Abstract
Study Objectives:
The impact of vitamin D on human health including sleep has been well described in adults. Its deficiency has been associated with multiple sleep disorders such as decrease in sleep duration, worsening of sleep quality, and even OSA. Such correlation is less evident in the pediatric population. In the current study, we examined the relationship between sleep architecture and vitamin D status in children referred to a sleep clinic.
Methods:
This was a retrospective-cohort study in a tertiary care children’s hospital over a 1-year period. Children who underwent an in-laboratory overnight-polysomnogram and had a 25-hydroxy vitamin D level obtained within 120 days of the sleep study were included. Patients with OSA or central sleep apnea were excluded. Data from polysomnograms and Pediatric Sleep Questionnaires were collected and analyzed.
Results:
A total of 39 patients (mean age, 6.6 years; 46% female) were included in the study. Twenty (51%) patients had vitamin D deficiency (25-hydroxy vitamin D level < 30 ng/mL). Children with vitamin D deficiency had less total sleep time (470.3 minutes ± 35.6 vs 420.3 minutes ± 61.7; P = .004) and poorer sleep efficiency (91.9% ± 5.6% vs 84.5% ± 9.5%; P = .015) compared with children with sufficient vitamin D. In addition, children with vitamin D deficiency had later weekday bedtimes (21:02 Pm ± 1:01 vs 20:19 Pm ± 0:55; P = .037) and later weekend bedtimes (21:42 Pm ± 0:59 vs 20:47 Pm ± 1:08; P = .016) than children with sufficient vitamin D, with a tendency for later wake time that did not reach statistical significance. The remainder of the polysomnogram findings and Pediatric Sleep Questionnaire data were not different between the 2 groups.
Conclusions:
Vitamin D deficiency in children was associated with objectively measured decreased sleep duration and poorer sleep efficiency. Furthermore, vitamin D deficiency was associated with delayed bedtimes, suggesting that vitamin D and circadian rhythm could be related. Future prospective studies in children would be helpful to learn if vitamin D deficiency leads to sleep disturbance or vice versa.
Citation:
Al-Shawwa B, Ehsan Z, Ingram DG. Vitamin D and sleep in children. J Clin Sleep Med. 2020;16(7):1119–1123.
Keywords: vitamin D, sleep architecture, sleep duration, sleep efficiency, delayed sleep phase, circadian rhythm, children
BRIEF SUMMARY
Current Knowledge/Study Rationale: Vitamin D deficiency in adults has been associated with multiple sleep disorders such as decrease in sleep duration, worsening of sleep quality, and even OSA. Such correlation is less evident in the pediatric population.
Study Impact: This study shows a positive association between vitamin D deficiency and sleep architecture and suggests a possible circadian influence, with vitamin D deficiency associated with delayed sleep onset. These findings may have implications for clinicians caring for children with clinical sleep problems.
INTRODUCTION
Vitamin D is an essential nutrient known to play an important role in the growth and bone health of the human body. It is ingested in the diet in the form of ergocalciferol (vitamin D2) or produced in the skin by the action of ultraviolet light in the form of cholecalciferol (vitamin D3). Both forms go through an activation process in the liver to form 25-hydroxy vitamin D (25-OH-vitD). Vitamin D deficiency is commonly assessed by measuring 25-OH-vitD in the serum. Over the past 2 decades, mounting evidence has shown the crucial role of vitamin D in many other aspects of human health including immune modulation, allergy, cardiovascular disease, cancer, infectious diseases, glucose metabolism/insulin resistance, and even sleep.1–9
The discovery of vitamin D receptors in the brain sparked research interest into the effect of vitamin D deficiency on a wide variety of neurological disorders, including sleep disorders.10 One study that examined the U.S. National Health and Nutrition Examination Survey data found that low vitamin D levels were associated with increased duration to fall asleep in adults.11 Subsequent epidemiological studies showed short sleep duration in association with vitamin D deficiency.12–15 This association with sleep duration remained even when sleep was objectively assessed via actigraphy or polysomnography in adults.16 Other studies showed worsening of sleep quality and daytime sleepiness with vitamin D deficiency.17–19 Several studies showed a strong correlation between vitamin D deficiency and OSA, especially in adults. Continuous positive airway pressure use in adult men with OSA affected vitamin D homeostasis in 1 study.20–23 Some evidence has shown that vitamin D therapy will improve sleep complaints in adults.24,25
Although there is increasing evidence of the association between vitamin D deficiency and different sleep complaints and disorders in adults, the correlation is less elucidated in children. In the current study, our aim was to describe sleep characteristics associated with vitamin D deficiency in children referred to a pediatric sleep clinic.
