Citation:
Won CHJ. When will we ditch the AHI? J Clin Sleep Med. 2020;16(7):1001–1003.
Obstructive sleep apnea (OSA) is a disorder that describes repetitive upper airway obstruction during sleep, leading to increased work of breathing, blood gas derangements, intrathoracic pressure swings, autonomic imbalances, and fragmented sleep. Even though the body is undergoing many physiologic disturbances (measured in exquisite detail over many hours), we continue to define the disease by one metric—the apnea-hypopnea index, or AHI. The AHI quantifies the average number of apneas and hypopneas per hour of sleep, regardless of associated hypoxemia, hypercarbia, arousals, hemodynamic changes, etc. An AHI ≥ 5 events/h diagnoses OSA, and an AHI ≥ 30 events/h arguably arbitrarily defines “severe OSA”. However, as anyone who reads sleep studies appreciates, not all apneas or hypopneas are the same (between individuals or even within an single night; Figure 1). By distilling OSA into this single metric, we disregard other potentially impactful disease characteristics, such as event duration, hypoxemia burden, arousal intensity, heart rate variability, sleep stage, or body position dependence. It is perhaps this overreliance on a single static metric, the AHI, that has led to inconsistent findings or only modest predictions of cardiovascular outcomes,1,2 or that has contributed to negative results in therapeutic trials in OSA.3–5
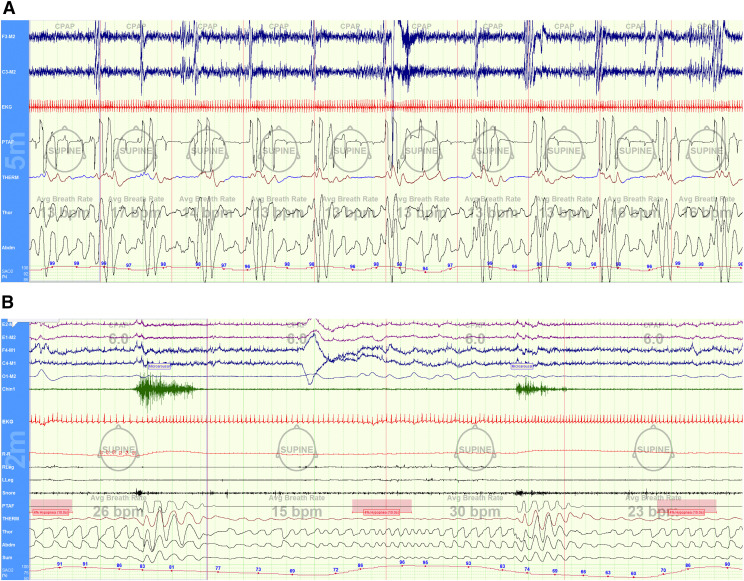
Figure 1. Each of these patients was categorized as “severe OSA” with AHI > 30 events/h.
In both clinical and research contexts, they may be considered similar. However, patient (A) had very short obstructive apneas (2-breaths duration) without significant oxygen desaturations; whereas patient (B) had very long apneas lasting over 20-breaths duration, which are associated with paradoxical breathing and very severe oxygen desaturations to a nadir SpO2 60%. Patient (A) showed frequent electroencephalogram disturbances and greater occurrences of heart rate variability. Patient (B) was notably having REM-related events.
