Abstract
Study Objectives:
There is a well-established association between headache disorders and sleep disturbances in children, but it is unknown whether sleep disturbance plays a role in pediatric intracranial hypertension. The objective of this study was to examine sleep issues related to pediatric intracranial hypertension.
Methods:
Patients with intracranial hypertension in the Pediatric Intracranial Hypertension Clinic were recruited between July 2017 and September 2018. Demographic data were collected from the electronic medical record in addition to patient and parent completed questionnaires. Information on sleep behaviors was gathered using the Children’s Sleep Habits Questionnaire, and control data were obtained from patient siblings. Statistical analyses were performed using paired t tests or two-sample t tests, as appropriate.
Results:
Sixty-two pairs of patients and matched sibling controls were compared. We found a statistically significant difference in total sleep disturbance score (control mean, 44.3; patient mean, 48.1; n = 33 pairs, t = −2.2, P = .035), as well as subscale scores of sleep onset delay (control mean, 1.4; patient mean, 1.7; n = 52 pairs, t = −2.53, P = .014), parasomnias (control mean, 8.5; patient mean, 9.5; n = 42 pairs, t = −2.59, P = .013), and sleep-disordered breathing (control mean, 3.1; patient mean, 3.4; n = 44 pairs, t = −2.61, P = .013). No difference was found in bedtime resistance, sleep duration, sleep anxiety, night awakenings, and daytime sleepiness subscales. Furthermore, no difference was found in total sleep disturbance score between patient subsets, including primary vs secondary intracranial hypertension, body mass index, pubertal status, presence of headaches, or intracranial hypertension treatment.
Conclusions:
This observational study suggests that pediatric intracranial hypertension is associated with a modest increase in sleep disturbances.
Citation:
Kornbluh AB, Thompson K, Mcmahen G, et al. Sleep disturbance in pediatric intracranial hypertension. J Clin Sleep Med. 2020;16(7):1099–1105.
Keywords: intracranial hypertension, pseudotumor cerebri, sleep, pediatric neurology
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is a known bidirectional relationship between headache disorders and sleep disturbances in children, but there is a paucity of literature describing the relationship between sleep and pediatric intracranial hypertension, a form of pediatric headache. This study was performed to characterize sleep-related issues in pediatric patients diagnosed with intracranial hypertension.
Study Impact: Our findings suggest that pediatric intracranial hypertension is associated with a modest increase in sleep disturbances, and, similar to other types of primary pediatric headache disorders, this may open avenues to interventions that could in turn lead to improvement in headaches and quality of life.
INTRODUCTION
Primary intracranial hypertension (PIH) is a condition of unknown causes that produces signs and symptoms of raised intracranial pressure (ICP) and, if left untreated, can result in irreversible vision loss. Headache is the most common symptom at PIH presentation.1,2 Coexisting primary headache disorders, including migraine and tension headache, have been found in conjunction with PIH, and many patients have persistent headaches throughout their clinical course that contribute to reduced quality of life and depression.2,3 There is a well-established predilection for obese postpubertal women, although PIH has also been documented in prepubertal and nonobese patients of both sexes. Among pediatric patients with PIH, subgroups are thought to exist, including a young group that is not overweight, an early adolescent group that is either overweight or obese, and a late adolescent group that is mostly obese.4 Nuances in the presentation, clinical features, comorbidities, and treatment in these less classic subpopulations are not as well characterized in the literature.