METHODS
This study was approved by the institutional review board. Patients who underwent a polysomnogram (PSG) between January 2016 and December 2016 and had serum 25-OH-vitD testing within 120 days of the PSG were included in the study. The following information was collected from patients’ charts: age, sex, race, body mass index z-score, 25-OH-vitD level, ferritin level, season at the time of vitamin D testing (ie, winter, spring, summer, or fall), and reason for the sleep study referral. PSG data were pulled electronically from the PSG software. Patients were excluded if they had an obstructive apnea-hypopnea index of more than 2 events/h or central apnea-hypopnea index of more than 5 events/h. Available Pediatric Sleep Questionnaire data were also abstracted. Patients were considered vitamin D–deficient if their 25-OH-vitD level was below 30 ng/mL.26
PSGs were performed in an American Academy of Sleep Medicine–accredited pediatric sleep laboratory with at least 6 hours of recording time. The patients were allowed to fall asleep spontaneously without the use of any sedating or hypnotic medications. The following PSG parameters were measured according to AASM guidelines: bilateral electro-oculogram, 6 channels of electroencephalogram (2 frontal, 2 occipital, and 2 central leads), chin and bilateral-legs electromyogram, chest and abdominal wall movements by inductance plethysmography, heart rate by electrocardiography, air flow by an oronasal thermistor, nasal pressure transducer, nasal end tidal carbon dioxide monitoring (also used to assess ventilation), arterial pulse oxygen saturation by pulse oximetry, and a digital time-synchronized video recording. All measures were digitized using a commercially available system (Nihon Kohden-Polysmith acquisition software 11.0, Nihon Kohden, California). The sleep technician followed patient behavior and confirmed sleep position by the infrared camera inside the room. Sleep studies were scored by a registered polysomnographic technologist and read by a board-certified sleep specialist based on AASM scoring criteria.
Statistical analysis
Categorical variables were analyzed using contingency tables where χ2 tests or Fisher exact tests were performed. Bivariate correlations were examined via Pearson correlation. Statistical comparisons for the means were done by t tests appropriately corrected for nonequal variance where needed. P values < .05 were considered statistically significant. Summary data are presented as mean ± standard deviation (SD) except where otherwise noted. All analyses were performed using SPSS software (version 24, IBM, USA).
RESULTS
A total of 39 children met the criteria for inclusion. Children ranged in age from 2–17 years, with an average age of 6.6 (SD = 3.8) years. Of the 39 participants, 18 (46%) were female. The mean vitamin D level for the entire cohort was 33.3 ng/mL (SD = 13.8), with 20 (51%) patients with vitamin D deficiency (vitamin D < 30 ng/mL). The average time between the date of the overnight sleep study and the date of sampling for vitamin D level was 7.0 (SD = 67.1) days. Serum ferritin levels were also available for patients, and the average level was 26.7 ug/mL (SD = 15.0), which was closely aligned with our previously published findings from our sleep center.27,28 The reasons for participation in the sleep study were concern for sleep apnea (71%), periodic limb movement disorder (5%), restless sleep (10%), and daytime sleepiness (12%).
