Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a common condition with significant symptoms and long-term adverse cognitive, mental health, vascular, and respiratory sequelae. Physical activity has been recognized as a key determinant for good health and has been associated with lower risk of these sequelae. We hypothesized that increased physical activity may be associated with a decreased prevalence of OSA.
Methods:
This cross-sectional study used baseline questionnaire data from the Ontario Health Study, a population-based cohort of residents of Ontario, Canada. Participants were adults who provided lifestyle, medical, socio-demographic, and sleep health information. The study sample consisted of 155,448 men (39.8%) and women (60.2%). The prevalence of physician-diagnosed OSA in this cohort was 6.9%. Logistic regression models were used to investigate the association of OSA with physical activity. Missing data were imputed using a multiple imputation by chained equation approach.
Results:
In multivariable analyses adjusted for potential confounding factors, increased total physical activity (metabolic equivalent [h/wk]) (odds ratio [OR] = .98, 95% confidence interval [CI] = .96 to 1.00), vigorous-intensity activity (OR = .98, 95% CI = .97 to 1.00), and walking (OR = .98, 95% CI = .96 to 1.00) were all associated (all P .045) with decreased prevalence of OSA. Moderate-intensity activity was not associated with risk of OSA (P = .826).
Conclusions:
Independent of known risk factors for OSA, including body mass index, increased levels of physical activity, including walking, were associated with a prevalence of OSA. Our results highlight the importance of physical activity as a preventive measure for sleep apnea.
Citation:
Hall KA, Singh M, Mukherjee S, Palmer LJ. Physical activity is associated with reduced prevalence of self-reported obstructive sleep apnea in a large, general population cohort study. J Clin Sleep Med. 2020;16(7):1179–1187.
Keywords: physical activity, population health, sleep apnea, sleep health
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is a common but underdiagnosed chronic condition requiring long-term clinical monitoring and treatment. Physical inactivity and sedentary behavior are increasingly recognized as risk factors for poor health. It is largely unknown whether such inactivity is associated with increased risk of OSA.
Study Impact: In a large, population-based, multi-ethnic sample of adults from Canada we found significant associations between increased physical activity and lower prevalence of OSA. Subjects diagnosed with OSA, on average, report performing less physical activity than those without a diagnosis of sleep apnea. Further research is needed to investigate the intensity and duration of physical activity required to reduce the risk of sleep apnea.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder, with a current estimated global prevalence of 13–33% and 6–19% in middle-aged men and women, respectively.1 OSA is severely under-diagnosed, with the majority of affected individuals in the community undiagnosed.2 OSA has been estimated to affect ∼6% of Canadian adults and ∼13% adults aged 60 to 79 years, with a significantly higher prevalence in men.3 OSA is characterized by repeated episodes of apnea (no airflow) and hypopnea (partially obstructed airflow) that occur during sleep. This results in sleep fragmentation and hypoxemia, which lead to excessive daytime sleepiness,4 impaired cognitive functioning,5 increased risk of motor vehicle and occupational accidents,6 and reduced quality of life.7 OSA is associated with early mortality8 and serious long-term cognitive,9 mental health,7,10 cardiovascular, cerebrovascular, metabolic, and respiratory sequelae.1,11–13 A key metric used to diagnose OSA and as a measure of disease severity is the apnea-hypopnea index (AHI), a count of the number of airway obstructions per hour of sleep.
OSA is a chronic condition that usually requires long-term monitoring and treatment. Continuous positive airway pressure (CPAP) therapy is considered the gold standard treatment for moderate to severe sleep apnea.14 However, poor patient adherence can reduce the effectiveness of CPAP.15 Treatment with oral appliances, such as mandibular advancement devices, or oropharyngeal surgery can be effective in selected patients.16 Patients may also benefit from lifestyle changes such as weight loss, exercise, and dietary control.17
Physical activity and exercise have long been recognized as key determinants of good health.18 Prior studies have shown a consistent beneficial association of physical activity with amelioration of OSA-associated clinical outcomes such as impaired cognition,19 depression,20 and cardiovascular disease (CVD).21 In contrast, the potential role of physical activity as an independent risk factor for OSA has been largely ignored in the sleep epidemiological literature. Prior research has generally addressed the association of physical activity with secondary outcomes in patients with OSA, such as disease severity and comorbid outcomes. Exercise programs for patients with OSA (usually with comorbid CVD) have been shown to mitigate adverse clinical outcomes associated with OSA, including cardiovascular disorders, glucose intolerance, and fatigue.22–24 In a network meta-analysis of 80 randomized controlled trials, exercise was shown to be the second most effective treatment for OSA behind CPAP in terms of reducing disease severity (ie, AHI) in those with diagnosed OSA.16 Exercise was found to be more effective than CPAP in OSA patients at reducing self-reported daytime sleepiness as measured by the Epworth Sleepiness Scale.16 However, the mechanisms by which exercise attenuates OSA severity remain unclear.25 Potential explanations for the beneficial effects of exercise on OSA severity include increased upper airway dilator muscle tone, reduced fluid accumulation in the neck, increased proportion of slow-wave sleep, reduced body weight, and reduced systemic inflammatory response.17
The Ontario Health Study (OHS) is a population-based cohort of volunteer adults resident in the Canadian province of Ontario. Using cross-sectional baseline questionnaire data from the OHS, we evaluated the association of physical activity with diagnosed OSA among participants. We hypothesized that, independent of potential confounding factors, increased physical activity would be associated with a lower prevalence of OSA. Ours is the largest research study to date to report the interrelationships between physical activity and physician diagnosed OSA.