The association between headache disorders and sleep disturbances in children is well documented, although the characteristics of sleep in patients with PIH are not thoroughly described.5 Current literature characterizing the relationship between sleep quality and PIH relates to obstructive sleep apnea, primarily through its association with obesity in adults.6 In OSA, it is thought that cycles of apnea result in subtle repetitive episodes of intermittent hypoxemia and hypercapnea, resulting in cerebral vasodilation and surges of arterial blood pressure. Nocturnal elevations in ICP can ultimately lead to the development of papilledema. Not only do anatomical factors in obese women with OSA lead to decreased jugular venous drainage and subsequent elevated ICP, but also it has been hypothesized that mouth breathing in childhood may increase the risk for OSA and raise the risk for increased resistance in the jugular veins and PIH.6 This association is not confined to the obese female patient population; men with PIH have a higher rate of sleep apnea than both women and matched controls, and it is recommended that male patients be evaluated for sleep-disordered breathing.7 Although there is evidence that CPAP may not be optimal treatment for patients with OSA and PIH because of its effects on ICP, cases have been reported in which PIH resolved when treated surgically, including a child with tonsillectomy/adenoidectomy and an adult with counterclockwise maxillomandibular advancement.8,9 Furthermore, weight loss, accomplished by both lifestyle changes and gastric surgery, is a well-established treatment for PIH and obesity-related comorbidities, including OSA.10,11
With the exception of OSA, and limited case reports describing patients with both PIH and narcolepsy, it is unclear whether sleep-disordered breathing plays a role in prepubertal and nonobese populations with PIH, and there is a paucity of literature describing the coexistence of other sleep disturbances in these patients.12,13 Currently, treatment goals for PIH, in addition to preserving vision, include symptomatic treatment for bothersome features like headache. Further establishing the characteristics of sleep and comorbid sleep disorders in children with intracranial hypertension (ICH) could have a significant impact on clinical management strategies.
The aim of this study is to evaluate pediatric patients diagnosed with ICH for sleep-related issues.
METHODS
Patients with ICH diagnosed at Nationwide Children’s Hospital and followed up in the pediatric ICH clinic were recruited between July 2017 and September 2018. Demographic data were collected from the electronic medical record in addition to patient- and parent-completed questionnaires. Information on sleep behaviors was gathered using the Children’s Sleep Habits Questionnaire (CSHQ), a comprehensive, parent-reported sleep screening tool that provides both a total sleep score as well as eight subscale scores that reflect key sleep domains encompassing the major medical and behavioral sleep disorders in school-age children.14 Control data were obtained from questionnaires about patient siblings when available to control for the large environmental influence on sleep quality in a patient’s household. To compare CSHQ outcomes and quantitative demographic variables across patients and controls, statistical analyses were performed using paired t tests using data from patients with a sibling control available. In the case of a categorical demographic variable, patient and control comparisons were made using McNemar’s test to compare paired proportions. For variables in which either the patient or control observation was missing, the pair was excluded from the analysis for this outcome. To compare patient CSHQ outcomes across categorical demographic variables, two-sample pooled t tests were used. A significance level of .05 was used for all analyses.
RESULTS
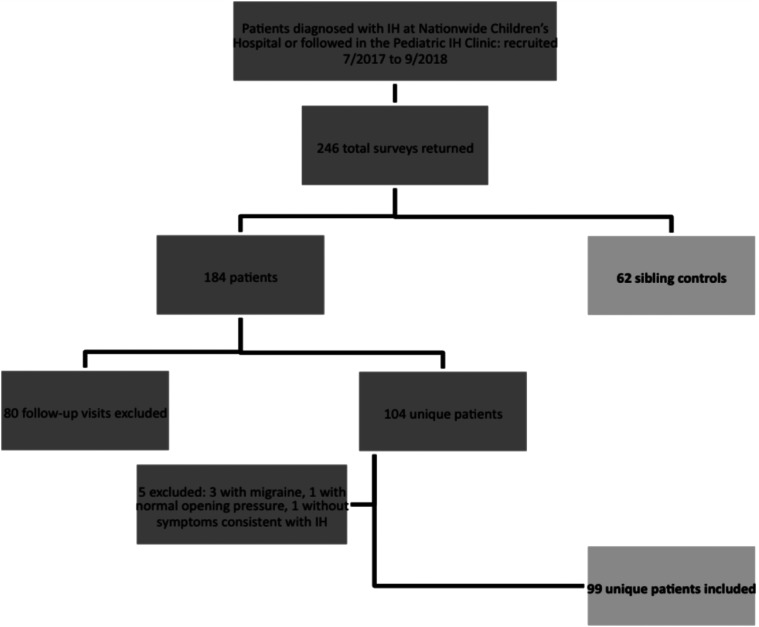
Of the surveys distributed between July 2017 and September 2018, 246 total surveys were returned for analysis. Responses from 184 patients and 62 sibling controls were evaluated; 104 unique patients, some newly diagnosed and some presenting for follow-up appointments, were included. Surveys completed by the same patient during follow-up visits (80 follow-ups in total) were excluded to avoid duplication of sleep data for an individual patient. Five patients from the unique patient group were excluded, including three with headache features more consistent with migraine rather than ICH, one with normal opening pressure and one without any symptoms of ICH. Ninety-nine unique patients were included for analysis (Figure 1).