Participant and PSG characteristics according to vitamin D status are provided in Table 1. There were no significant differences between body mass index z-scores, ferritin levels, sex distribution, race, season, reason for PSG, time between sleep study and blood draw, or age in children with vitamin D deficiency vs those without. Children with vitamin D deficiency exhibited significantly less total sleep time (470.3 minutes ± 35.6 vs 420.3 minutes ± 61.7; P = .004) and poorer sleep efficiency (91.9% ± 5.6% vs 84.5% ± 9.5%; P = .015) compared with children with sufficient vitamin D levels. In contrast, there were no significant between-group differences in sleep latency, percentages of sleep stages N1, N2, N3, and R, obstructive apnea-hypopnea index, central apnea index, oxygen saturation mean, oxygen saturation nadir, percentage of end tidal carbon dioxide > 50%, total arousal index, periodic limb movement arousal index, periodic limb movement index, or total limb movement index (all P > .2). Similar results were found in the bivariate correlation analysis, with vitamin D levels significantly associated with total sleep time (r = .362; P = .023) and sleep efficiency (r = .396; P = .013) but no association with any other examined variable. There were 27 Caucasian participants and 12 non-Caucasian participants, and they did not differ in vitamin D level (33.6 ± 13.9 vs 32.6 ± 14.2; P = .838). Likewise, vitamin D level did not differ by season (df = 3; F = 1.3; P = .283). These characteristics, along with participant age, were entered into a multivariable model to assess the relationship with vitamin D level; in this model, age, sex, race, season, and reason for PSG were all unrelated to vitamin D level (all P > .5).
Table 1.
Participants and polysomnographic characteristics according to vitamin D deficiency status.
| Characteristic | Serum 25-OH-vitD Categories | P | |
|---|---|---|---|
| 25-OH-vitD ≥ 30 ng/mL (n = 19) | 25-OH-vitD < 30 ng/mL (n = 20) | ||
| Age, y | 5.7 (3.0) | 7.4 (4.34) | .194 |
| Sex, % female | 47% (9/19) | 45% (9/20) | .882 |
| Race, % Caucasian | 79% (15/19) | 60% (12/20) | .200 |
| BMI z-score | .91 (1.1) | .55 (1.4) | .390 |
| Time from PSG to blood draw, days | 2.3 (58.0) | −15.8 (75.1) | .405 |
| Serum ferritin | 30.3 (18.1) | 23.3 (10.7) | .149 |
| TST, minutes | 470.3 (35.6) | 420.3 (61.7) | .004* |
| Sleep efficiency, % | 91.9 (5.6) | 84.5 (9.5) | .015* |
| Sleep latency, minutes | 19.1 (29.4) | 18.3 (17.1) | .914 |
| Stage N1 sleep, %TST | 4.2 (2.4) | 5.3 (4.7) | .386 |
| Stage N2 sleep, %TST | 43.9 (12.7) | 42.7 (9.4) | .733 |
| Stage N3 sleep, %TST | 34.1 (9.4) | 34.0 (12.0) | .958 |
| Stage R sleep, %TST | 18.5 (5.3) | 18.7 (4.2) | .920 |
| OAHI, events/h | .6 (.5) | .7 (.4) | .491 |
| CAI, events/h | .9 (.7) | 1.1 (1.0) | .629 |
| O2 mean, % | 96.4 (1.1) | 96.5 (1.5) | .856 |
| O2 nadir, % | 90.2 (3.4) | 89.2 (5.2) | .483 |
| %EtCO2 > 50 | 1.9 (3.7) | 6.1 (14.8) | .233 |
| Total AI, events/h | 8.8 (2.6) | 10.0 (7.1) | .481 |
| PLM AI, events/h | 4.8 (2.6) | 6.2 (5.0) | .304 |
| PLMI, events/h | 3.1 (5.1) | 5.1 (9.2) | .352 |
| Total LMI, events/h | 10.3 (9.3) | 13.4 (13.2) | .405 |
Values are presented as mean (SD) unless otherwise indicated. *Indicate statistical significance P < .05 N for NREM and R for REM sleep stages. AI = arousal index, BMI = body mass index, CAI = central apnea index, EtCO2 = end tidal carbon dioxide, LMI = limb movement index, O2 = oxygen saturation, OAHI = obstructive apnea-hypopnea index, PLM = periodic limb movement, PLMI = Periodic limb movement index, PSG = polysomnogram, SD = standard deviation, TST = total sleep time.