Several studies have attempted to identify polysomnographic traits that may better predict cardiovascular disease. For example, Oldenburg et al6 showed the amount of total sleep time with SpO2 < 90% (TST90) better predicted all-cause mortality in OSA patients with heart failure even after adjustment for confounding factors. In their study, the risk of death increased by 16% for every hour of TST90. Azarbarzin et al7 computed a measurement of “hypoxic burden” by measuring the area under the oxygen desaturation curve during an obstructive event. They found that hypoxic burden from OSA was better than AHI at predicting cardiovascular endpoints. Butler et al8 found that shorter duration of obstructive events predicted all-cause mortality over and beyond that predicted by the AHI. Event duration is a heritable trait reflecting arousability. Those who tend to have shorter obstructive events may be prone to greater sleep fragmentation and sympathetic activity, which are detrimental to cardiovascular health. Finally, data is amassing to suggest that the pathogenesis and sequelae of OSA are sleep-stage dependent. Obstructive events during REM sleep have been shown to better predict cardiovascular outcomes than the overall AHI or non-REM events.9
Similarly, in this issue of the Journal of Clinical Sleep Medicine, Wang et al10 compared oxygen desaturation rates (ODR) as a novel parameter of intermittent hypoxemia in patients with severe OSA (AHI ≥ 30 events/h). They found that the rate of change in SpO2 during an obstructive apnea varied between individuals such that patients could be categorized as having either “fast” or “slow” ODR. They found that those that desaturated quickly experienced higher sleep and awake blood pressures, as well as greater blood pressure and heart rate variability. The authors hypothesized that a quick descent of SpO2 sensitizes chemoreflex responses and sympathetic activity, while slow desaturations generate hypoxemia-conditioning that results in less exaggerated hemodynamic responses. A notable confounder was that the fast ODR group was more obese than the slow group. Obesity is an independent risk factor for hypertension, and it is possible obesity itself may have contributed to these findings. However, when they compared the ODR with other polysomnography measurements, the authors found that the ODR outperformed the AHI, oxygen desaturation index, TST90, or the minimum and mean SpO2 in predicting cardiovascular outcomes. The Wang et al study recapitulates the need to characterize OSA beyond the AHI, and exemplifies a movement toward precision medicine.
The importance of precision medicine is that it allows for better risk stratification and targeted treatment. Positive airway pressure therapy, while highly efficacious in reducing AHI, is not well tolerated by many patients, nor does it uniformly attenuate cardiovascular risk. Medicine of the future is unlikely to continue this one-therapy-fits-all approach. Instead, we may identify alterative therapeutic strategies that target disease phenotypes. In personalized medicine, there may be more of a role for adjunctive oxygen, respiratory stimulants, or hypnotics, for example. As the Wang et al study demonstrates, we need better methods for understanding disease traits and acquiring personalized diagnostics.
Unfortunately, the overreliance on AHI as the sole metric for defining OSA has led to the pursuit of oversimplified diagnostic tools. While our approach to therapeutics moves toward better understanding of disease phenotypes, our diagnostic strategies stray toward simplified testing, like overnight oximetry, snore pattern detectors, and nontouch breathing sensors, that aim to corroborate AHI. As a result, we miss the opportunity to fully characterize OSA and to better understand disease traits that may affect cardiovascular or other risks, and importantly, that may better inform treatment strategies and outcomes.
DISCLOSURE STATEMENT
The author has seen and approved the manuscript. The author reports no conflicts of interest.
REFERENCES
- 1.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. 10.1016/S0140-6736(05)71141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 3.Barbé F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. 10.1001/jama.2012.4366 [DOI] [PubMed] [Google Scholar]
- 4.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. . Engl J Med. 2016;375(10):919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 5.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37(21):1695–1703. 10.1093/eurheartj/ehv624 [DOI] [PubMed] [Google Scholar]
- 7.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019;40(14):1149–1157. 10.1093/eurheartj/ehy624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler MP, Emch JT, Rueschman M, et al. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am J Respir Crit Care Med. 2019;199(7):903–912. 10.1164/rccm.201804-0758OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mokhlesi B, Finn LA, Hagen EW, et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190(10):1158–1167. 10.1164/rccm.201406-1136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, Meng Z, Ding N, et al. Oxygen desaturation rate as a novel intermittent hypoxemia parameter in severe obstructive sleep apnea is strongly associated with hypertension. J Clin Sleep Med. 2020;16(7):1055–1062. 10.5664/jcsm.8396 [DOI] [PMC free article] [PubMed] [Google Scholar]