Figure 1. Flow diagram of patients.
Of the total 246 total surveys returned, ultimately 99 unique patient visits and 62 sibling controls were assessed.
The vast majority of patients included were diagnosed with PIH. Thirty-six patients were diagnosed with secondary intracranial hypertension (SIH), a diagnosis made in patients with evidence of increased ICP resulting from an identified cause. Of the patients with SIH, the most common causes were neoplastic (n = 7), infectious (n = 6), and drug-induced (n = 6) (Table 1).
Table 1.
Causes of secondary intracranial hypertension (SIH).
| Causes of SIH | No. of Patients |
|---|---|
| Neoplastic (acute lymphocytic lymphoma, meningiosarcoma) | 7 |
| Infectious (viral meningitis, adenovirus, and influenza encephalitis, Staphylococcus toxic shock, Lemierre syndrome) | 6 |
| Drug-induced (minocycline, lithium, doxycycline, Accutane) | 6 |
| Cerebrospinal fluid pleocytosis, unknown cause | 3 |
| Periodic fever syndromes (Muckle-Wells) | 3 |
| Chiari malformation | 2 |
| Arachnoid cyst | 2 |
| Autoimmune disorders (scleroderma, Behçet) | 2 |
| Cerebral sinus venous thrombosis | 2 |
| Pineal gland cyst, associated with mild mass effect | 1 |
| Hydrocephalus, unspecified | 1 |
| Demyelinating disorder, unknown | 1 |
There were 36 patients included with SIH. Various causes fell within 12 different categories.
There was no significant difference in sex, pubertal status, or average age across controls and patients. The average age of the patient group was 12.5 years, and the average age of the sibling control group was 12.2 years (t = −.38, P = .7063), but patient ages spanned from the youngest patient (1.9 years) to the oldest patient (21.1 years) (our hospital treats patients up to age 22 years). There was evidence of a difference in body mass index comparison across patients and controls (80.4% sibling controls nonobese vs 48.1% patients nonobese; 51.9% obese patients vs 19.6% obese sibling controls; X2 = 13.24, P = .0003). In addition, each questionnaire asked if there was a perceived problem with sleep in both patients and their sibling controls; evidence was found of a difference in self-reported sleep problems in controls and patients (76.5% sibling controls without self-reported sleep problems vs 47.9% patients without self-reported sleep problems; 52.1% patients with self-reported sleep problems vs 23.5 sibling controls with self-reported sleep problems; X2 = 6.76, P = .0093) (Table 2).
Table 2.
Characteristics of study population.
| Characteristics | Patients | Sibling Controls | Significance | |
|---|---|---|---|---|
| Sex | Male | 38.5% | 51.9% | P = .1936 |
| (n = 20) | (n = 27) | |||
| Female | 61.5% | 48.1% | ||
| (n = 32) | (n = 25) | |||
| Body mass index | Nonobese | 48.1% | 80.4% | P = .0003 |
| (n = 22) | (n = 37) | |||
| Obese | 51.9% | 19.6% | ||
| (n = 24) | (n = 9) | |||
| Pubertal status | Prepubertal | 37.3% | 42.9% | P = .3657 |
| (n = 18) | (n = 21) | |||
| Postpubertal | 62.7% | 57.1% | ||
| (n= 30) | (n = 27) | |||
| Average age | Current age | 12.5 y | 12.2 y | P = .7063 |
| Subjective sleep assessment | No sleep problem | 47.9% | 76.5% | P = .0093 |
| (n = 23) | (n = 36) | |||
| Yes sleep problem | 52.1% | 23.5% | ||
| (n = 24) | (n = 11) | |||
Evidence was found of a difference in the percentages of obese vs nonobese as well as self-reported sleep problems in both controls and patients, but no differences were noted in sex, prepubertal vs postpubertal, or average age across controls and patients.