Data were also examined for the subset of individuals who completed the sleep questionnaire (n ranged from 25–35 depending on symptom queried). In terms of reported sleep complaints, there were no significant differences in the prevalence of restless sleep, restless legs when in bed, growing pains that were unexplained, growing pains that were worse in bed, brief kicks of the legs, repeated kicks of the legs during sleep, teeth grinding, enuresis, sleepwalking, nightmares, waking up screaming, difficulty falling asleep, sleep latency, head banging or body rocking, waking up more than twice per night, trouble falling back to sleep, feeling unrefreshed in the morning, problems with daytime sleepiness, teacher complaints of daytime sleepiness, napping during the day, difficulty waking up in the morning, or morning headache (all P > .05 via χ2). The only difference in reported symptoms was in sleep talking, with 94% (16/17) of children with sufficient vitamin D reporting sleep talking compared with 61% (11/18) of children with vitamin D deficiency (χ2 = 5.4; P = .020).
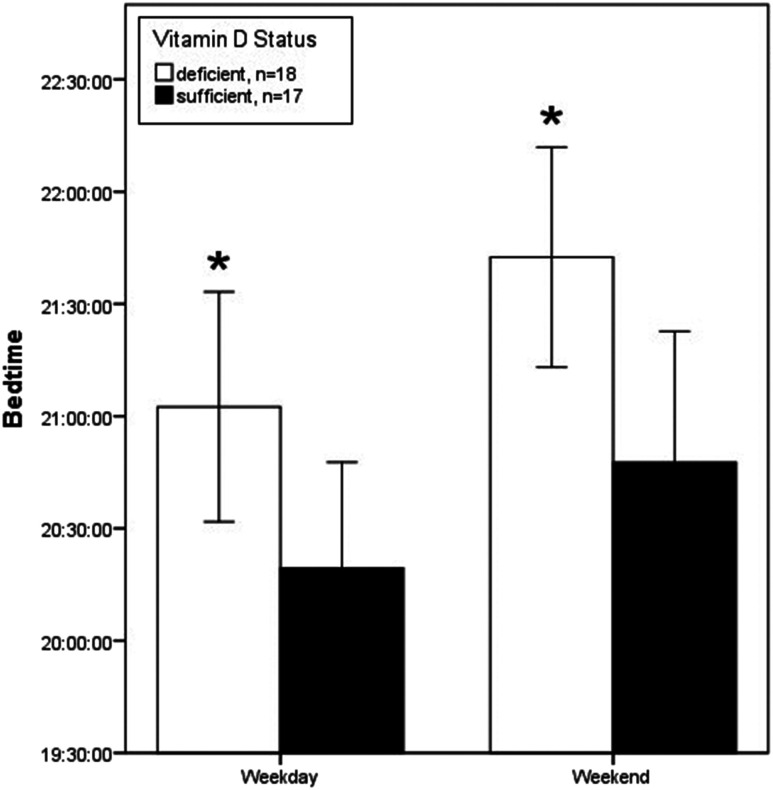
We examined parent-reported sleep schedules at home for the 35 children with data available. Children with vitamin D deficiency had later weekday bedtimes (21:02 Pm ± 1:01 vs 20:19 Pm ± 0:55; P = .037) and weekend bedtimes (21:42 Pm ± 0:59 vs 20:47 Pm ± 1:08; P = .016) than children with sufficient vitamin D levels (Figure 1). Although there was a trend for later wake times in children with vitamin D deficiency than in children with sufficient vitamin D during both weekdays (7:18 Am ± 1:16 vs 6:42 Am ± 0:27; P = .075) and weekends (8:10 Am ± 1:22 vs 7:33 Am ± 1:33; P = .231), this trend did not meet statistical significance.
