vitamin D status, and sleep in shift workers. Park and his colleagues16 conducted a study with a large number of full-time workers and found no direct effect of vitamin D on sleep quality in those with shift work. However, the authors evaluated sleep quality based on self-report questionnaires without objective sleep data collection. In addition, shift workers with and without night shifts were classified as a single “shift workers” category. Therefore, further well-designed studies are required for clarifying the effect of serum vitamin D level on sleep in relation to night shift work.
Thus, the objective of the current study was to examine the relationship between serum vitamin D levels and working conditions and explore the impact of vitamin D level on sleep in night-shift and daytime workers. To overcome methodological shortcomings of precedent studies, including the lack of objective sleep assessments and no clear distinction between day and night shifts, this study was performed based on a large number of night-shift and daytime workers using actigraphic sleep measures.
METHODS
Study population
A total of 844 employees of Seoul National University Bundang Hospital (SNUBH) participated in the current study from November 2017 to January 2019. Our study population comprised 412 shift workers and 432 daytime workers aged from 20 to 65 years. In this study, those who worked ≥ 6 night shifts (working hours of 6:00 pm to 8:00 am, 7:00 pm to 7:00 am, or 10:00 pm to 7:00 am) in a month were categorized as “night-shift workers.” Fixed daytime workers (working hours of 9:00 am to 6:00 pm) and shift workers with 2-shift work schedules (working hours of 7:00 am to 3:00 pm or 2:00 pm to 10:00 pm) without night shifts were defined as “daytime workers.” Those with any psychiatric disorders or sleep disorders including sleep-related breathing disorder and restless legs syndrome were excluded. Participants with premorbid severe medical illness such as malignancy, uncontrolled cardiovascular or cerebrovascular disease, hepatic or renal impairment, and respiratory disease were also excluded from the present study. Informed consent was obtained from all participants. This study was approved by the Institutional Review Board of SNUBH (B-1710/424-301).
Serum vitamin D measurement
Serum 25-hydroxyvitamin D [25(OH)D] concentration was adopted as an indicator for serum vitamin D level because it is widely used for assessing and monitoring vitamin D conditions.17 Vitamin D deficiency was defined as serum 25(OH)D < 10 ng/mL, according to the definition by the World Health Organization.18
Blood samples were collected during the daytime (from 10:00 am to 2:00 pm), and they were properly processed and transported to the testing institute (Seoul Clinical Laboratories, Seoul, South Korea). Serum concentration of 25(OH)D was measured using a chemiluminescent immunoassay method. Vitamin D is metabolized in the liver and kidney. Vitamin D metabolism is interconnected to serum calcium and phosphate.19 For this reason, serum levels of calcium, phosphate, creatinine, blood urea nitrogen, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, and gamma glutamyl transpeptidase were also measured as confounding variables. Additionally, seasonal variation of serum vitamin D level was considered as a major confounder due to seasonal difference in sunlight UV-B intensity. Our study participants were categorized into 2 groups according to the season of blood test: “spring and winter (December to May)” group and “summer and autumn (June to November)” group. Precedent studies have revealed that mean levels of serum 25(OH)D in Korean adults are significantly higher in summer and autumn compared with those in spring and winter.20,21
Demographic and clinical characteristics
Demographic and anthropometric data were obtained, including age, sex, body mass index, marital status, education level, current smoking status, drinking habits, caffeine consumption, physical activity, and vitamin D supplementation. As for drinking habits, participants were grouped as positive if they had consumed any alcohol at least once a week. With regard to physical activity, those who performed any kind of exercise for at least 30 minutes each, twice a week were classified as positive. In addition, medical comorbidities such as hypertension, diabetes, gastrointestinal diseases, and chronic pain were examined.
Clinical information was gathered through self-report questionnaires regarding sleep quality, daytime sleepiness, chronotypes, depressive and anxiety symptoms, fatigue, resilience, and quality of life. The Pittsburgh sleep-quality index (PSQI) was used to estimate self-reported sleep quality. A total PSQI score > 5 is considered to demonstrate self-reported poor sleep quality.22 Daytime sleepiness was evaluated with Epworth Sleepiness Scale (ESS). ESS scores of 11–24 represent excessive daytime sleepiness (EDS).23 Chronotypes of participants were assessed with Morningness–Eveningness Questionnaire (MEQ). Those with MEQ scores of 59–86, 42–58, and 16–41 are defined as morning, intermediate, and evening types, respectively.24 Hospital Anxiety and Depression Scale (HADS), which consists of 14 items (7 items each for depression and anxiety), was also used, with higher scores indicating severe symptoms of anxiety and depression.25 The Fatigue Severity Scale (FSS) was also adopted in this study, with global FSS score ≥ 36 meaning abnormal fatigue level.26 The Connor–Davidson Resilience Scale (CD-RISC) was used to evaluate psychological resilience. Its total score ranges from 0 to 100, with higher scores reflecting greater resilience.27 Quality of Life Scale abbreviated version (QOL-BREF) was introduced for the assessment of quality of daily life. The QOL-BREF scale has 4 domains (physical health, psychological health, social relationship, and environmental health). The score of each domain ranges from 0 to 100, with higher scores denoting better life quality in the domain of interest.28 Although there is no established cut-off point for defining poor quality of life, a couple of studies have indicated that a score of less than 60 can adequately detect life quality impairment.29,30
Actigraphic sleep assessment