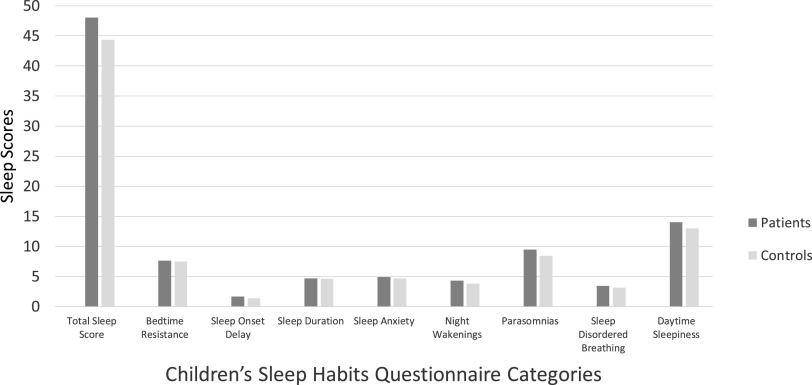
We found a statistically significant difference in the CSHQ total sleep disturbance score between patients and sibling controls (control mean, 44.3; patient mean, 48.1; n = 33 pairs, t = −2.2, P =.035). Breaking down the CSHQ into its components, significant differences across patients and siblings controls included subscale scores of sleep-onset delay (controls, mean 1.4; patients, mean 1.7; n = 52 pairs, t = −2.53, P = .014), parasomnias (controls, mean 8.5; patients, mean 9.5; n = 42 pairs, t = −2.59, P = .013), and sleep-disordered breathing (controls, mean 3.1; patients, mean 3.4; n = 44 pairs, t = −2.61, P = .013). No significant difference was found in bedtime resistance, sleep duration, sleep anxiety, night awakenings, and daytime sleepiness subscales (Figure 2).
Figure 2. Children’s Sleep Habits Questionnaire (CSHQ) scores in patients vs sibling controls.
There was a statistically significant difference in the total sleep disturbance score as well as subscale scores of sleep-onset delay, parasomnias, and sleep-disordered breathing in patients compared with sibling controls.
Specific patient characteristics were further subdivided based on the cause of ICH (primary vs secondary), body mass index (obese vs nonobese), pubertal status (prepubertal vs postpubertal), symptoms (headaches or headache-free), and treatment (taking medications to treat ICH or in remission and not currently taking therapies). Medications used to treat ICH and its associated symptoms included acetazolamide, furosemide, steroids, and topiramate. No significant difference was noted in total sleep disturbance score between patient subsets, including PIH vs SIH, obese vs nonobese, prepubertal vs postpubertal, headaches vs headache-free, or treated vs untreated patients. Significant differences were observed in several CSHQ subscales. Bedtime-resistance scores were higher in nonobese patients compared with obese patients (mean, 8.1 vs. 7.0; P = .019), prepubertal compared with postpubertal patients (mean, 8.4 vs. 6.9; P = .003), and patients not on treatment compared with those taking medications (mean, 8.3 vs. 7.1; P = .047). Sleep-duration scores were lower in prepubertal compared with postpubertal patients (mean, 4.1 vs. 5.1; P = .020) and in patients not taking treatment compared with those taking medications (mean, 4.3 vs. 6.1; P < .001). Similar to bedtime-resistance differences, the sleep anxiety subscale scores were higher in nonobese patients compared with obese patients (mean, 5.4 vs. 4.4; P = .006), prepubertal compared with postpubertal patients (mean, 5.6 vs. 4.4; P = .002), and patients not receiving treatment compared with those taking medications (mean, 5.4 vs. 4.4; P = .028). Higher parasomnia subscale scores were noted in nonobese patients compared with obese patients (mean, 9.6 vs. 8.5; P = .018) and prepubertal compared with postpubertal patients (mean, 9.6 vs. 8.6; P = .035). Daytime sleepiness subscale scores were significantly lower in prepubertal patients compared with postpubertal patients (mean, 12.8 vs. 14.5; P = .048) and patients without headaches compared with those with headaches (mean, 12.5 vs. 14.5; P = .027). CSHQ subscales without significant differences among patient groups included sleep-onset delay, night awakenings, and sleep-disordered breathing. The only patient category subset without any differences in the CSHQ subscales was patients with PIH vs SIH (Table 3).