METHODS
Study design
The OHS is a population-based cohort study comprising a large, ethnically diverse sample from the province of Ontario, Canada.26 The current study analyzed the self-reported data collected at baseline recruitment from adult (age ≥ 18 years) residents of Ontario using a web-based questionnaire. Data collected included health-related behavior, personal medical history, education history, socio-demographic characteristics, and health service utilization. Data provided as part of this study were collected from study participants between September 29, 2010 and April 29, 2013. Institutional review ethics board approval was obtained (Women’s College Hospital REB#2013-0010-E). Informed consent was obtained from all volunteer participants.
Questionnaire data
All questions asked (with the exception of current age and sex) had the additional possible answers of “don’t know” and “prefer not to answer.”
Sleep-related variables
OSA status was recorded based on the question: “Has a doctor ever told you that you had any of the following conditions?” The “Yes” responses were treated as “diagnosed OSA”; responses of “No” were treated as “no diagnosed OSA.” Self-reported sleep duration was recorded based on the response to the question: “On average, how many hours per day do you usually sleep, including naps?” The response was recorded as a continuous variable in hours and minutes. Sleep quality was determined by the response to the question: “How often do you have trouble going to sleep or staying asleep?” Possible responses were “never,” “part of the time,” “some of the time,” “most of the time,” or “all of the time.”
Physical activity
The International Physical Activity Questionnaire (IPAQ)27 was used to assess physical activity. Four physical activity measures were derived from IPAQ activity scores, one for each type of physical activity: vigorous-intensity activity, moderate-intensity activity, walking, and total physical activity (the sum of the 3 activity scores). The questions asked were “During the last 7 days, on how many days did you do vigorous physical activities like heavy lifting, digging, aerobics or fast bicycling?’; “During the last 7 days, on how many days did you do moderate physical activities like carrying light loads, bicycling at a regular pace or doubles tennis?”; and “During the last 7 days, on how many days did you walk for at least 10 minutes at a time?”. All physical activity measures were recorded as metabolic equivalents (MET-min/wk).26 The IPAQ sedentary score was calculated as “Total sitting min/week,” based on the question “During the last 7 days, on how much time did you spend sitting on a weekday?”.
As recommended, any measure of physical activity (walking, moderate or vigorous activity) greater than 180 minutes (3 hours) per day was truncated at 180 minutes.28 All variables were converted to MET-h/wk for ease of interpretation in analyses and were loge-transformed before analysis to reduce heteroscedasticity (adding 1 to allow transformation of zero values).
Socio-demographic variables
Current age and sex were self-reported by participants. Participants body mass index (BMI; kg/m2) was calculated using self-reported height and weight; BMI values of less 14 and greater than 58 were truncated to 14 or 58.29 Comorbidities of participants investigated were self-reported physician diagnosed conditions (ie, “Has a doctor ever told you that you had any of the following conditions?”). Conditions reported included: high blood pressure (hypertension), blood glucose disorder (high blood glucose/sugar), diabetes (gestational, type 1, or type 2), high cholesterol, depression, stroke, and cardiovascular conditions (heart disease and myocardial infarction). Ethnic background (ie, “What is your ethnic background?” was self-reported as membership of one or more of the following categories as per Canadian census definitions: 1 = Aboriginal, 2 = Arab, 3 = Black, 4 = Chinese, 5 = Filipino, 6 = Japanese, 7 = Korean, 8 = Latin American/Hispanic, 9 = South Asian, 10 = Southeast Asian, 11 = West Asian, 12 = White. Where more than one ethnicity was recorded, ethnicity was defined as “Mixed.” Further socio-demographic variables assessed included geographic residential status (rural or urban, according to Canada Post designations), education level (ie, “What is the highest level of education you have completed?”, with responses: 1 = none, 2 = elementary, 3 = high school, 4 = trade, 5 = diploma, 6 = certificate, 7 = bachelor's, 8 = graduate degree), current working status (ie, “Which of the following best describes your current employment status?”, with responses 1 = full-time employed/self-employed, 2 = part-time employed/self-employed, 3 = retired, 4 = looking after home and/or family, 5 = unable to work because of sickness or disability, 6 = unemployed, 7 = Doing unpaid or voluntary work, 8 = Student), current shift work status (ie, “Which of the following best describes your working schedule in your current job?”, with responses 1 = regular daytime shift, 2 = regular evening shift, 3 = regular night shift, 4 = rotating shift, 5 = split shift, 6 = irregular schedule/on call, responses of 2–6 were coded as 1 = shift worker, response of 1 were coded as 0 = not a shift worker), smoking status (ie, “Do you smoke cigarettes daily, occasionally, or not at all?”, with responses 1 = do not smoke, 2 = occasionally smoke, 3 = smoke daily). Alcohol consumption (ie, “On average, over the last year, how often did you drink alcohol?”) was assessed as: 0 = never, 1 = less than monthly, 2 = about once a month, 3 = 2 to 3 times a month, 4 = once a week, 5 = 2 to 3 times a week, 6 = 4 to 5 times a week, 7 = 6 to 7 times a week.
Statistical analysis
Data were analyzed using the R statistical software (R Core Team, Vienna, Austria; version 3.5.1).30 Descriptive statistics for normally distributed continuous variables used mean and standard deviation; median and interquartile range (IQR) were used for nonnormal continuous variables; frequencies and percentages were used for categorical variables. The association of dichotomous physician diagnosed OSA status were analyzed using χ2, Student’s t test, or a Mann–Whitney U test, depending upon the distribution of the independent variable being compared.