Of enrolled study participants, those who volunteered were included for objective sleep assessments using wrist actigraphy. Participants were instructed to wear an accelerometer (wGT3X-BT, ActiGraph, LLC, Pensacola, FL) on their nondominant wrist for 14 consecutive days. During the period of actigraphic recordings, individuals kept a daily sleep diary of the time they spent in bed, which was considered as time in bed. The wGT3X-BT estimated sleep-wake status by capturing and recording physical activity. ActiLife 6 software (ActiGraph, LLC, Pensacola, FL) was used to analyze raw data and calculate the following 4 sleep parameters: total sleep time (TST; the number of minutes spent asleep), sleep efficiency (SE, the ratio of TST to time in bed), sleep onset latency (the number of minutes taken to fall asleep), and wake after sleep onset (WASO, the number of minutes of wakefulness after sleep onset). A total of 376 participants underwent actigraphic recordings. Data of participants who made proper use of devices as instructed for at least 7 days were analyzed. Finally, data from 150 night-shift workers and 203 daytime workers were included for the analysis of objective sleep estimates. According to sleep parameters from actigraphy, 3 types of sleep disturbances were defined as follows: TST ≤ 6 hours, SE ≤ 85%, and WASO ≥ 30 min. These cut-off values were determined based on treatment goals of the practice guideline for chronic insomnia disorders in adults.31
Statistical analysis
All statistical analyses were carried out using SPSS version 25.0 for Windows (SPSS, Chicago, IL). Two-tailed P value of < .05 was considered statistically significant for all statistical analyses. Demographic and clinical characteristics were compared between 2 groups using independent t test for continuous variables and chi-square test for categorical variables. Scores of PSQI, ESS, MEQ, HADS, FSS, CD-RISC, and QOL-BREF were analyzed by analysis of covariance. For evaluating differences in serum vitamin D levels according to working conditions and season of blood test, independent t test and analysis of covariance adjusted for possible confounders were used. To identify associated factors for vitamin D deficiency, age, sex, season of blood test, current smoking status, drinking habits, use of vitamin D supplement, and night shift work were included as independent variables for multiple logistic regression analysis. Partial correlation analysis controlling for age and sex was also conducted to examine relationships between serum vitamin D levels and scores of PSQI, ESS, FSS, QOL-BREF, and objective sleep parameters by actigraphy. Finally, odds ratios were determined for both sleep disturbances and life quality impairments by vitamin D deficiency by multiple logistic regression analysis controlling for potential covariates.
RESULTS
Demographic and clinical characteristics
Results of comparison of demographic and clinical information between daytime workers and night-shift workers are described in Table 1. Night-shift workers were female-dominant and younger. They had less body mass index and higher education level. Proportions of being married and current smoker were higher in daytime workers. They consumed more caffeine and less alcohol compared with those with night shifts. In addition, regular physical activity was more common in daytime workers. As expected, night-shift workers experienced poorer sleep quality, more severe degree of daytime sleepiness, anxiety/depressive symptoms, fatigue, and worse quality of life. They were at increased risk of self-reported poor sleep quality (PSQI score > 5) and developing EDS (ESS score > 10). Also, a circadian preference for evening and lower psychological resilience were more frequently found in night-shift workers. With regard to objective sleep estimates, night-shift workers showed greater WASO and lower SE compared with daytime workers. However, there was no significant difference between those with and without actigraphic assessments in age, sex and serum vitamin D levels.
Table 1.
Comparison of demographic and clinical characteristics between night-shift workers and daytime workers.
| Characteristics | Daytime Workers (n = 432) | Night-Shift Workers (n = 412) | P |
|---|---|---|---|
| Sex, male | 43 (10.0) | 25 (6.1) | .038 |
| Age, years | 36.39 ± 11.38 | 29.02 ± 6.99 | < .001 |
| Body mass index, kg/m2 | 21.83 ± 2.82 | 21.08 ± 2.79 | < .001 |
| Marital status, married | 212 (49.1) | 91 (22.1) | < .001 |
| Education, college graduates | 346 (80.1) | 390 (94.7) | < .001 |
| Current smoking | 24 (5.6) | 6 (1.5) | .001 |
| Drinking | 206 (47.7) | 259 (62.9) | < .001 |
| Caffeine consumption, cups/day | 1.29 ± .98 | 1.14 ± .85 | .014 |
| Physical activity | 165 (38.2) | 129 (31.3) | .036 |
| Medical comorbidities | 72 (16.7) | 59 (14.3) | .347 |
| PSQI*, score | 6.17 ± .16 | 8.44 ± .16 | < .001 |
| Self-reported poor sleep quality (PSQI > 5) | 209 (48.4) | 325 (78.9) | < .001 |
| ESS*, score | 7.85 ± .16 | 8.48 ± .16 | .007 |
| Excessive daytime sleepiness (ESS > 10) | 80 (18.5) | 124 (30.1) | < .001 |
| MEQ*, score | 46.97 ± .36 | 42.75 ± .37 | < .001 |
| HADS*, score | 10.95 ± .28 | 13.41 ± .29 | < .001 |
| FSS*, score | 29.68 ± .54 | 34.78 ± .55 | < .001 |
| CD-RISC*, score | 64.04 ± .68 | 61.92 ± .70 | .036 |
| QOL-BREF-physical health*, score | 67.59 ± .73 | 61.50 ± .75 | < .001 |
| QOL-BREF-psychological health*, score | 64.53 ± .76 | 61.14 ± .78 | .003 |
| QOL-BREF-social relationship*, score | 65.09 ± .78 | 62.09 ± .80 | .010 |
| QOL-BREF-environmental health*, score | 67.83 ± .70 | 64.33 ± .72 | .001 |
| Actigraphy*,† | |||
| Sleep onset latency, min | 5.70 ± .29 | 5.60 ± .34 | .834 |
| Total sleep time, min | 391.16 ± 3.05 | 399.52 ± 3.58 | .085 |
| Sleep efficiency, % | 85.94 ± .33 | 83.94 ± .39 | < .001 |
| Wake after sleep onset, min | 58.87 ± 1.67 | 70.47 ± 1.97 | < .001 |
Data are presented as mean ± standard deviation for numerical data and number (percentage) for categorized data. *Data are presented as adjusted means ± standard error. Analysis of covariance was performed after controlling for age and sex. †Analysis was performed for 150 night-shift workers and 203 daytime workers. CD-RISC = Connor–Davidson resilience scale, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, HADS = Hospital Anxiety and Depression Scale, MEQ = Morningness–Eveningness Questionnaire, PSQI = Pittsburgh Sleep Quality Index, QOL-BREF = Quality of Life Scale Abbreviated Version.