Table 3.
Sleep scores stratified by subgroups.
| Total Sleep Score | Bedtime Resistance | Sleep- Onset Delay | Sleep Duration | Sleep Anxiety | Night Awakenings | Parasomnias | Sleep-Disordered Breathing | Daytime Sleepiness | |
|---|---|---|---|---|---|---|---|---|---|
| PIH | 47.5 (P = .975) | 7.3 (P = .444) | 1.8 (P = .145) | 4.8 (P = .707) | 4.6 (P = .182) | 4.2 (P = .311) | 8.7 (P = .206) | 3.5 (P = .770) | 13.9 (P = .952) |
| SIH | 47.5 (P = .975) | 7.7 (P = .444) | 1.6 (P = .145) | 4.6 (P = .707) | 5.1 (P = .182) | 4.5 (P = .311) | 9.4 (P = .206) | 3.6 (P = .770) | 14.0 (P = .952) |
| Nonobese | 48.9 (P = .270) | 8.1 (P = .019) | 1.6 (P = .255) | 4.4 (P =.161) | 5.4 (P = .006) | 4.5 (P = .253) | 9.6 (P = .018) | 3.4 (P = .371) | 13.8 (P = .792) |
| Obese | 46.4 (P = .270) | 7.0 (P = .019) | 1.8 (P = .255) | 5.0 (P = .161) | 4.4 (P = .006) | 4.1 (P = .253) | 8.5 (P = .018) | 3.6 (P = .371) | 14.0 (P = .792) |
| Prepubertal | 47.4 (P = .953) | 8.4 (P = .003) | 1.6 (P = .088) | 4.1 (P = .020) | 5.6 (P = .002) | 4.5 (P = .416) | 9.6 (P = .035) | 3.5 (P = .753) | 12.8 (P = .048) |
| Postpubertal | 47.5 (P = .953) | 6.9 (P = .003) | 1.9 (P = .088) | 5.1 (P = .020) | 4.4 (P = .002) | 4.2 (P = .416) | 8.6 (P = .035) | 3.6 (P = .753) | 14.5 (P = .048) |
| Headache-free | 44.8 (P = .089) | 7.3 (P = .669) | 1.6 (P = .318) | 4.7 (P = .950) | 4.7 (P = .636) | 4.0 (P = .271) | 8.4 (P = .103) | 3.5 (P = .952) | 12.5 (P = .027) |
| Headaches | 48.8 (P = .089) | 7.5 (P = .669) | 1.8 (P = .318) | 4.7 (P = .950) | 4.9 (P = .636) | 4.4 (P = .271) | 9.2 (P = .103) | 3.5 (P = .952) | 14.5 (P = .027) |
| No medications | 50.8 (P = .929) | 8.3 (P = .047) | 1.8 (P = .405) | 4.3 (P < .001) | 5.4 (P = .028) | 4.5 (P = .625) | 9.7 (P = .822) | 3.9 (P = .451) | 14.5 (P = .505) |
| Medications | 51.1 (P = .929) | 7.1 (P = .047) | 2.0 (P = .405) | 6.1 (P < .001) | 4.4 (P = .028) | 4.7 (P = .625) | 9.5 (P = .822) | 3.6 (P = .451) | 15.1 (P = .505) |
There was no difference in the total sleep disturbance score between patient subsets, but statistically significant differences included bedtime resistance scores in nonobese vs obese patients, prepubertal vs. postpubertal patients, and no medications vs. medications; sleep duration scores in prepubertal vs postpubertal and no medications vs. medications; sleep anxiety scores in nonobese vs. obese patients, prepubertal vs. postpubertal patients, and no medications vs. medications; parasomnias score in nonobese vs. obese patients and prepubertal vs. postpubertal patients; and daytime sleepiness score in prepubertal vs. postpubertal and headache-free vs. patients with headaches. PIH = primary intracranial hypertension; SIH = secondary intracranial hypertension.