To investigate the multivariable association of OSA with the 4 physical activity measures, logistic regression models were fitted. The principal outcome was OSA status. The principal, continuous explanatory variables were loge-transformed walking, loge-transformed moderate-intensity activity, loge-transformed vigorous-intensity activity, and loge-transformed total physical activity. Log transformations of the physical activity measures were used as the dependent variables in the logistic regression models because the distributions of the 4 variables were skewed. Potential additional covariates investigated in multivariable modeling included age, sex, BMI, physician diagnosis of morbidities (high blood pressure [hypertension], high blood glucose, diabetes [gestational, type 1, or type 2], high cholesterol level, depression, stroke, sleep apnea, cardiovascular conditions [heart disease and previous myocardial infarction], ethnicity, smoking status, alcohol consumption, highest educational level, residential location [rural/urban], employment status, and current shift worker status).
Three logistic regression models were fitted; first, with each of the 3 individual physical activity measures and then with the combined measure, “total physical activity,” the sum of the individual measures. Six models were thus fit in total. Model 1 was an unadjusted model, including only the physical activity measures; model 2 was adjusted for all significant covariates, excluding BMI; and model 3 was adjusted for all covariates including BMI. The models were fit using a purposeful selection method.31 Interaction terms for sex:BMI, age:BMI, physical activity measures:BMI, and physical activity measures:sex were investigated.
In secondary analyses, we investigated the relationship between OSA status and sleep duration and between OSA status and sleep quality. We also investigated the relationship between OSA status and 3 variables commonly associated with OSA: sex, age, and BMI.
Questions answered as “Prefer not to answer” or “Don’t know” were coded as missing. All tests were 2 sided, and we considered P < .05 to be statistically significant.
Missing data
The study population consisted of 155,448 participants. Of these, 100,693 (64.8%) had missing values in one or more variables, with missing data ranging from 0% (rural status) to 29% (OSA status) (Table S1 in the supplemental material). To account for missing data, our primary analyses were conducted on an imputed data set where missing values were generated using multiple imputation by chained equations (MICE).32 In the MICE procedure, a series of regression models are run whereby each variable with missing data is modeled conditional upon the other variables in the data.33 Imputed data sets were generated under the missing at random assumption that the probability of data being missing is dependent on the observed data.34 The imputation model included all variables of interest, including the outcome variables. Linear regression was used for imputation of continuous variables, logistic regression for dichotomous variables and multiple logistic regression for categorical variables with more than 2 categories. Following recommendations by van Buuren,35 35 datasets were imputed and combined according to Rubin’s rules.36
We also performed a complete case sensitivity analysis without imputed data.
RESULTS
Cohort characteristics
Table 1 describes the demographic characteristics of the study population. A total of 7,666 participants (6.9%) reported being previously diagnosed with OSA. The entire cohort included a lower proportion of men (39.8%). The mean age was 46 years (standard deviation [SD] = 15, range: 18 to 99 years), and the mean BMI was 26.9 kg/m2 (SD = 5.7). For participants with OSA, a higher proportion were men (62.4%), the mean age was 54 years (SD = 11.7), and the mean BMI was 31.8 kg/m2 (SD = 7.1). Those with OSA were also more sedentary (median time spent sitting per week = 46 hours [IQR = 33–64]) than those without OSA (median = 42 hours [IQR = 29 to 57] (P < .001). Participants with OSA had much higher rates of physician-diagnosed morbidities (frequency from 2.7 to 45.8%) (Table 1) (all P < .001). Summary statistics derived from imputed data set were very similar to those in the original dataset (Table S2).
Table 1.
Characteristics of the study sample in the entire cohort and by OSA status.
| OSA (n = 7,666) | No OSA (n = 102,889) | Total Cohort (n = 155,448) | |
|---|---|---|---|
| Men, n (%)# | 4,784 (62.4) | 40,357 (39.2) | 61,771 (39.8) |
| Women, n (%)# | 2,882 (37.6) | 62,529 (60.8) | 93,582 (60.2) |
| Age, mean years (SD)# | 53.6 (11.7) | 45.7 (14.9) | 46.1 (15.0) |
| BMI, mean kg/m2 (SD)# | 31.8 (7.1) | 26.6 (5.4) | 26.9 (5.7) |
| Time spent sitting, median h/wk [IQR]# | 46.4 [33.0–64.0] | 42.0 [29.0–57.0] | 42.0 [30.0–58.0] |
| High blood glucose, n (%)# | 1,604 (20.9) | 5,641 (5.5) | 9,461 (6.1) |
| Myocardial infarction, n (%)# | 435 (5.7) | 1,744 (1.7) | 2,911 (1.9) |