Serum vitamin D levels
Table 2 shows the comparison of vitamin D status between daytime workers and night-shift workers. There was a significant difference in mean serum concentration of 25(OH)D between the 2 groups. However, this significance was lost after adjusting for possible confounders such as age, sex, season of blood test, and other blood parameters. In addition, the prevalence of vitamin D deficiency did not differ substantially between night-shift workers and daytime workers. Since vitamin D supplement use was more common in daytime workers compared with that in night-shift workers, 25(OH)D levels were examined after excluding those on vitamin D supplement for additional analysis. Still, no significant difference was found in serum 25(OH)D concentration between night-shift workers and daytime workers after adjusting for confounders (14.06 ng/mL vs 14.17 ng/mL, P = .847).
Table 2.
Comparison of serum vitamin D levels and other blood parameters between night-shift workers and daytime workers.
| Characteristics | Daytime Workers (n = 432) | Night-Shift Workers (n = 412) | P |
|---|---|---|---|
| 25-hydroxyvitamin D, ng/mL | |||
| Crude model | 14.95 ± 8.37 | 13.22 ± 5.79 | < .001 |
| Adjusted model | 14.25 ± .36 | 13.96 ± .37 | .597 |
| Blood parameters | |||
| Calcium, mg/dL | 9.41 ± .34 | 9.27 ± .34 | < .001 |
| Phosphate, mg/dL | 3.79 ± .54 | 4.00 ± .65 | < .001 |
| Creatinine, mg/dL | 0.68 ± .12 | 0.65 ± .10 | .001 |
| Blood urea nitrogen, mg/dL | 12.46 ± 3.29 | 12.32 ± 2.82 | .511 |
| Alanine transaminase, IU/L | 18.88 ± 13.47 | 16.57 ± 11.29 | < .001 |
| Aspartate aminotransferase, IU/L | 22.17 ± 7.29 | 20.42 ± 5.46 | .007 |
| Alkaline phosphatase, IU/L | 57.92 ± 17.69 | 53.37 ± 13.07 | < .001 |
| Total bilirubin, mg/dL | 0.62 ± .22 | 0.64 ± .28 | .247 |
| Gamma glutamyl transpeptidase, IU/L | 18.76 ± 17.73 | 14.50 ± 9.40 | < .001 |
| Season of blood test, n (%) | < .001 | ||
| Spring and winter (December to May) | 247 (57.2) | 300 (72.8) | |
| Summer and autumn (June to November) | 185 (42.8) | 112 (27.2) | |
| Vitamin D supplement use, n (%) | 25 (5.8) | 11 (2.7) | .025 |
| Vitamin D deficiency, n (%) | 146 (33.8) | 133 (32.3) | .661 |
Data are presented as mean ± standard deviation (SD) for numerical data and number (percentage) for categorized data. Crude model: data are presented as mean ± SD. Independent t test was performed. Adjusted model: data are presented as adjusted means ± standard error. Analysis of covariance was performed after controlling for age, sex, season of blood test, serum levels of calcium, phosphate, creatinine, blood urea nitrogen, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, and gamma glutamyl transpeptidase.
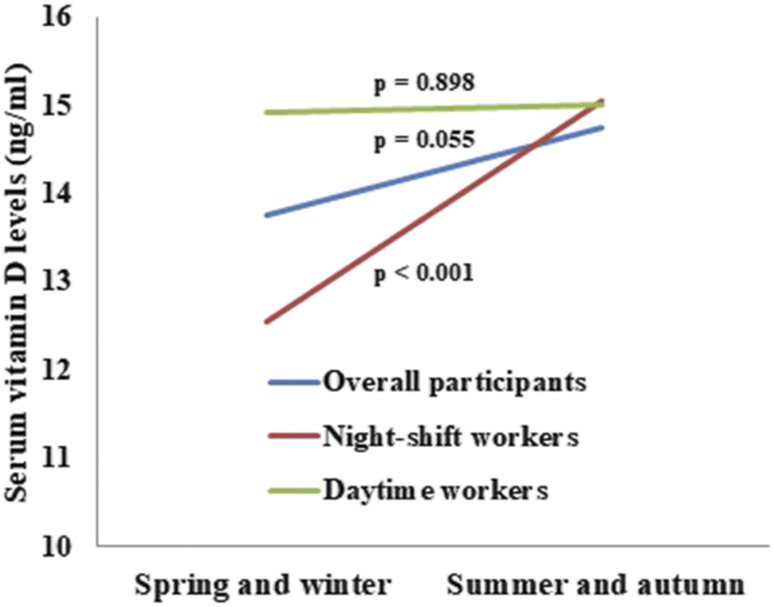
Figure 1 describes the differences of serum vitamin D levels according to the season of blood test after controlling for age, sex, serum levels of calcium, phosphate, creatinine, blood urea nitrogen, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, and gamma glutamyl transpeptidase. In night-shift workers, the mean serum 25(OH)D concentration of those who underwent blood tests during spring and winter was significantly lower than that of those with blood tests during summer and autumn (12.56 ng/mL vs 15.05 ng/mL, P < .001). Meanwhile, there was no significant difference in serum vitamin D level according to the season of blood test in daytime workers (15.01 ng/mL vs 14.90 ng/mL, P = .898). In all participants, there was a trend-level difference in serum vitamin D level between participants with blood tests during winter and spring and those with blood tests during summer and autumn (13.76 ng/mL vs 14.75 ng/mL, P = .055).
