Abstract
Study Objectives:
Because existing data investigating obstructive sleep apnea (OSA) and insulin resistance (IR) are inconsistent, we examine OSA and IR in a pediatric obesity clinic.
Methods:
Children (2–18 years) in the obesity clinic (2013–2017) undergoing polysomnography (PSG), anthropometric measurements, and fasting laboratory tests were included. Linear regression assessed OSA defined by the obstructive apnea-hypopnea index (oAHI) with the homeostatic model assessment of insulin resistance (HOMA-IR). Secondary aims assessed oxygen desaturation index (ODI) and age interactions with HOMA-IR. Logistic regression models and receiver operating characteristic analysis were performed to investigate optimal oAHI and ODI cutoffs relative to HOMA-IR ≥ 3.
Results:
Eighty children were included (mean age, 11.4 ± 4.0 years; 56% female; 46% Caucasian; median body mass index [BMI], 34.6 kg/m2 [interquartile ratio, 29.9–40.1], median BMI z-score, 2.5 [interquartile ratio, 2.3–2.8); 46% with oAHI ≥ 5 events/h. HOMA-IR was higher in the OSA group (oAHI ≥ 5 events/h): 5 vs 3.8 (P = .034). After adjustment for sex, race, and BMI z-score, oAHI ≥ 5 events/h retained significance with HOMA-IR (P = .041). HOMA-IR increased in older children (age ≥ 12 years) when adjusting for waist circumference z-score and waist-height ratio (statistical interaction, P = .020 and .034, respectively). Receiver operating characteristic showed optimal cut points of oAHI and ODI for predicting significant IR 4.9 (area under the curve, 0.70; 95% confidence interval, 0.57–0.83; sensitivity, 0.76; specificity, 0.66) and 4.6 (area under the curve, 0.68; 95% confidence interval, 0.55–0.80; sensitivity, 0.70; specificity, 0.67), respectively.
Conclusions:
In a clinic-based pediatric cohort with obesity, OSA is associated with increased IR even after adjusting for confounders including obesity defined by the BMI z-score. Age ≥ 12 years was associated with AHI relative to IR after adjustment for waist circumference z-score and waist-height ratio. Significant IR could be discriminated by oAHI ≥ 4.9 with moderate sensitivity/specificity. Future studies are needed to verify these findings.
Citation:
Siriwat R, Wang L, Shah V, Mehra R, Ibrahim S. Obstructive sleep apnea and insulin resistance in children with obesity. J Clin Sleep Med. 2020;16(7):1081–1090.
Keywords: children, insulin resistance, obesity, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Children with obesity are at risk for obstructive sleep apnea (OSA) and insulin resistance (IR). This study was performed to understand the relationship between OSA and IR while examining adiposity exclusively in children with obesity.
Study Impact: In a clinic-based study of children with obesity, OSA severity, as measured by the obstructive apnea-hypopnea index, is a significant predictor of IR independent of body mass index z-score. Additionally, this study uniquely evaluates waist circumference z-score as a measure of adiposity in this population and identifies that the older age group age had a stronger association of OSA and IR. Furthermore, using a cutoff of obstructive apnea-hypopnea index ≥ 4.9 events/h may offer clinicians a way to predict pediatric patients with obesity at risk of IR with modest sensitivity and specificity.
INTRODUCTION
Pediatric obesity is a major public health problem, with approximately 17% of children and adolescents 2–19 years of age with obesity (ie a body mass index [BMI] of ≥95th percentile).1,2 The consequences of childhood obesity include negative influences on cardiovascular health such as hypertension and metabolic derangements such as the metabolic syndrome and insulin resistance (IR)/type 2 diabetes in early life and in adulthood.3,4 Identification of modifiable risk factors such as obstructive sleep apnea (OSA), a disorder characterized by repetitive collapse of the upper airway associated with intermittent bouts of hypoxia, sleep fragmentation, autonomic nervous system fluctuations, and intrathoracic pressure alterations, is imperative to minimize immediate and downstream health detriment.
The prevalence of OSA is 2–3% in children,5 and some studies demonstrate the prevalence as high as 60% in children and adolescents with obesity.6,7 Similar to obesity, OSA is associated with increased cardiovascular risk and increased mortality if left untreated.8–11 Obesity and OSA share common cardiometabolic pathophysiologic effects on health through mechanisms such as increase in blood glucose and IR,12,13 as well as alterations in inflammatory cytokines and adipokines.14,15 This reflects the likely bidirectional nature of the obesity-OSA association characterized not only by obesity risk for OSA but also OSA affecting weight gain and metabolic derangements, leading to obesity.