| High cholesterol, n (%)# | 3,106 (40.5) | 18,106 (17.6) | 24,120 (15.5) |
| High blood pressure, n (%)# | 3,514 (45.8) | 19,211 (18.7) | 31,428 (20.2) |
| Heart disease, n (%)# | 723 (9.4) | 2,868 (2.8) | 4,363 (2.8) |
| Diabetes, n (%)# | 1,403 (18.3) | 5,392 (5.2) | 9,266 (6.0) |
| Depression, n (%)# | 1,742 (22.7) | 8,896 (8.7) | 15,569 (10.0) |
| Stroke, n (%)# | 206 (2.7) | 892 (.9) | 1,506 (1.0) |
| Frequency of alcohol consumption, n (%)# | |||
| Never drink | 607 (7.9) | 5,267 (5.1) | 8,135 (5.2) |
| Less than monthly | 1,684 (22.0) | 19,847 (19.3) | 29,065 (18.7) |
| About once a month | 629 (8.2) | 8,662 (8.4) | 12,827 (8.3) |
| 2 to 3 times a month | 921 (12.0) | 13,184 (12.8) | 19,801 (12.7) |
| Once a week | 845 (11.0) | 12,179 (11.8) | 18,365 (11.8) |
| 2 to 3 times a week | 1,122 (14.6) | 16,941 (16.5) | 25,740 (16.6) |
| 4 to 5 times a week | 654 (8.5) | 9,004 (8.8) | 13,836 (8.9) |
| 6 to 7 times a week | 793 (10.3) | 9,094 (8.8) | 14,278 (9.2) |
| Smoking status (ever smoked) | 4,210 (54.9) | 43,285 (42.1) | 65,514 (42.2) |
| Highest level of education completed, n (%)# | |||
| None | 16 (.2) | 238 (.2) | 312 (.2) |
| Elementary school | 173 (2.3) | 1,696 (1.7) | 2,307 (1.5) |
| High school | 1,735 (22.6) | 22,437 (21.8) | 32,486 (20.9) |
| Trade/vocation school/technical | 718 (9.4) | 6,029 (5.9) | 8,698 (5.6) |
| Diploma | 1,940 (25.3) | 25,356 (24.6) | 36,485 (23.5) |
| Certificate | 322 (4.2) | 3,751 (3.7) | 5,692 (3.7) |
| Bachelor’s degree | 1,670 (21.8) | 27,823 (27.0) | 42,308 (27.2) |
| Graduate degree | 956 (12.5) | 13,553 (13.2) | 23,160 (14.9) |
| Working status, n(%)# | |||
| Full-time employed/self-employed | 3,576 (46.7) | 53,846 (52.3) | 80,168 (51.6) |
| Part-time employed/self-employed | 718 (9.4) | 12,341 (12.0) | 18,337 (11.8) |
| Doing unpaid or voluntary work | 57 (.7) | 926 (.9) | 1,474 (1.0) |
| Looking after home and/or family | 158 (2.1) | 4,560 (4.4) | 6,094 (3.9) |
| Retired | 1,833 (23.9) | 15,531 (15.1) | 24,477 (15.8) |
| Unable to work due to illness/disability | 765 (10.0) | 3,078 (3.0) | 5,270 (3.4) |
| Student | 84 (1.1) | 5,687 (5.5) | 8,895 (5.7) |
| Unemployed | 303 (4.0) | 4,016 (3.9) | 5,786 (3.7) |
| Shift work status, n (%) | 1,044 (13.6) | 14,941 (14.5) | 21,942 (14.1) |
| Rural status, n (%) | 878 (11.5) | 11,277 (11.0) | 16,357 (10.5) |
| Ethnicity, n (%)# | |||
| Aboriginal | 81 (1.1) | 660 (.6) | 998 (.6) |
| Arab | 52 (.7) | 592 (.6) | 894 (.6) |
| Black | 96 (1.3) | 1,405 (1.4) | 2,106 (1.4) |
| Chinese | 225 (2.9) | 5,015 (4.9) | 6,047 (3.9) |
| Filipino | 50 (.7) | 671 (.7) | 1,010 (.7) |
| Japanese | 17 (.2) | 278 (.3) | 346 (.2) |
| Korean | 10 (.1) | 345 (.3) | 419 (.3) |
| Latin American/Hispanic | 53 (.7) | 725 (.7) | 1,135 (.7) |
| Mixed | 669 (8.7) | 8,488 (8.3) | 12,454 (8.0) |
| South Asian | 164 (2.1) | 3,536 (3.4) | 5,230 (3.4) |
| South East Asian | 14 (.2) | 451 (.4) | 650 (.4) |
| West Asian | 15 (.2) | 382 (.4) | 607 (.4) |
| White | 5,889 (76.8) | 76,328 (74.2) | 117,475 (75.6) |
Entire cohort data set was evaluated prior to imputation. BMI = body mass index, IQR = interquartile range. #P value < .001.
The association between physical activity and OSA
Table 2 summarizes the distribution of physical activity scores based on OSA status. Univariate analyses indicated that participants with OSA had lower median values and smaller IQRs for all physical activity measures compared with those without (all P < .001). Median total physical activity was 18.1 MET-h/wk [IQR = 2.0 to 47.6] in those with OSA compared with 25.8 MET-h/wk [IQR = 6.6–59.2] in those without. Univariate analyses suggested that male sex, increased BMI, and increased age were all significantly associated with diagnosed OSA. Specifically, men had 2.56 times (95% CI 2.44 to 2.68) the prevalence of diagnosed OSA than women; participants who were overweight had 2.85 times (95% CI 2.66 to 3.09), and participants with obesity were 7.58 times (95% CI 7.07 to 8.13) the prevalence of diagnosed OSA than participants with normal weight; 41- to 60-year-old participants have 3.53 times (95% CI 3.30 to 3.78), 61- to 80-year-old participants have 4.46 times (95% CI 4.15 to 4.79), and participants over 80 years have 2.59 times (95% CI 1.92 to 3.49) the prevalence of diagnosed OSA then 18- to 40-year-old participants (Table 3).
Table 2.
Physical activity characteristics of the study sample by OSA status: univariate analysis.
| Physical Activity Measure, Median MET-h/wk [IQR] | OSA (n = 7,666) | No OSA (n = 102,889) | Total cohort (n = 155,448) |
|---|---|---|---|
| Walking# | 8.3 [2.2–23.1] | 11.0 [3.3–23.1] | 11.0 [3.3–23.1] |
| Moderate-intensity activity# | 0.0 [0.0–10.0] | 0.0 [0.0–12.0] | 0.7 [0.0–12.0] |
| Vigorous-intensity activity# | 0.0 [0.0–10.0] | 0.0 [0.0–12.0] | 0.0 [0.0–12.0] |
| Total physical activity# | 18.1 [2.0–47.6] | 25.8 [6.6–59.2] | 26.0 [7.4–58.5] |
IQR = interquartile range, MET = metabolic equivalent, OSA = obstructive sleep apnea. #OSA vs no OSA, P value < .001.