Figure 1. Differences of serum vitamin D levels according to the season of blood test.
Night-shift work was not associated with vitamin D deficiency (odds ratio [OR]: 0.81, 95% confidence interval [CI]: 0.59–1.12, P = .197) after multiple logistic regression analysis using all participants. In contrast, blood test during spring and winter season was found to be a risk factor for the development of vitamin D deficiency with the same analysis method (OR: 1.59, 95% CI: 1.14–2.21, P = .006).
Impacts of serum vitamin D on sleep and quality of life
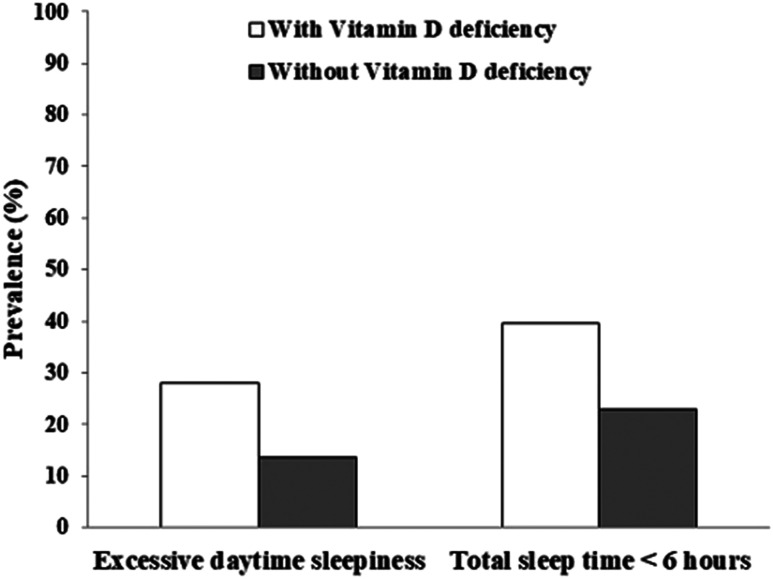
Table 3 compares clinical factors between those with and without vitamin D deficiency among daytime workers. Those with deficient vitamin D showed shorter total sleep time and more severe symptoms of daytime sleepiness. In addition, they had higher proportion of those with short sleep duration (TST ≤ 6 hours) (39.7% vs 23.0%) and EDS (28.1% vs 13.6%) (Figure 2). They also reported more evening-type orientation and worse life quality in domains of psychological health and social relationship. Impacts of vitamin D deficiency among night-shift workers are described in Table 4. No significant difference was observed between the 2 groups in all clinical parameters evaluated, including self-reported sleep quality, actigraphy-measured sleep variables, and other psychiatric/psychological characteristics.
Table 3.
Comparison of clinical characteristics between those with and without vitamin D deficiency among daytime workers.
| Characteristics | With Vitamin D Deficiency (n = 146) | Without Vitamin D Deficiency (n = 286) | P |
|---|---|---|---|
| Sex, male | 7 (4.8) | 36 (12.6) | .010 |
| Age*, years | 34.60 ± 11.16 | 37.30 ± 11.41 | .019 |
| PSQI, score | 6.29 ± .24 | 6.04 ± .17 | .400 |
| Self-reported poor sleep quality (PSQI > 5) | 69 (47.3) | 140 (49.0) | .761 |
| ESS, score | 8.07 ± .24 | 7.36 ± .17 | .018 |
| Excessive daytime sleepiness (ESS > 10) | 41 (28.1) | 39 (13.6) | < .001 |
| MEQ, score | 47.30 ± .59 | 48.79 ± .42 | .042 |
| HADS, score | 11.21 ± .43 | 10.73 ± .31 | .369 |
| FSS, score | 29.21 ± .90 | 27.86 ± .64 | .225 |
| CD-RISC, score | 65.06 ± 1.13 | 64.64 ± .80 | .763 |
| QOL-BREF-physical health, score | 66.04 ± 1.15 | 68.01 ± .81 | .164 |
| QOL-BREF-psychological health, score | 62.53 ± 1.20 | 65.58 ± .85 | .040 |
| QOL-BREF-social relationship, score | 61.84 ± 1.27 | 65.31 ± .91 | .028 |
| QOL-BREF-environmental health, score | 66.33 ± 1.17 | 67.78 ± .83 | .318 |
| Actigraphy† | |||
| Sleep onset latency, min | 5.05 ± .52 | 5.78 ± .37 | .256 |
| Total sleep time, min | 375.20 ± 5.32 | 391.03 ± 3.76 | .017 |
| Sleep efficiency, % | 85.76 ± .54 | 85.88 ± .38 | .863 |
| Wake after sleep onset, min | 57.88 ± 2.67 | 58.82 ± 1.89 | .775 |
Data are presented as adjusted means ± standard error for numerical data and number (percentage) for categorized data. Analysis of covariance was performed after controlling for age and sex. *Data are presented as means ± standard deviation. †Analysis was performed for 203 daytime workers. CD-RISC = Connor–Davidson resilience scale, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, HADS = Hospital Anxiety and Depression Scale, MEQ = Morningness–Eveningness Questionnaire, PSQI = Pittsburgh Sleep Quality Index, QOL-BREF = Quality of Life Scale Abbreviated Version.
Figure 2. Prevalence of excessive daytime sleepiness and short sleep duration in daytime workers.
Table 4.