Adults with OSA are at greater risk of having type 2 diabetes, IR, and metabolic syndrome.16–18 In children, emerging data suggest insufficient sleep caused by OSA may lead to metabolic alterations including IR.19,20 For example, significantly higher levels of fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR) levels, and low-density lipoprotein cholesterol were identified in adolescents with OSA.21 These adolescents also had an increased systolic and diastolic blood pressure after adjusting for BMI percentile.21 In another study, OSA severity was associated with increased fasting insulin, blood glucose, and HOMA-IR even after controlling for age and BMI z-score.22
Although many studies showed an association between OSA and IR, some have not. Treatment for OSA with either adenotonsillectomy in children or continuous positive airway pressure in small randomized controlled trials of primarily children without obesity have shown inconsistent improvements in IR.23,24 A large cohort study in children who snore found no significant correlation between sleep parameters and serum insulin, serum glucose, insulin/glucose ratio, or HOMA-IR for either children with or without obesity.25 Another study in children demonstrated no difference in whole-body insulin sensitivity in those with and without OSA.26
Given inconsistencies in the literature, we chose to investigate the association of OSA and IR, leveraging prospective data collection in a high-risk pediatric obesity clinic. We hypothesized that increased severity of OSA, measured by the obstructive apnea-hypopnea index (oAHI) and oxygen desaturation index (ODI), will be predictive of measures of IR. Our secondary aims were to evaluate the interaction of age on the relationship between oAHI and ODI and IR. We also assessed oAHI and ODI cutoff values that rendered higher risk of having IR. To our knowledge, there are limited data in this high-risk group of children with obesity.
METHODS
Recruitment
This is a retrospective examination of data collected from a tertiary care pediatric obesity management clinic at Cleveland Clinic Children’s Hospital. Consecutive patients 2–18 years of age with normal development who underwent overnight polysomnography (PSG) between January 1, 2013 and March 31, 2017 and who also had anthropometric measurements and laboratory testing for IR were included. Exclusion criteria were genetic or craniofacial abnormalities, and those children treated for IR with metformin before laboratory studies. Demographics including age, sex, and race were collected. Comorbidities associated with obesity (ie hypertension, prediabetes, dyslipidemia) and medical history (such as attention deficit hyperactivity disorder) were collected. The study was conducted with approval from the Institutional Review Board of the Cleveland Clinic Foundation.
Data Collection
Comorbidities were collected by review of the medical history in the electronic medical record. Additional comorbidity data were collected by clinical evaluation in the obesity clinic for obesity-related complications, such as diabetes and hypertension. Nonalcoholic fatty liver disease was defined by upper quadrant abdominal ultrasound showing >5% fatty deposition.
Laboratory data on IR consisted of fasting morning glucose and insulin levels. Laboratory data within 6 months of the PSG were selected, with the median time frame of 2 months. Serum fasting glucose was measured using glucose hexokinase (Roche Cobas 8000 702 Platform, Roche, Basel, Switzerland), with an intra-assay coefficient of variation ≤ 5%. Fasting insulin was measured by enzymatic methods under Centers for Disease Control and Prevention guidelines27 using the chemiluminescence immunoassay (CLIA) (ADVIA Centaur XP Immunoassay system, an automated in vitro diagnostic analyzer, catalog 078-A011-03, Siemens Healthcare Diagnostics, Tarrytown, NY), with a coefficient of variation ≤ 8.0%.
HOMA-IR, our primary outcome measure, was calculated as a product of fasting insulin and glucose using a standard equation (fasting insulin [μIU/mL] × fasting blood glucose [mg/dL]/405).28,29 Based on this score and taking into consideration previously published studies, clinically significant insulin resistance was defined using the HOMA-IR cut-point ≥ 3 in children.30,31
Anthropometric measurements comprised of height, weight, waist-height ratio (WHR), waist circumference (WC), and neck circumference were also collected.32,33 Height and weight were recorded for each patient for calculation of BMI (kg/m2) and BMI z-score. BMI z-score was calculated as the number of standard deviations the child’s BMI differs from the median according to normative values. Compared with BMI, the BMI z-score is preferred by the US Preventative Services Task Force, because it is the only widely available measure that could be used to compare relative degree of excess weight across age groups.2 WC was measured after palpating the iliac crest in the midaxillary lines while participants/parents placed their hands on the opposite shoulders.34 The WC z-score was based on tables using the National Health and Nutrition Examination Survey III. We used an online link to calculate the WC z-score that uses data from these tables: https://apps.cpeggcep.net/WCz_cpeg. We included the WC because it is more strongly associated with cardiometabolic risks than the BMI z-score, likely because of the relationship with central adiposity.35
Polysomnography
All patients included in the analytic sample underwent polysomnography (PSG) using a commercially available PSG system (Nihon Kohden’s Polysmith, Tokyo, Japan) at the Cleveland Clinic sleep laboratory. Physiologic PSG parameters were collected as follows: electroencephalographic activity in wakefulness and in sleep staging, electro-oculograms, chin and lower limb electromyography, electrocardiogram, air flow signals using nasal pressure transducer and air flow thermistor, respiratory effort channels using chest wall and abdominal plethysmography, end-tidal carbon dioxide, and pulse oximetry to measure oxygen saturation. PSG variables considered for analyses included the obstructive apnea-hypopnea index (oAHI), oxygen desaturation index (ODI), arousal index, sleep stages, and total sleep time.