Patients undergoing treatment for ICH, including acetazolamide, furosemide, or other medications, were compared with sibling controls. Comparison of the treated patient population vs control group total sleep scores showed no significant differences (treated patient mean, 46.0; control mean, 43.2; n = 21 pairs, t = −1.4, P = .188). Similarly, patients not currently undergoing ICH treatment were compared with the control group, and no difference in total sleep score (untreated patient mean, 51.8; control mean, 46.3; n = 12 pairs, t = −1.8, P = .107) was found.
DISCUSSION
Although it has been well established that children with headache disorders frequently have sleep disturbances, and improving sleep can result in improved headaches, the pediatric population with ICH has not been previously studied to determine whether sleep disturbance and its treatment play a role in their headaches. In more commonly studied pediatric headache disorders, a bidirectional relationship between poor sleep and headache is recognized. Indeed, there may be an intrinsic pathophysiologic relationship between headache and sleep disorders, as disrupted sleep architecture with reduced REM sleep and slow-wave sleep in severe and chronic migraine headaches has been demonstrated via polysomnography.15 The relationship between pediatric headache and sleep has been extensively defined in the literature on pediatric migraine. For example, in a prospective French study that sought to delineate common triggers in childhood migraine, lack of sleep was identified as the most common culprit.16 A Canadian study with similar design to ours concluded that children with migraine had higher total sleep, sleep delay, and daytime sleepiness scores than did their sibling controls. Patients with more severe migraines had a greater level of sleep disruption compared with those with milder headaches, and worse total sleep scores predicted greater behavioral problems.17 Regarding the role of sleep in migraine treatment, providers frequently encourage healthy lifestyle habits, with an emphasis on proper sleep hygiene not only as a crucial component for migraine prevention but also for the overall success of other migraine treatments.18 The effectiveness of this nonpharmacologic approach was demonstrated in a study that randomly assigned pediatric patients with migraines with poor sleep hygiene to a group who received sleep hygiene counseling vs a group without counseling; this study found that the mean duration and frequency of migraine attacks were significantly reduced upon follow-up in the group who received instruction to improve sleep hygiene.19 The success of nonpharmacologic headache management strategies, including good sleep hygiene, may be particularly helpful in the initial treatment of migraine in the younger preschool-age migraine population.20
The relationship between sleep and headache extends beyond pediatric migraine. An Iranian study demonstrated a link between sleep quality and pain intensity in both children with migraine as well as tension-type headache.21 Another study comparing children with tension-type headache and new daily persistent headache vs those with migraine found that sleep disturbance was greatest in patients with tension-type headache and new daily persistent headache rather than migraine.22 Even though headache is the most common presenting feature and often the most debilitating symptom in pediatric ICH, little is known regarding the role of sleep disorders in this population. This observational study suggests that pediatric ICH is associated with a modest increase in sleep disturbances. The mean total sleep score in patients (48.1) compared with their sibling controls (44.3) was significantly higher (P = .035). Perhaps as expected, sleep-disordered breathing significantly contributed to this difference; but, in addition, there were significant differences in the categories of sleep-onset delay and parasomnias as well. In the CSHQ, a cutoff score of 41 has the best diagnostic confidence (sensitivity of .80, specificity of .72), with higher scores suggesting worse sleep. Both our patient and control groups yielded total sleep scores >41, which may speak to a higher baseline rate of sleep disorders in this population.14
In further examining the subpopulations of patients with pediatric IH, we found that the factors most often associated with sleep-disordered patterns included the presence of obesity, pubertal status, the presence of headaches, and the use of medications to treat ICH. CSHQ total score and subscores were not different based on diagnosis of PIH vs SIH. Differences in sleep scores stratified by subgroups affecting bedtime resistance, sleep duration, sleep anxiety, parasomnias, and daytime sleepiness were most affected by pubertal status and BMI. Our findings may further support the paradigm of distinct subgroups among pediatric ICH patients: a young group with normal body mass index, an early adolescent group that is either overweight or obese, and a mostly obese late adolescent group.4 No statistically significant difference was noted in total sleep scores when comparing patients with ICH undergoing medical treatment vs controls, and likewise no difference was found when comparing untreated patients vs controls. Overall, the trends, however, are similar to those of the total patient group in that patients with ICH had higher total sleep scores than did sibling controls.