Comparison of clinical characteristics between those with and without vitamin D deficiency among night-shift workers.
| Characteristics | With Vitamin D Deficiency (n=133) | Without Vitamin D Deficiency (n=279) | P |
|---|---|---|---|
| Sex, male | 4 (3.0) | 21 (7.5) | .072 |
| Age*, years | 29.84 ± 8.26 | 28.63 ± 6.28 | .136 |
| PSQI, score | 8.28 ± .29 | 8.58 ± .20 | .394 |
| Self-reported poor sleep quality (PSQI > 5) | 105 (78.9) | 220 (78.9) | 1.000 |
| ESS, score | 8.38 ± .29 | 8.91 ± .20 | .137 |
| Excessive daytime sleepiness (ESS > 10) | 37 (27.8) | 87 (31.2) | .566 |
| MEQ, score | 41.31 ± .62 | 41.40 ± .43 | .910 |
| HADS, score | 13.46 ± .53 | 13.47 ± .37 | .988 |
| FSS, score | 35.47 ± .94 | 36.57 ± .65 | .338 |
| CD-RISC, score | 60.42 ± 1.20 | 61.48 ± .83 | .467 |
| QOL-BREF-physical health, score | 62.63 ± 1.37 | 61.33 ± .94 | .437 |
| QOL-BREF-psychological health, score | 60.46 ± 1.40 | 61.43 ± .96 | .570 |
| QOL-BREF-social relationship, score | 62.44 ± 1.39 | 63.40 ± .96 | .572 |
| QOL-BREF-environmental health, score | 63.60 ± 1.23 | 65.52 ± .84 | .198 |
| Actigraphy† | |||
| Sleep onset latency, min | 5.43 ± .60 | 5.97 ± .36 | .444 |
| Total sleep time, min | 410.89 ± 6.32 | 405.41 ± 3.79 | .460 |
| Sleep efficiency, % | 84.52 ± .77 | 83.91 ± .46 | .497 |
| Wake after sleep onset, min | 70.40 ± 3.95 | 71.16 ± 2.37 | .869 |
Data are presented as adjusted means ± standard error for numerical data and number (percentage) for categorized data. Analysis of covariance was performed after controlling for age and sex. *Data are presented as means ± standard deviation. †Analysis was performed for 150 night-shift workers. CD-RISC = Connor–Davidson resilience scale, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, HADS = Hospital Anxiety and Depression Scale, MEQ = Morningness–Eveningness Questionnaire, PSQI = Pittsburgh Sleep Quality Index, QOL-BREF = Quality of Life Scale Abbreviated Version.
Correlation analysis results revealed that age was directly proportional to serum 25(OH)D level in both daytime workers (r = .211, P < .001) and night-shift workers (r = .103, P = .036). In daytime workers, serum 25(OH)D concentration was significantly correlated with objective total sleep time (r = .226, P = .001) and the score of social relationship domain of QOL-BREF (r = .105, P =.029). Also, a trend-level inverse correlation was observed between serum vitamin D level and the severity of daytime sleepiness (r = −.083, P = .085). In contrast, among night shift workers, vitamin D status exhibited no significant association with any sleep parameter or life quality index after partial correlation analysis.
Table 5 presents adjusted odds ratios for sleep disturbances and life quality impairments by vitamin D deficiency after controlling for relevant factors that might affect sleep and quality of life, respectively. In daytime workers, vitamin D deficiency was significantly associated with increased risk of EDS (OR: 2.20, 95% CI: 1.30–3.74, P = .003) and short sleep duration (OR: 3.07, 95% CI: 1.51–6.26, P = .002). In this group, vitamin D deficiency was observed to be a risk factor for impaired quality of life related to psychological health (OR: 1.83, 95% CI: 1.07–3.29, P = .028) and social relationship (OR: 1.78, 95% CI: 1.10–2.88, P = .020). However, vitamin D deficiency was not related to sleep disturbance or poor life quality among night-shift workers.
Table 5.
Adjusted odds ratios for sleep disturbances and life quality impairment by vitamin D deficiency.
| Sleep Disturbances and Life Quality Impairment | Vitamin D Deficiency | |||
|---|---|---|---|---|
| Daytime Workers (n = 432) | Night-Shift Workers (n = 412) | |||
| OR (95% CI) | P | OR (95% CI) | P | |
| Self-reported poor sleep quality (PSQI > 5) | 0.78 (.50–1.22) | .281 | .98 (.55–1.74) | .934 |
| Excessive daytime sleepiness (ESS > 10) | 2.20 (1.30–3.74) | .003 | .88 (.54–1.42) | .592 |
| Total sleep time ≤ 6 hours* | 3.07 (1.51–6.26) | .002 | 1.10 (.32–3.74) | .879 |
| Sleep efficiency ≤ 85%* | 1.62 (.86–3.06) | .137 | .76 (.35–1.64) | .489 |
| Wake after sleep onset ≥ 30 min* | 0.61 (.22–1.73) | .352 | .47 (.02–13.43) | .659 |
| QOL-BREF-psychological health score < 60† | 1.83 (1.07–3.29) | .028 | 1.07 (.60–1.91) | .818 |
| QOL-BREF-social relationship score < 60† | 1.78 (1.10–2.88) | .020 | 1.06 (.60–1.88) | .844 |
Multiple logistic regression analysis was performed controlling for age, sex, body mass index, current smoking status, drinking habits, caffeine consumption, physical activity, medical comorbidities, MEQ scores and HADS scores. *Analysis was performed for 150 night-shift workers and 203 daytime workers. †Analysis was performed after controlling for age, sex, body mass index, current smoking status, drinking habits, physical activity, medical comorbidities, CD-RISC = Connor–Davidson resilience scale, CI, confidence interval, ESS = Epworth Sleepiness Scale, FSS = Fatigue Severity Scale, HADS = Hospital Anxiety and Depression Scale, MEQ = Morningness–Eveningness Questionnaire, OR = odds ratio, PSQI = Pittsburgh Sleep Quality Index, QOL-BREF = Quality of Life Scale Abbreviated Version.