Table 3.
Pooled univariable logistic regression results describing the association between common predictors of OSA and having diagnosed OSA.
| Predictor | OR (95% CI) | P Value |
|---|---|---|
| Sex | ||
| Female | Reference | |
| Male | 2.56 (2.44, 2.68) | < .001 |
| Age, years | ||
| 18–40 | Reference | |
| 41–60 | 3.53 (3.30, 3.78) | < .001 |
| 61–80 | 4.46 (4.15, 4.79) | < .001 |
| 80+ | 2.59 (1.92, 3.49) | < .001 |
| BMI | ||
| Normal (< 25 kg/m2) | Reference | |
| Overweight (25 – 29.9 kg/m2) | 2.85 (2.66, 3.06) | < .001 |
| Obese (30+ kg/m2) | 7.58 (7.07, 8.13) | < .001 |
BMI = body mass index, OR = odds ratio, OSA = obstructive sleep apnea.
Logistic regression models (Table 4) fitted on the imputed data confirmed the univariate observations. The unadjusted model (Model 1) showed that total physical activity, as a combined measure of the 3 types of physical activity, was associated with a decreased prevalence of diagnosed OSA (OR .88, 95% CI .87 to .90). Adjusting for all risk factors except for BMI (Model 2) showed that increasing total physical activity was independently associated with a decreased prevalence of diagnosed OSA (OR .94, 95% CI .92 to .95). When BMI was added to the model (Model 3), the odds ratio increased to .98 (95% CI .96 to 1.00). In the models fitted with the individual physical activity measures, walking and vigorous-intensity physical activity were independently associated with a decreased prevalence of diagnosed OSA in all models, with ORs in the model adjusting for all significant risk factors (Model 3) higher than for total physical activity (walking: OR .98, 95% CI .96 to 1.00; vigorous-intensity activity: OR .98, 95% CI .97 to 1.00). Moderate-intensity activity was not significantly associated with OSA (OR 1.00, 95% CI .98, 1.02).
Table 4.
Pooled multivariable logistic regression results predicting the likelihood of having sleep apnea using imputed data.
| Predictor | Model 1 Univariate | Model 2a Adjusted (−BMI) | Model 3b Adjusted (+BMI) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | aOR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Individual physical activity measuresc, MET-h/wk | ||||||
| Loge walking | .93 (.92, .95) | < .001 | .96 (.94, .97) | < .001 | .98 (.96, 1.00) | .045 |
| Loge moderate-intensity activity | .99 (.98, 1.01) | .534 | 1.00 (.98, 1.02) | .872 | 1.00 (.98, 1.02) | .835 |
| Loge vigorous-intensity activity | .89 (.88, .90) | < .001 | .95 (.94, .97) | < .001 | .98 (.97, 1.00) | .041 |
| Combined physical activity measured, MET-h/wk | ||||||
| Loge total physical activity | .88 (.87, .90) | < .001 | .94 (.92, .95) | < .001 | .98 (.96, 1.00) | .005 |
aOR = adjusted odds ratio, BMI = body mass index, CI = confidence interval, MET = metabolic equivalent. aModel adjusted for sex, age, age2, time spent sitting, depression, high blood pressure, high cholesterol, blood glucose, stroke, heart disease, diabetes mellitus, myocardial infarction, alcohol frequency, smoking status, ethnicity, working status, and education level; bmodels adjusted for BMI, BMI2, sex, age, age2, time spent sitting, depression, high blood pressure, high cholesterol, blood glucose, stroke, heart disease, diabetes mellitus, myocardial infarction, alcohol frequency, smoking status, ethnicity, working status, and education level; cmodels fit with all 3 physical activity measures as individual covariates; dmodels fit with one combined physical activity measure: (loge walking + loge moderate-intensity activity + loge vigorous-intensity activity).
The independently significant covariates in the final multivariate model (Model 3) were BMI, BMI,2 sex, age, age,2 time spent sitting, comorbidities, ethnicity, alcohol frequency, smoking status, working status, and education level (Table S3). One significant interaction was identified: BMI:vigorous-intensity activity (OR 1.01, 95% CI 1.00 to 1.01, P < .001). This suggested that BMI acts as an effect modifier on the association between vigorous-intensity activity and OSA risk; vigorous activity was most effective in those of lower BMI in reducing prevalence of OSA.
Results and inferences from the complete case sensitivity analyses were comparable to those found using the imputed data (Table S4); the ORs for all physical activity intensities were slightly lower in all models.
Secondary analyses found that participants with a diagnosis of OSA had significantly lower average sleep duration (7.11 hours, SD = 1.51) than participants without OSA (7.34 hours, SD = 1.20) (P < .001). Self-reported sleep quality also differed significantly by OSA status (P < .001), with 31% of participants with OSA reporting trouble sleeping most or all of the time, compared with 17% of participants without OSA (Table 5).
Table 5.
Sleep duration and quality by OSA status.
| Sleep-Related Characteristics | OSA (n = 7,666) | No OSA (n = 102,889) | Total cohort (n = 155,448) | P Value |
|---|---|---|---|---|
| Sleep duration, mean hours (SD) | 7.12 (1.55) | 7.34 (1.19) | 7.32 (1.22) | <.001* |
| Trouble sleeping, n (%) | <.001† | |||
| All the time | 851 (11.1) | 4,220 (4.1) | 7,372 (4.8) | |
| Most of the time | 1,545 (20.3) | 12,837 (12.6) | 20,650 (13.5) | |
| Some of the time | 2,283 (30.0) | 33,782 (33.2) | 50,463 (33.0) | |
| Part of the time | 1,465 (19.2) | 24,469 (24.0) | 36,549 (23.9) | |
| Never | 1,477 (19.4) | 26,509 (33.2) | 37,752 (24.7) |
*t test; †χ2 test.