This study had several limitations. Although the CSHQ tool has demonstrated good reliability and validity, the questionnaires may reflect parental bias and were at times incompletely or incorrectly completed; the tool is validated for ages 4 to 10 years.14,23 When analyzing our cohort of 4- to 10-year-olds, there does not appear to be any trends between patients vs controls; however, interpretation is very limited owing to small sample size (only five pairs with three completed surveys within this age group). Our study also included patients at different points in their ICH disease process: some newly diagnosed, some undergoing active treatment, and others having completed their treatment course. It is not known whether sleep disturbances can continue beyond treatment for ICH in a manner similar to that of headaches, which may persist despite achieving PIH remission. Subgroup analyses comparing treated and untreated patients with the control group were limited by smaller sample sizes. Future directions should track sleep quality trends as each patient progresses through different stages of the ICH clinical course to determine response in sleep characteristics to ICH treatment. Several patients were referred for polysomnography as a result of this study, and future work should include sleep study results to incorporate objective sleep data with questionnaire information. The difference between patient and control total sleep score was statistically significant, differing by four points in the sleep scale. Several patient subscale scores, however, also met statistical significance; but at times the absolute numerical values comparing controls vs patients were separated by less than a one-point difference in sleep scale. Ultimately, the clinical significance of these findings is unclear, and future avenues of research should examine the impact of these sleep disturbances on quality of life. Nevertheless, this observational study suggests that pediatric ICH is associated with a modest increase in sleep disturbances; and, similar to other types of primary pediatric headache disorders, this finding may open avenues to interventions that could in turn lead to improvement in headaches and quality of life.
DISCLOSURE STATEMENT
All authors have seen and approved the final version of this manuscript. Work for this study was performed at the Nationwide Children’s Hospital, Columbus, Ohio. The authors report no conflicts of interest.
ABBREVIATIONS
- CSHQ
Children’s Sleep Habits Questionnaire
- ICH
intracranial hypertension
- ICP
intracranial pressure
- PIH
primary intracranial hypertension
- SIH
secondary intracranial hypertension
REFERENCES
- 1.Aylward SC, Reem RE. Pediatric intracranial hypertension. Pediatr Neurol. 2017;66:32–43. 10.1016/j.pediatrneurol.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Thurtell MJ, Bruce BB, Newman NJ, Biousse V. An update on idiopathic intracranial hypertension. Rev Neurol Dis. 2010;7:e56–e68. [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SV, Friedman DI. The Idiopathic Intracranial Hypertension Treatment Trial: a review of the outcomes. Headache. 2017;57(8):1303–1310. 10.1111/head.13144 [DOI] [PubMed] [Google Scholar]
- 4.Sheldon CA, Paley GL, Xiao R, et al. Pediatric idiopathic intracranial hypertension: age, gender, and anthropometric features at diagnosis in a large, retrospective, multisite cohort. Ophthalmology. 2016;123(11):2424–2431. 10.1016/j.ophtha.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isik U, Ersu RH, Ay P, Save D, Arman AR, Karakoc F, Dagli E. Prevalence of headache and its association with sleep disorders in children. Pediatr Neurol. 2007;36(3):146–151. 10.1016/j.pediatrneurol.