DISCUSSION
The present study demonstrated that night shift work had no noticeable association with vitamin D status since no difference was observed in mean serum 25(OH)D concentration between night-shift workers and daytime workers. With regard to the relationship between serum vitamin D and sleep, vitamin D deficiency was closely associated with sleep problems, including excessive daytime sleepiness and short sleep duration among daytime workers. Deficient vitamin D also resulted in life quality impairments in participants without night shift work. However, inadequate vitamin D had no direct effect on sleep or quality of life in night-shift workers.
In our study population, sleep problems, such as self-reported poor sleep quality, sleep maintenance difficulties, and excessive daytime sleepiness, were more common among night-shift workers. Night-shift workers also experienced more severe depression, anxiety, fatigue, and impaired quality of life compared with their day-working counterparts, although they did not have additional diagnoses of depressive or anxiety disorders because we excluded workers with any psychiatric disorders. Many studies have documented harmful health consequences of shift work, such as disturbed sleep/wakefulness,32 depression,16 anxiety,33 fatigue,34 and quality of life.35 Shift work-induced circadian misalignment and stressful working conditions may lead to chronic fatigue, anxiety, depression, neuroticism, and sleep disorders.36
Our results regarding serum vitamin D levels were inconsistent with findings of previous studies, suggesting that shift work might correlate with low vitamin D status.13–15 Differences in statistical analysis methods could be a plausible explanation for such discrepancy. As mentioned above, most of precedent reports were based on the comparison of serum 25(OH)D concentrations by crude analysis without adjusting for confounding variables such as age, sex, seasonal variation, or factors related to vitamin D metabolism. Likewise, significant relationship between night shift work and serum vitamin D levels was found in the present study based on a crude analysis. However, this relationship disappeared after controlling for possible confounders. Similar to our findings, Itoh et al37 observed no significant difference in serum 25(OH)D concentrations among Japanese workers with and without shift work after adjusting for age. Nakamura and his colleagues38 also found no impact of working night shifts on serum vitamin D status after controlling for age and sunshine duration. Another possible interpretation of our negative finding could be that the influence of sex and age may offset the impact of shift work on vitamin D status. A large Korean population-based study showed that women and young adults were more vulnerable to vitamin D deficiency, possibly due to frequent use of sunblock, dress style, and preference for indoor activity.21 Since the majority of our study participants were young women under age 40 years, shift work-related differences in serum vitamin D levels might have been attenuated due to underlying prevailing low serum vitamin D in our study population.
This study revealed a significant seasonal difference in serum vitamin D level among night-shift workers, in line with findings from existing surveys based on the general adult population in Korea.20,21 Interestingly, this seasonal variation was not found among daytime workers. One hypothesis is that night-shift workers might be more vulnerable to the effect of short day-length in winter and spring since they already have less opportunity for sunlight exposure than their daytime counterparts. In addition, daytime workers might have spent less time outside during summer day due to hot and humid weather, which can attenuate the association between vitamin D and seasonal variation of UV-B intensity. Meanwhile, regarding a diurnal rhythm of vitamin D, there is some evidence that the serum vitamin D levels reach the peak at midday and decline in the evening.39,40 However, we obtained all the blood samples during the daytime and thereby the effects of circadian variation of vitamin D might be minimal.
Daytime workers with vitamin D deficiency had an increased risk of manifesting EDS in the present study. Inconsistent with our findings, several studies have reported a significant relationship between symptoms of daytime somnolence and vitamin D. McCarty and colleagues41 revealed an inverse correlation between 25(OH)D levels and sleepiness in those with serum 25(OH)D ≥ 20 ng/mL. Another study based on a large US adult population showed that serum vitamin D levels are inversely associated with daytime sleepiness in adults.42 Also, 2 case studies showed therapeutic effects of vitamin D supplementation for excessive daytime sleepiness in patients with low serum vitamin D.43,44 Although the physiological mechanism behind this relationship has not been clearly established yet, EDS could be a phenomenon of immune dysregulation due to insufficient vitamin D. Precedent studies on this topic have suggested that effects of vitamin D on components of inflammatory pathways, including tumor necrosis factor-alpha, nuclear factor-kappa B, and prostaglandin D2, may decrease central nervous system homeostatic sleep pressure.41,45,46
We discovered a positive correlation between serum vitamin D levels and objectively measured total sleep time among daytime workers. Consistent with this result, vitamin D deficiency was found to be an associated factor for a decrease in total sleep time. A number of previous studies could support these results. The improvement of self-reported sleep duration due to vitamin D supplementation was observed in 20- to 50-year-old people with sleep disorders9 and chronic pain patients.10 A study of Massa et al47 also verified the relationship between low serum vitamin D and short sleep duration objectively measured by actigraphy. In addition, a systemic review of 9 studies on this issue indicated that vitamin D deficiency is linked to short sleep duration.7
Contrary to our expectations, insufficient vitamin D had no significant influence on sleep among night-shift workers. One plausible explanation could be that considerable adverse effects of night-shift work on sleep might have masked the modest impact of inadequate serum vitamin D. Although there are numerous reports dealing with shift work, sleep, and vitamin D, the exact relationships among these 3 variables are not clearly established yet. A pathway analysis demonstrated that while shift work per se may cause both poor sleep quality and poor vitamin D status, serum vitamin D levels were not correlated with sleep quality among shift workers,16 supporting our results. Furthermore, to the authors’ knowledge, the mean serum 25(OH)D concentration of 13.22 ng/mL in night-shift workers was the lowest among all study samples in precedent studies on this topic. Therefore, the relationship between vitamin D and sleep quality might be attenuated due to these extraordinarily low vitamin D levels in our study participants with night-shift work.
Our data suggested that vitamin D deficiency could be associated with life quality impairments concerning psychological health and social relationship in daytime workers. Some previous studies have found that vitamin D supplementation can improve self-reported quality of life, such as mental health and social functioning,10,48 supporting our results. One possible biological mechanism of these findings could be that adequate vitamin D can reduce psychological stress by downregulating inflammatory mediators such as nuclear factor-kappa B.49 However, contrary to our findings, a recent systematic review focusing on vitamin D and health-related quality of life reported that long-term vitamin D supplementation has no beneficial effects on quality of life.50 Further research is required to elucidate the exact relationship between vitamin D and life quality.