Figure 1. Mean bedtime during weekdays and weekends according to vitamin D status.
Vitamin D deficiency defined as 25-OH-vitD < 30 ng/mL. *P < .05. Error bars are 95% confidence interval.
Several linear models were constructed with vitamin D status and participant age as predictors, and bedtime during both weekdays (df = 1; F = 4.4; P = .042) and weekends (df = 1; F = 5.5; P = .024) remained statistically significantly associated with vitamin D status. Furthermore, significant associations were found between vitamin D status and sleep time (beta = .537; t = 3.9; P < .001) and sleep efficiency (beta = .437; t = 2.8; P = .007) in a regression model that included race and season. Similarly, significant associations between vitamin D status and weekday bedtime (beta = −.351; t = −2.0; P = .044) and weekend bedtime (beta = −.402; t = −2.4; P = .021) remained with models that included race and season. Wake time on weekdays (beta = −.337; t = −1.9; P = .056) and weekends (beta = −.232; t = −1.44; P = .161) did not meet significance.
DISCUSSION
In pediatric patients referred for sleep evaluation, this study showed that vitamin D deficiency correlated with shorter sleep duration and less sleep efficiency even after controlling for body mass index and age. However, vitamin D deficiency was not associated with the rest of the polysomnographic findings including sleep stages, periodic limb movements, and arousal index. In the patients who completed a Pediatric Sleep Questionnaire, vitamin D deficiency was associated with delayed bedtime by almost an hour, and this was consistent on both weekday and weekend nights. There was also a tendency for these patients to wake up later, but this difference did not reach statistical significance.
Most prior studies examining the association between vitamin D deficiency and sleep characteristics have been conducted in adults. Our current findings are supported by a study conducted in 657 adult individuals that demonstrated an association between vitamin D deficiency, OSA, and short sleep duration defined as sleeping less than 6 hours; these findings persisted even after controlling for age, sex, ethnicity, obesity, smoking, hypertension, and diabetes.12 In another cohort of 2,966 elderly men who had actigraphy and vitamin D measures (25-OH-vitD), participants with sleep duration of less than 5 hours were more likely to have vitamin D deficiency with an odds ratio of 1.73 (1.02–2.92) for the group with 25-OH-vitD of 20–30 ng/mL and 2.15 (1.21–3.79) for the group with 25-OH-vitD of less than 20 ng/mL.16 Furthermore, in that study, lower serum 25-OH-vitD levels were associated with sleep efficiency of less than 70% in multivariate adjusted models that included age, season, body mass index, and physical and cognitive function.16 Therapeutic trials of vitamin D supplementation in adults have shown improvement in sleep duration and other sleep quality measures.24,25,29
A recent study by Yong et al14 even postulated that vitamin D deficiency may have long-lasting effects on sleep duration. They found that patients with low cord blood level of vitamin D at birth have increased risk of short sleep trajectory (less than 10.5 hours/night) in the preschool years (between ages 2 and 5–6 years).
The mechanisms by which vitamin D could affect sleep are not completely understood. Animal studies have demonstrated vitamin D receptors in brain areas that are also involved in sleep regulation, such as the hypothalamus, raphe nuclei, and midbrain central gray; this overlap suggestions a role for vitamin D in sleep regulation.30 This intersection is also the case in the human brain, where vitamin D receptors are present in the same structures.10 Therefore, vitamin D deficiency could directly affect sleep by acting on these centers. In addition, vitamin D deficiency could indirectly affect sleep duration and quality of sleep by increasing the risk of chronic nonspecific musculoskeletal pain, which in turn could adversely affect sleep quality and sleep duration.31
The finding of delayed bedtime by almost an hour in patients with vitamin D deficiency is of great interest. This finding could explain the decrease in sleep duration noted in patients with vitamin D deficiency and may suggest that vitamin D status serves as one of the modulators of circadian rhythm. Along these lines, previous research showed that supplementation with high doses of vitamin D negatively affects melatonin production.32 This effect may in turn affect circadian rhythmicity. Vitamin D could also have a direct effect on vitamin D receptors in the suprachiasmatic nucleus, which is the master circadian clock in the brain.