DISCUSSION
Principal findings
In a large, general population-based prospective cohort study, we assessed the association between measures of physical activity and physician-diagnosed OSA. To our knowledge, the current study is substantially larger than any similar study published to date.24,37,38 We found that most forms of physical activity had a significant protective association with OSA prevalence, independent of potential confounding covariates including BMI. The subtypes of physical activity were assessed separately; increased walking and vigorous-intensity activity were both associated with reduced OSA prevalence. Moderate-intensity activity failed to reach statistical significance once we accounted for all other covariates.
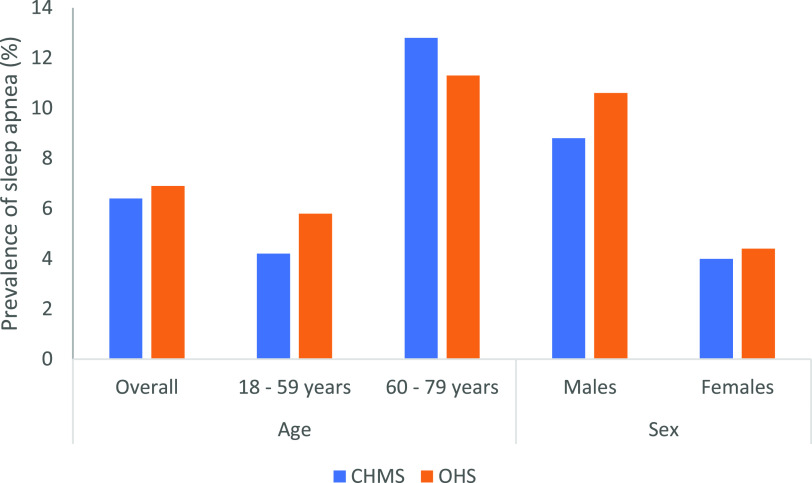
The prevalence of diagnosed OSA in our sample of adults aged 18 to 79 years was 6.9%, consistent with previous findings from the Canadian Health Measures Survey (CHMS), 2016 and 2017,3 which found a prevalence of 6.4% (Figure 1). The self-reported key parameters among those with diagnosed OSA in the OHS participants, a higher proportion of men (62.4%), a mean age of 54 years (SD = 11.7), and mean BMI of 31.8 kg/m2 (SD = 7.1), were also consistent with previous samples of patients with OSA drawn from general population cohorts.39–41 This is also consistent with prevalence of objectively diagnosed OSA, which has been determined to be from 9% to 38% in the general population.1
Figure 1. Comparison of prevalence of sleep apnea by age group and sex in the Ontario Health Study (OHS) and Canadian Health Measures Survey (CHMS), cycle 5 (2016 to 2017).3.
Comparison with previous studies
Our findings are consistent with previous studies on the association of physical activity with OSA disease severity.24,38,42 Some previous studies reported that only vigorous-intensity activity had a significant association with reduced risk of OSA,24 and others38 reported that only moderate-to-vigorous activity was associated with reduced risk of OSA. In contrast, we found that the risk of OSA was independently associated with both walking and vigorous-intensity activity. The type of physical activity being measured was variable in previous studies, with some studies using self-reported activity,38,42,43 as done in the current study, and others using structured exercise with the intensity formally measured in a laboratory setting.44
Several studies suggest that the association between physical activity and OSA may be bidirectional,43,45 with patients less likely to engage in physical activity due to the symptoms of sleep apnea. Specific impairments in sleep apnea patients impacting aerobic exercise capacity include a lower Vo2max,44 dyspnea, lower limb muscle weakness, cardiac dysfunction, respiratory muscle dysfunction, hypoxemia, lack of motivation, and peripheral vascular disease.46, It remains unclear whether 1) the amount of physical activity directly decreases OSA susceptibility, 2) OSA susceptibility directly impacts the extent of physical activity engaged in, or 3) a combination of both.
Limitations and strengths of the current study
This study has several potential limitations. First, the volunteer nature of the OHS means that selection bias is possible. However, comparison of age, sex, BMI, ethnicity, and the primary outcome and explanatory variables used in this study to the CHMS47 suggest that the OHS sample comprises a reasonably good approximation to the general adult population of Canada (data not shown) (Figure 1). Second, the findings are based upon self-reported measures and information bias may thus be a factor. Prior studies have shown that the IPAQ survey, used in the current study, is prone to bias as it significantly overestimates time spent doing physical activity compared with more objective methods such as accelerometry.48 Similarly, recall bias is a potential issue in the reporting of OSA status, which is indicated on the questionnaire as a medical professional telling them they have the condition; however, no details are provided as to the severity or currency of the diagnosis or whether any treatment has been provided. However, the consistency of prevalence estimates in the OHS sample and the CHMS (Figure 1) suggest any information bias is likely minor in nature. Previous research has also shown that the accuracy of a self-reported history of physician-diagnosed chronic health conditions is good for prevalent chronic diseases, with high specificity and sensitivity.49,50 In addition, secondary analyses found a strong association between OSA status and shorter average sleep duration and poorer quality sleep, measures often associated with OSA.2,51 Finally, due to the sample being cross-sectional we were unable to make temporal inferences on the observed associations between physical activity and sleep apnea. Another potential form of bias arises from missing data. However, this was dealt with formally in the analysis by imputing missing values.