This study has several limitations. First, we cannot generalize our findings to all workers with and without night-shift work since most of our study population were female health care workers under age 40 years. In particular, nearly 70% of night-shift workers in our study sample were female nurses in their twenties. They could not represent night-shift workers in the whole working population. Second, we could not obtain information on exposure to sunlight such as the amount of outdoor activity and dietary intake of vitamin D. Hence, residual confounding effects of these potential covariates could affect our results on serum vitamin D levels. Third, objective sleep data were collected from those who volunteered and less than a half of all participants underwent actigraphic assessment. Although we found no difference in basic demographics between participants with and without objective sleep evaluation, selection bias could not be ruled out. Finally, there were individual differences in the frequency of night shifts during the actigraphic recording among night-shift workers. These individual differences in the frequency of night shifts could affect the outcome of our analysis, but these differences might be unavoidable in the real-world work setting.
In conclusion, there was no direct relationship between night-shift work and serum vitamin D levels. Furthermore, insufficient vitamin D was associated with sleep problems in daytime workers, but not in night-shift workers. Shift work-related sleep disturbances in night-shift workers may offset the adverse effect of insufficient vitamin D on sleep. Considering adverse impacts of vitamin D deficiency, vitamin D supplementation should be highly recommended in workers with daytime working hours. Moreover, in addition to the correction of insufficient vitamin D, sleep-promoting shift work schedules such as flexible working hours and less frequent night shifts might be helpful for ameliorating sleep problems in night-shift workers. Further studies are needed to clarify beneficial effects of vitamin D optimization for improving sleep and daytime sleepiness in workers with various working conditions.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Seoul National University Bundang Hospital. Financial support: This study was funded by the Engineering Research Center of Excellence (ERC) Program supported by National Research Foundation (NRF), Korean Ministry of science & ICT (MSIT) (Grant No. NRF-2017R1A5A1014708). The authors report no conflicts of interest.
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- CD-RISC
Connor–Davidson Resilience Scale
- CI
confidence interval
- EDS
excessive daytime sleepiness
- ESS
Epworth Sleepiness Scale
- FSS
Fatigue Severity Scale
- HADS
Hospital Anxiety and Depression Scale
- MEQ
Morningness–Eveningness Questionnaire
- OR
odds ratio
- PSQI
Pittsburgh Sleep Quality Index
- QOL-BREF
Quality of Life Scale Abbreviated Version
- SE
sleep efficiency
- SNUBH
Seoul National University Bundang Hospital
- TST
total sleep time
- UV-B
ultraviolet B
- WASO
wake after sleep onset
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 2.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. 10.1161/HYPERTENSIONAHA.107.087288 [DOI] [PubMed] [Google Scholar]
- 3.Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2018;202(2):100–107. 10.1192/bjp.bp.111.106666 [DOI] [PubMed] [Google Scholar]
- 4.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. 10.2105/AJPH.2004.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyles D, Liu P, Josh P, Cui XJN. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. J Neurosci. 2014;268:1–9. 10.1016/j.neuroscience.2014.02.042 [DOI] [PubMed] [Google Scholar]
- 6.Stumpf W, O’Brien LJH. 1,25 (OH)2 vitamin D 3 sites of action in the brain. Histochemistry. 1987;87(5):393–406. 10.1007/BF00496810 [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between Vitamin D deficiency and sleep disorders: A systematic review and meta-analysis. Nutrients. 2018;10(10):1395. 10.3390/nu10101395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79(2):132–135. 10.1016/j.mehy.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 9.Majid MS, Ahmad HS, Bizhan H, Hosein HZM, Mohammad A. The effect of vitamin D supplement on the score and quality of sleep in 20–50 year-old people with sleep disorders compared with control group. Nutr Neurosci. 2018;21(7):511–519. 10.1080/1028415X.2017.1317395 [DOI] [PubMed] [Google Scholar]
- 10.Huang W, Shah S, Long Q, Crankshaw AK, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. 2013;29(4):341–347. 10.1097/AJP.0b013e318255655d [DOI] [PubMed] [Google Scholar]
- 11.Kling RN, McLeod CB, Koehoorn M. Sleep problems and workplace injuries in Canada. Sleep. 2010;33(5):611–618. 10.1093/sleep/33.5.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, Lerner D. The cost of poor sleep: workplace productivity loss and associated costs. J Occup Environ Med. 2010;52(1):91–98. 10.1097/JOM.0b013e3181c78c30 [DOI] [PubMed] [Google Scholar]
- 13.Olivieri M, Biscardo CA, Valenza D, Verlato G. Night shift, vitamin D and occupational allergies in bakers. Eur Respir J. 2014;44(Suppl 58):4539. [Google Scholar]
- 14.Jeong H, Hong S, Heo Y, et al. Vitamin D status and associated occupational factors in Korean wage workers: data from the 5th Korea national health and nutrition examination survey (KNHANES 2010–2012). Ann Occup Environ Med. 2014;26(1):28. 10.1186/s40557-014-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alefishat E, Farha RA. Determinants of vitamin D status among Jordanian employees: focus on the night shift effect. Int J Occup Med Environ Health. 2016;29(5):859–870. 10.13075/ijomeh.1896.00657 [DOI] [PubMed] [Google Scholar]
- 16.Park H, Suh B, Lee SJ. Shift work and depressive symptoms: the mediating effect of vitamin D and sleep quality. Chronobiol Int. 2019;36(5):689–697. 10.1080/07420528.2019.1585367 [DOI] [PubMed] [Google Scholar]