PSG data were scored by a single scorer who was blinded to the metabolic variables and rereviewed to ensure consistency. The respiratory events (apnea and hypopnea) were identified and scored according to the American Academy of Sleep Medicine pediatric criteria as defined in the American Academy of Sleep Medicine Manual for Scoring of Sleep and Associated Events.36 Hypopnea were scored if the event was associated with a 30% reduction in amplitude of the nasal pressure transducer, lasting for at least 2 breaths, and was associated with an arousal/awakening or 3% desaturation. Apneas were defined as ≥90% reduction in airflow lasting at least 2 breaths in duration The oAHI was defined as the total number of obstructive and mixed apnea and hypopnea per hour of sleep. Clinically significant OSA was classified as oAHI ≥ 5 events/h (primary predictor) because of associations of this cutoff with metabolic syndrome in adolescents,21 elevated C-reactive protein levels, and adverse clinical outcomes in young children/adolescents.37 The ODI was defined as the number of times per sleep hour with oxygen desaturation of 3% or more.38
Other PSG and sleep variables examined included percentage (%) of time in slow-wave sleep, sleep efficiency (percentage of the sleep period spent asleep), total sleep time, arousal index, and reported habitual sleep duration during both weekday and weekend.
Statistical methods
Linear regression was used to assess the relationship of HOMA-IR with OSA predictors of interest. Models were unadjusted (model 1); adjusted for sex, race, and BMI z-score (model 2); adjusted for sex, race, and WC z-score (model 3); or adjusted for sex, race, and WHR (model 4). Age was not considered as a covariate given the inherent consideration of age in the z-score calculations. Linear models were performed with oAHI as a categorical variable (<5 vs ≥5 events/h) defined as the primary predictor or oAHI alternatively considered as a continuous variable (ie, interpreted as change in HOMA-IR per 5-unit increase in oAHI). In secondary analyses, we considered ODI as a predictor of HOMA-IR using the same methods as described for oAHI. In our secondary analyses, we also examined WHR, as prior data have suggested that this may be superior to BMI to discriminate obesity-related cardiometabolic risk in adults.39 The interaction between oAHI and age group (dichotomized at 12 years based on the sample median) was tested to assess the difference in the relationship between oAHI and HOMA-IR in patients <12 years vs 12–19 years old. No multicollinearity was identified by variance inflation factor before modeling. Beta estimates and 95% confidence intervals are presented. Outcome HOMA-IR was log-transformed to satisfy normal distribution and transformed back for presentation of estimates and for ease of interpretation.
Receiver operating characteristic (ROC) analysis was used to investigate the optimal cutoff of oAHI and ODI on outcome HOMA-IR ≥ 3. The ROC curve is a plot of the true positive rate (sensitivity) against the false-positive rate (specificity) for the different possible cutoff values of a diagnostic test based on a logistic regression model. The area under the curve (AUC) demonstrated the overall discriminatory power of a diagnostic test over the whole range of testing values. Sensitivity and specificity have been calculated at all possible cutoff points to find the optimal cutoff value. The optimal cutoff value was selected based on the distance to (0, 1), Youden index (sensitivity + specificity − 1), absolute value of difference between sensitivity and specificity, and correct classification rate (weighted average of sensitivity and specificity).
All analyses were performed in SAS software (version 9.4; SAS, Inc, Cary, NC), and a significance level of 0.05 was assumed for all tests.