Our study has several strengths and limitations. The disturbance in sleep architecture and its relation to vitamin D deficiency were objectively measured using an overnight diagnostic PSG. Vitamin D levels were obtained within consistent timeframes of the PSG, and complete Pediatric Sleep Questionnaire reports were available for most of the patients. The study’s major limitations are its retrospective nature, small sample size, and inability to control for missing data such as dietary intake, sunlight exposure, and socioeconomic factors. Moreover, children were referred to a sleep clinic and therefore at risk a priori of a sleep disorder. Another limitation of this study is the association between vitamin D and sleep disturbance could be reciprocal where sleep disturbance may lead to vitamin D deficiency by affecting duration of light exposure, vitamin D metabolism, or even vitamin D uptake. Larger studies would also be able to better parse which factors are related to vitamin D and various aspects of the sleep schedule; it may be that bedtime is determined by external factors and that sleep duration and sleep efficiency are more strongly determined by intrinsic factors. Certainly, more large-scale studies in children would better elucidate the association between vitamin D levels and sleep. Nevertheless, we feel the results of our study are applicable to sleep specialists caring for children.
In conclusion, this study suggests that vitamin D may play a role in pediatric sleep, especially sleep duration and efficiency, and may have a role in circadian rhythm. Further larger and more controlled studies are recommended to explore these possibilities, which may be a major factor in the sleep health, daytime function, and school performance of children.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Children’s Mercy Hospital. Dr. Ingram has served on a medical advisory board for Jazz Pharmaceuticals and received research support from Wake-Up-Narcolepsy. All remaining authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Medical Writing Center at Children’s Mercy Kansas City for editing this manuscript.
ABBREVIATIONS
- AI
arousal index
- BMI
body mass index
- CAI
central apnea index
- EtCO2
end tidal carbon dioxide
- LMI
limb movement index
- O2
oxygen saturation
- OAHI
obstructive apnea-hypopnea index
- PLM
periodic leg movements
- PLMI
periodic limb movement in sleep index
- PSG
polysomnogram
- TST
total sleep time
- 25-OH-vitD
25-hydroxy vitamin D
REFERENCES
- 1.McCarty DE, Chesson ALJr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18(4):311–319. 10.1016/j.smrv.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between vitamin D deficiency and sleep disorders: a systematic review and meta-analysis. Nutrients. 2018;10(10):1395. 10.3390/nu10101395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann NY Acad Sci. 2007;1117(1):94–105. 10.1196/annals.1402.036 [DOI] [PubMed] [Google Scholar]
- 4.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–1720S. 10.1093/ajcn/80.6.1717S [DOI] [PubMed] [Google Scholar]
- 5.Motiwala SR, Wang TJ. Vitamin D and cardiovascular risk. Curr Hypertens Rep. 2012;14(3):209–218. 10.1007/s11906-012-0262-y [DOI] [PubMed] [Google Scholar]
- 6.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29(9):3687–3698. [PubMed] [Google Scholar]
- 7.Grant WB. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer? An examination using Hill’s criteria for causality. Dermatoendocrinol. 2009;1(1):17–24. 10.4161/derm.1.1.7388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15(1):84. 10.1186/s12871-015-0063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly A, Brooks LJ, Dougherty S, Carlow DC, Zemel BS. A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child. 2011;96(5):447–452. 10.1136/adc.2010.187591 [DOI] [PubMed] [Google Scholar]
- 10.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. 10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Shiue I. Low vitamin D levels in adults with longer time to fall asleep: US NHANES, 2005-2006. Int J Cardiol. 2013;168(5):5074–5075. 10.1016/j.ijcard.2013.07.195 [DOI] [PubMed] [Google Scholar]