A major strength of the current study is that OHS is a large cohort from the general population that was consistent for most variables of interest with estimates derived from contemporaneous Canadian representative samples.47 The OHS sample is diverse and came from a range of sociodemographic and ethnic backgrounds and from all adult age groups. This suggests findings may be generalizable, particularly to the Canadian general population, but possibly also to similar countries such as Australia and the United Kingdom. Since the OHS collected a very broad range of data, many potentially confounding socio-demographic and health-related risk factors could be accounted for when assessing associations between physical activity and OSA.
Implications of the study
OSA is associated with substantial social and economic costs globally due to its high prevalence in the community; the profound clinical effects on an individual’s cardiometabolic, cognitive, and general functioning; and the increased risk of adverse health complications. Therefore, understanding the role of risk factors for OSA and its associated adverse health outcomes is imperative.
The findings from the current study support the potential importance of physical activity to reduce the risk of OSA. Our results suggest that an increase in total physical activity of 15 MET-h/wk was associated with a reduction in the predicted risk of OSA of ∼10% for men and ∼10% in women. This behavioral modification is achievable, as 15 MET-h/w equates to a relatively modest increase in physical activity, eg, an increase of 8 minutes of vigorous activity per day plus an increase of 20 minutes of walking per day. Health promotion and OSA prevention efforts should therefore encourage and promote healthy lifestyle choices, including engaging in more physical activity. Previous studies have also suggested that physical activity is an efficient intervention in reducing OSA severity,16,17,52 improving cardiorespiratory fitness52 and quality of life in patients with OSA17 and showing that the improvement of OSA severity obtained after exercise therapy could be maintained years after the intervention. Our findings also support the routine use of IPAQ surveys or other physical activity assessments to monitor physical activity levels in patients with OSA.
CONCLUSION
We demonstrated that walking and vigorous-intensity physical activity are associated with a decreased risk of OSA independent of other risk factors in a large population-based prospective cohort study. These results build on prior findings that physical activity can be a contributing factor to OSA severity. To further clarify the intensity and amount of activity required to make a difference to the risk of OSA, new longitudinal randomized control trials should be conducted.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at University of Adelaide, South Australia. This work was supported by the Ontario Institute for Cancer Research; Cancer Care Ontario; Public Health Ontario and the Canadian Partnership Against Cancer. The Ontario Health Study is part of the Canadian Partnership for Tomorrow Project, which is made up of 5 regional health studies across Canada. The data and biosamples used for this research were made available by the Ontario Health Study with the financial support from the Canadian Partnership Against Cancer and Health Canada, and the Ontario Institute for Cancer Research. The views expressed herein represent the views of the authors and do not necessarily represent the views of Health Canada or the Government of Ontario. The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors thank the participants in the Ontario Health Study.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CHMS
Canadian Health Measures Survey
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CVD
cardiovascular disease
- IPAQ
International Physical Activity Questionnaire
- IQR
interquartile range
- MET
metabolic equivalent of task
- MICE
multiple imputation by chained equations
- OSA
obstructive sleep apnea
- OR
odds ratio
- SD
standard deviation
REFERENCES
- 1.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. 2017;34:70–81. 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 2.Gibson GJ. Obstructive sleep apnoea syndrome: underestimated and undertreated. Br Med Bull. 2004;72(1):49–64. 10.1093/bmb/ldh044 [DOI] [PubMed] [Google Scholar]
- 3.Sheet HF. Sleep Apnea in Canada, 2016 and 2017. Statistics Canada Catalogue no. 82-625-X. https://www150.statcan.gc.ca/n1/pub/82-625-x/2018001/article/54979-eng.htm. Accessed October 24, 2018.
- 4.Lavie P. Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep. 1983;6(4):312–318. 10.1093/sleep/6.4.312 [DOI] [PubMed] [Google Scholar]
- 5.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep. 2000;23(Suppl 4):S102–S108. [PubMed] [Google Scholar]
- 6.Terán-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340(11):847–851. 10.1056/NEJM199903183401104 [DOI] [PubMed] [Google Scholar]
- 7.Gall R, Isaac L, Kryger M. Quality of life in mild obstructive sleep apnea. Sleep. 1993;16(Suppl 8):S59–S61. 10.1093/sleep/16.suppl_8.S59 [DOI] [PubMed] [Google Scholar]
- 8.Lavie P. Mortality in sleep apnoea syndrome: a review of the evidence. Eur Respir Rev. 2007;16(106):203–210. 10.1183/09059180.00010610 [DOI] [Google Scholar]
- 9.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18(1):61–70. 10.1111/j.1440-1843.2012.02255.x [DOI] [PubMed] [Google Scholar]
- 10.Vandeputte M, de Weerd A. Sleep disorders and depressive feelings: a global survey with the Beck depression scale. Sleep Med. 2003;4(4):343–345. 10.1016/S1389-9457(03)00059-5 [DOI] [PubMed] [Google Scholar]
- 11.Malik JA, Masoodi SR, Shoib S. Obstructive sleep apnea in Type 2 diabetes and impact of continuous positive airway pressure therapy on glycemic control. Indian J Endocrinol Metab. 2017;21(1):106–112. 10.4103/2230-8210.196005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 13.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121(8):1014–1021. 10.1161/CIRCULATIONAHA.109.900357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotenberg BW, Vicini C, Pang EB, Pang KP. Reconsidering first-line treatment for obstructive sleep apnea: a systematic review of the literature. J Otolaryngol. 2016;45:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catcheside PG. Predictors of continuous positive airway pressure adherence. F1000 Med Rep. 2010;2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iftikhar IH, Bittencourt L, Youngstedt SD, et al. Comparative efficacy of CPAP, MADs, exercise-training, and dietary weight loss for sleep apnea: a network meta-analysis. Sleep Med. 2017;30:7–14. 10.1016/j.sleep.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 17.Andrade FM, Pedrosa RP. The role of physical exercise in obstructive sleep apnea. J Bras Pneumol. 2016;42(6):457–464. 10.1590/s1806-37562016000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. 10.1016/j.gaitpost.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuch FB, Vancampfort D, Sui X, et al. Are lower levels of cardiorespiratory fitness associated with incident depression? A systematic review of prospective cohort studies. Prev Med. 2016;93:159–165. 10.1016/j.ypmed.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 21.Wahid A, Manek N, Nichols M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(9):e002495. 10.1161/JAHA.115.002495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto U, Mohri M, Shimada K, et al. Six-month aerobic exercise training ameliorates central sleep apnea in patients with chronic heart failure. J Card Fail. 2007;13(10):825–829. 10.1016/j.cardfail.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Ueno LM, Drager LF, Rodrigues AC, et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep. 2009;32(5):637–647. 10.1093/sleep/32.5.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quan SF, O’Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11(3):149–157. 10.1007/s11325-006-0095-5 [DOI] [PubMed] [Google Scholar]
- 25.Van Offenwert E, Vrijsen B, Belge C, Troosters T, Buyse B, Testelmans D. Physical activity and exercise in obstructive sleep apnea. Acta Clin Belg. 2019;74(2):92–101. 10.1080/17843286.2018.1467587 [DOI] [PubMed] [Google Scholar]
- 26.Ontario Health Study . About the Study. https://www.ontariohealthstudy.ca/about-the-study/. Accessed June 1, 2019, 2019.