- 17.Cashman KD, van den Heuvel EG, Schoemaker RJ, Prévéraud DP, Macdonald HM, Arcot J. 25-Hydroxyvitamin D as a biomarker of vitamin D status and its modeling to inform strategies for prevention of vitamin D deficiency within the population. Adv Nutr. 2017;8(6):947–957. 10.3945/an.117.015578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Scientific Group on the Prevention and Management of Osteoporosis. . Prevention and management of osteoporosis: report of a WHO scientific group(WHO technical report series; 921) Geneva: World Health Organization, 2000. [Google Scholar]
- 19.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. 10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu A, Kim J, Kwon O, Oh S-y, Kim J, Yang YJ. Associations between serum 25-hydroxyvitamin D and consumption frequencies of vitamin D rich foods in Korean adults and older adults. Korean J Community Nutr. 2014;19(2):122–132. 10.5720/kjcn.2014.19.2.122 [DOI] [Google Scholar]
- 21.Nah EH, Kim S, Cho H-I. Vitamin D levels and prevalence of vitamin D deficiency associated with sex, age, region, and season in Koreans. Lab Med Online. 2015;5(2):84–91. 10.3343/lmo.2015.5.2.84 [DOI] [Google Scholar]
- 22.Buysse DJ, Reynolds CFIII, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 24.Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52(2):69–77. 10.1016/S0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 26.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 27.Connor KM, Davidson JR. Development of a new resilience scale: The Connor‐Davidson resilience scale (CD‐RISC). Depress Anxiety. 2003;18(2):76–82. 10.1002/da.10113 [DOI] [PubMed] [Google Scholar]
- 28.Group TW. The World Health Organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–1585. 10.1016/S0277-9536(98)00009-4 [DOI] [PubMed] [Google Scholar]
- 29.Silva PAB, Soares SM, Santos JFG, Silva LB. Cut-off point for WHOQOL-bref as a measure of quality of life of older adults. Rev Saude Publica. 2014;48(3):390–397. 10.1590/S0034-8910.2014048004912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva SM, Santana ANC, Silva NNBd, Novaes MRCG. VES-13 and WHOQOL-bref cutoff points to detect quality of life in older adults in primary health care. Rev. Saude Publica. 2019;53:26. 10.11606/S1518-8787.2019053000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med (Lond.). 2003;53(2):89–94. 10.1093/occmed/kqg046 [DOI] [PubMed] [Google Scholar]
- 33.Araújo JF. The impact of different shift work schedules on the levels of anxiety and stress in workers in a petrochemicals company. Estud Psicol Campinas. 2009;26(1):15–23. 10.1590/S0103-166X2009000100002 [DOI] [Google Scholar]
- 34.Shen J, Botly LC, Chung SA, Gibbs AL, Sabanadzovic S, Shapiro CM. Fatigue and shift work. J Sleep Res. 2006;15(1):1–5. 10.1111/j.1365-2869.2006.00493.x [DOI] [PubMed] [Google Scholar]
- 35.Kim YG, Yoon DY, Kim JI, et al. Effects of health on shift-work: general and psychological health, sleep, stress, quality of life. Korean J Occup Environ Med. 2002;14(3):247–256. 10.35371/kjoem.2002.14.3.247 [DOI] [Google Scholar]
- 36.Costa G. Shift work and health: current problems and preventive actions. Saf. Health Work. 2010;1(2):112–123. 10.5491/SHAW.2010.1.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh H, Weng Z, Saito H, et al. Association between night-shift work and serum 25-hydroxyvitamin D levels in Japanese male indoor workers: a cross-sectional study. Ind Health. 2011;49(5):658–662. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Nashimoto M, Matsuyama S, Yamamoto MJN. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: a cross sectional study. Nutrition. 2001;17(11-12):921–925. 10.1016/S0899-9007(01)00662-1 [DOI] [PubMed] [Google Scholar]
- 39.Masood T, Kushwaha RS, Singh R, et al. Circadian rhythm of serum 25 (OH) vitamin D, calcium and phosphorus levels in the treatment and management of type-2 diabetic patients. Drug Discov Ther. 2015;9(1):70–74. 10.5582/ddt.2015.01002 [DOI] [PubMed] [Google Scholar]
- 40.Jones KS, Redmond J, Fulford AJ, et al. Diurnal rhythms of vitamin D binding protein and total and free vitamin D metabolites. J Steroid Biochem Mol Biol. 2017;172:130–135. 10.1016/j.jsbmb.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8(6):693–697. 10.5664/jcsm.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beydoun MA, Gamaldo AA, Canas JA, et al. Serum nutritional biomarkers and their associations with sleep among US adults in recent national surveys. PLoS One. 2014;9(8):e103490. 10.1371/journal.pone.0103490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6(6):605–608. 10.5664/jcsm.27996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson K, Sattari M. Vitamin D deficiency and fatigue: an unusual presentation. Springerplus. 2015;4(1):584. 10.1186/s40064-015-1376-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25 (OH) D concentrations in healthy women. J Inflamm (Lond). 2008;5(1):10. 10.1186/1476-9255-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57(1):63–69. 10.1161/HYPERTENSIONAHA.110.160929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massa J, Stone KL, Wei EK, et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS sleep study. Sleep. 2015;38(2):251–257. 10.5665/sleep.4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzaei A, Zabihiyeganeh M, Jahed SA, Khiabani E, Nojomi M, Ghaffari S. Effects of vitamin D optimization on quality of life of patients with fibromyalgia: A randomized controlled trial. Med J Islam Repub Iran. 2018;32(1):167–172. 10.14196/mjiri.32.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22(4):982–1001. 10.1096/fj.07-9326rev [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann MR, Senior PA, Mager DR. Vitamin D supplementation and health-related quality of life: a systematic review of the literature. J Acad Nutr Diet. 2015;115(3):406–418. 10.1016/j.jand.2014.10.023 [DOI] [PubMed] [Google Scholar]