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 6.Wardly DE. Intracranial hypertension associated with obstructive sleep apnea: a discussion of potential etiologic factors. Med Hypotheses. 2014;83(6):792–797. 10.1016/j.mehy.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Bruce BB, Kedar S, Van Stavern GP, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72(4):304–309. 10.1212/01.wnl.0000333254.84120.f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalyoussef E, Brooks NO, Quraishi H, Turbin R, Frohman L. Idiopathic intracranial hypertension in a child with obstructive sleep apnea cured by tonsillectomy/adenoidectomy. J Neuroophthalmol. 2013;33(4):413–414. 10.1097/WNO.0000000000000059 [DOI] [PubMed] [Google Scholar]
- 9.Wardly D, Wolford LM, Veerappan V. Idiopathic intracranial hypertension eliminated by counterclockwise maxillomandibular advancement: a case report. Cranio. 2017;35:259–267. 10.1080/08869634.2016.1201634 [DOI] [PubMed] [Google Scholar]
- 10.Sugerman HJ, Felton WL, Salvant JB, Sismanis A, Kellum JM. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology. 1995;45(9):1655–1659. 10.1212/WNL.45.9.1655 [DOI] [PubMed] [Google Scholar]
- 11.Sugerman HJ, Felton WL, Sismanis A, Kellum JM, DeMaria EJ, Sugerman EL. Gastric surgery for pseudotumor cerebri associated with severe obesity. Ann Surg. 1999;229(5):634–640. 10.1097/00000658-199905000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goald HJ, Lloyd RA. Lumbar subarachnoid fallopian shunt in the treatment of pseudotumor cerebri in a patient with narcolepsy. J Natl Med Assoc. 1968;60(3):181–183. [PMC free article] [PubMed] [Google Scholar]
- 13.Rossor T, Lim M, VanDenEshof K, Gringas P. Pseudotumor cerebri syndrome in a patient with narcolepsy type 1. Eur J Paediatr Neurol. 2018;22(1):194–198. 10.1016/j.ejpn.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 14.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. 10.1093/sleep/23.8.1d [DOI] [PubMed] [Google Scholar]
- 15.Vendrame M, Kaleyias J, Valencia I, et al. Polysomnographic findings in children with headaches. Pediatr Neurol. 2008;39(1):6–11. 10.1016/j.pediatrneurol.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 16.Solotareff L, Cuvellier JC, Duhamel A, Vallee L, Tich SNT. Trigger factors in childhood migraine: a prospective clinic‐based study from North of France. J Child Neurol. 2017;32(8):754–758. 10.1177/0883073817705251 [DOI] [PubMed] [Google Scholar]
- 17.Heng K, Wirrell E. Sleep disturbance in children with migraine. J Child Neurol. 2006;21(9):761–766. 10.1177/08830738060210092201 [DOI] [PubMed] [Google Scholar]
- 18.Guidetti V, Dosi C, Bruni O. The relationship between sleep and headache in children: implications for treatment. Cephalalgia. 2014;34(10):767–776. 10.1177/0333102414541817 [DOI] [PubMed] [Google Scholar]
- 19.Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia. 1999;19(Suppl 25):57–59. 10.1177/0333102499019S2516 [DOI] [PubMed] [Google Scholar]
- 20.Eidlitz-Markus T, Haimi-Cohen Y, Steier D, Zeharia A. Effectiveness of nonpharmacologic treatment for migraine in young children. Headache. 2010;50(2):219–223. 10.1111/j.1526-4610.2009.01534.x [DOI] [PubMed] [Google Scholar]
- 21.Cheraghi F, Shamsaei F, Yeganeh F, Roshanaei G, et al. Comparison of the quality of sleep and intensity of headache between migraine, tension headache, and healthy children. Iran J Child Neurol. 2018;12(4):45–54. [PMC free article] [PubMed] [Google Scholar]
- 22.Rabner JA, Kaczynski KJ, Simons LE, LeBel A. Pediatric headache and sleep disturbance: a comparison of diagnostic groups. Headache. 2018;58(2):217–228. 10.1111/head.13207 [DOI] [PubMed] [Google Scholar]
- 23.Goodlin-Jones BL, Sitnick SL, Tang K, Liu J, Anders TF. “The Children’s Sleep Habits Questionnaire in Toddlers and Preschool Children”. JDBP. 2008;29(2):82–88. [DOI] [PubMed] [Google Scholar]