- 27.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 28.Sjöström M, Ainsworth B, Bauman A, Bull F, Craig C, Sallis J. Guidelines for data processing and analysis of the Intentional Physical Activity Questionnaire (IPAQ) - Short and long forms. Stockholm, Sweden: Karolinska Institute; 2005.
- 29.Craig CL, Cameron C, Bauman A. Socio-Demographic and Lifestyle Correlates of Obesity: Technical Report on the Secondary Analyses using the 2000-2001 Canadian Community Health Survey. Ottawa, Canada: Canadian Institute for Health Information; 2005. [Google Scholar]
- 30.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: The R Foundation; 2018. [Google Scholar]
- 31.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. New York: John Wiley & Sons; 2013. 10.1002/9781118548387 [DOI] [Google Scholar]
- 32.Raghunathan TELJ, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–95. [Google Scholar]
- 33.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 35.van Burren S. Chapter 2: Multiple imputation. In: Flexible Imputation of Missing Data. 2nd ed. Boca Raton, FL: CRC/Chapman & Hall; 2012:49–51. [Google Scholar]
- 36.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. 10.1002/9780470316696 [DOI] [Google Scholar]
- 37.van der Spuy IA-OX, Zhao G, Karunanayake CA-O, Pahwa PA-O. Predictors of sleep apnea in the Canadian population. Can Respir J. 2018;2018:1–11. 10.1155/2018/6349790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murillo R, Reid KJ, Arredondo EM, et al. Association of self-reported physical activity with obstructive sleep apnea: Results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Prev Med. 2016;93:183–188. 10.1016/j.ypmed.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Eng J Med. 1993;328(17):1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 40.Redline S, Tosteson T, Tishler PV, Carskadon MA, Millman RP, Milliman RP. Studies in the genetics of obstructive sleep apnea. Familial aggregation of symptoms associated with sleep-related breathing disturbances. Am Rev Respir Dis. 1992;145(2 Pt 1):440–444. 10.1164/ajrccm/145.2_Pt_1.440 [DOI] [PubMed] [Google Scholar]
- 41.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 42.da Silva RP, Martinez D, Pedroso MM, et al. Exercise, occupational activity, and risk of sleep apnea: a cross-sectional study. J Clin Sleep Med. 2017;13(2):197–204. 10.5664/jcsm.6446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35(7):1088–1092. 10.1249/01.MSS.0000074566.94791.24 [DOI] [PubMed] [Google Scholar]
- 44.Beitler JR, Awad KM, Bakker JP, et al. Obstructive sleep apnea is associated with impaired exercise capacity: a cross-sectional study. J Clin Sleep Med. 2014;10(11):1199–1204. 10.5664/jcsm.4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson L, McArdle N, Eastwood PR, et al. Physical inactivity is associated with moderate-severe obstructive sleep apnea. J Clin Sleep Med. 2015;11(10):1091–1099. 10.5664/jcsm.5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ucok K, Aycicek A, Sezer M, et al. Aerobic and anaerobic exercise capacities in obstructive sleep apnea and associations with subcutaneous fat distributions. Lung. 2009;187(1):29–36. 10.1007/s00408-008-9128-0 [DOI] [PubMed] [Google Scholar]
- 47.Statistics Canada . Canadian Health Measures Survey (CHMS). https://www.statcan.gc.ca/eng/survey/household/5071. Accessed February 2, 2019.
- 48.Grimm EK, Swartz AM, Hart T, Miller NE, Strath SJ. Comparison of the IPAQ-Short Form and accelerometry predictions of physical activity in older adults. J Aging Phys Act. 2012;20(1):64–79. 10.1123/japa.20.1.64 [DOI] [PubMed] [Google Scholar]
- 49.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. 10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 50.Oksanen T, Kivimäki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. Self-report as an indicator of incident disease. Ann Epidemiol. 2010;20(7):547–554. 10.1016/j.annepidem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 51.Drager LF, Santos RB, Silva WA, et al. OSA, short sleep duration, and their interactions with sleepiness and cardiometabolic risk factors in adults. Chest. 2019;155(6):1190–1198. 10.1016/j.chest.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 52.Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. 10.1007/s00408-013-9511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.