- 12.Piovezan RD, Hirotsu C, Feres MC, et al. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS One. 2017;12(7):e0180901. 10.1371/journal.pone.0180901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong QH, Li SX, Li H, Chen Q, Li XY, Xu GZ. 25-Hydroxyvitamin D status and its association with sleep duration in Chinese schoolchildren. Nutrients. 2018;10(8):1013. 10.3390/nu10081013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong CY, Reynaud E, Forhan A, et al. Cord-blood vitamin D level and night sleep duration in preschoolers in the EDEN mother-child birth cohort. Sleep Med. January 2019;53:70–74. 10.1016/j.sleep.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 15.Bertisch SM, Sillau S, de Boer IH, Szklo M, Redline S. 25-Hydroxyvitamin D concentration and sleep duration and continuity: multi-ethnic study of atherosclerosis. Sleep. 2015;38(8):1305–1311. 10.5665/sleep.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massa J, Stone KL, Wei EK, et al. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS sleep study. Sleep. 2015;38(2):251–257. 10.5665/sleep.4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8(6):693–697. 10.5664/jcsm.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung YS, Chae CH, Kim YO, et al. The relationship between serum vitamin D levels and sleep quality in fixed day indoor field workers in the electronics manufacturing industry in Korea. Ann Occup Environ Med. June 2017;29:25. 10.1186/s40557-017-0187-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng TS, Loy SL, Cheung YB, et al. Plasma vitamin D deficiency is associated with poor sleep quality and night-time eating at mid-pregnancy in Singapore. Nutrients. 2017;9(4):340. 10.3390/nu9040340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liguori C, Romigi A, Izzi F, et al. Continuous positive airway pressure treatment increases serum vitamin D levels in male patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11(6):603–607. 10.5664/jcsm.4766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheirandish-Gozal L, Peris E, Gozal D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Med. 2014;15(4):459–463. 10.1016/j.sleep.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salepci B, Caglayan B, Nahid P, et al. Vitamin D deficiency in patients referred for evaluation of obstructive sleep apnea. J Clin Sleep Med. 2017;13(4):607–612. 10.5664/jcsm.6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neighbors CLP, Noller MW, Song SA, et al. Vitamin D and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. March 2018;43:100–108. 10.1016/j.sleep.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 24.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6(6):605–608. 10.5664/jcsm.27996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79(2):132–135. 10.1016/j.mehy.2012.03.031 [DOI] [PubMed] [Google Scholar]
- 26.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 27.Ingram DG, Al-Shawwa B. Serum ferritin in the pediatric sleep clinic: what’s normal anyway? J Clin Sleep Med. 2019;15(11):1699–1700. 10.5664/jcsm.8050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Shawwa B, Ehsan Z, Perry GV, Ingram DG. Limb movements during sleep in children: effects of age, sex, and iron status in more than 1,000 patients referred to a pediatric sleep center. J Clin Sleep Med. 2020;16(1):49–54. 10.5664/jcsm.8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Shah S, Long Q, Crankshaw AK, Tangpricha V. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin J Pain. 2013;29(4):341–347. 10.1097/AJP.0b013e318255655d [DOI] [PubMed] [Google Scholar]
- 30.Stumpf WE, O’Brien LP. 1,25 (OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87(5):393–406. 10.1007/BF00496810 [DOI] [PubMed] [Google Scholar]
- 31.Benson J, Wilson A, Stocks N, Moulding N. Muscle pain as an indicator of vitamin D deficiency in an urban Australian Aboriginal population. Med J Aust. 2006;185(2):76–77. 10.5694/j.1326-5377.2006.tb00475.x [DOI] [PubMed] [Google Scholar]
- 32.Golan D, Staun-Ram E, Glass-Marmor L, et al. The influence of vitamin D supplementation on melatonin status in patients with multiple sclerosis. Brain Behav Immun. 2013;32:180–185. 10.1016/j.bbi.2013.04.010 [DOI] [PubMed] [Google Scholar]