RESULTS
A total of 80 patients with a mean age of 11.4 ± 4.0 years met inclusion criteria and were analyzed. Forty-five (56.3%) were female, and thirty-seven (46.3%) were Caucasian. All patients were obese (BMI z-score> 95th percentile), with a median BMI of 34.6 kg/m2 (interquartile range, 29.9–40.1) and a median BMI z-score of 2.5 (interquartile range, 2.3–2.8). Forty-three patients (54%) had an oAHI < 5 events/h (non-OSA group), and 37 patients (46%) had an oAHI ≥ 5 events/h (OSA group). Overall, comorbidities were similar in both groups: 19 (23.8%) with hypertension, 15 (18.8%) with asthma, 19 (23.8%) with attention deficit hyperactivity disorder, and 17 (21.3%) with nonalcoholic fatty liver disease. There were no group differences in sex, race, or habitual sleep duration on weekdays and weekends. The OSA group had a higher age, BMI, WHR, waist and neck circumference, arousal index, fasting glucose, and HOMA-IR than those in the non-OSA group. Contrary to the differences in BMI distribution across OSA and non-OSA groups, there were no statistically significant differences of BMI z-score and WC z-score between OSA and non-OSA groups (Table 1).
Table 1.
Descriptive subject characteristics.
| Factor | Overall (n = 80) | AHI < 5 Events/h (n = 43) | AHI ≥ 5 Events/h (n = 37) | P |
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 11.4 ± 4.0 | 10.4 ± 3.5 | 12.5 ± 4.3 | .017a |
| Sex (% male) | 35 (43.8) | 16 (37.2) | 19 (51.4) | .20b |
| Race/ethnicity (%) | .40c | |||
| White | 46.3 | 16 (37.2) | 21 (56.8) | |
| Black | 36.3 | 17 (39.5) | 12 (32.4) | |
| Hispanic | 11.3 | 6 (14.0) | 3 (8.1) | |
| Asian | 1.3 | 1 (2.3) | 0 (0.0) | |
| Other | 5.0 | 3 (7.0) | 1 (2.7) | |
| BMI z-score | 2.5 [2.3, 2.8] | 2.5 [2.3, 2.7] | 2.7 [2.4, 3.1] | .055d |
| Waist circumference z-score | 2.2 [2.0, 2.5] | 2.1 [2.0, 2.4] | 2.3 [2.0, 2.6] | .34d |
| Neck circumference (cm) | 36.3 [34.0, 40.0] | 35.0 [32.0, 38.0] | 38.5 [35.5, 41.9] | .002d |
| Waist-height ratio | 0.69 [0.60–0.78] | 0.66 [0.59–0.73] | 0.72 [0.62–0.82] | .004a |
| Comorbidity (%) | ||||
| Hypertension | 19 (23.8) | 8 (18.6) | 11 (29.7) | .24b |
| Asthma | 15 (18.8) | 9 (20.9) | 6 (16.2) | .59b |
| ADHD | 19 (23.8) | 10 (23.3) | 9 (24.3) | .91b |
| Depression | 11 (13.8) | 4 (9.3) | 7 (18.9) | .21b |
| Anxiety | 13 (16.3) | 6 (14.0) | 7 (18.9) | .55b |
| Allergic rhinitis | 9 (11.3) | 6 (14.0) | 3 (8.1) | .49c |
| GERD | 7 (8.8) | 5 (11.6) | 2 (5.4) | .44c |
| NAFLD | 17 (21.3) | 9 (20.9) | 8 (21.6) | .94b |
| PSG Variables | ||||
| Total sleep time (min) | 391.0 [361.5, 448.0] | 415.0 [371.0, 454.0] | 381.0 [353.0, 416.0] | .060d |
| Sleep efficiency (%) | 87.6 [75.0, 92.7] | 88.7 [75.7, 92.4] | 83.8 [72.4, 94.1] | .67d |
| Sleep latency (min) | 26.5 [12.8, 55.3] | 27.5 [14.5, 47.0] | 24.0 [8.0, 65.0] | .73d |
| AHI (events/h) | 4.0 [1.9, 9.4] | 2.0 [1.00, 2.9] | 10.3 [6.9, 21.9] | <.001d |
| ODI (O2 desaturations/h) | 3.9 [2.1, 9.2] | 2.4 [1.05, 3.2] | 9.2 [6.2, 18.7] | <.001d |
| Mean SpO2 (%) | 96.0 [95.0, 97.0] | 97.0 [96.0, 98.0] | 96.0 [94.0, 96.0] | <.001d |
| Arousal index (events/h) | 13.1 [10.3, 18.3] | 11.6 [8.5, 15.1] | 16.3 [12.6, 24.6] | <.001d |
| Habitual sleep duration | ||||
| Weekday sleep duration (h) | 8.5 [8.0, 10.0] | 9.0 [8.0, 10.0] | 8.0 [8.0, 9.5] | .10d |
| Weekend sleep duration (h) | 10.0 [8.0, 11.0] | 10.0 [8.0, 11.0] | 10.0 [8.5, 10.0] | .56d |
| Labs/metabolic indices | ||||
| Time since labs (mo) | 2.0 [1.00, 7.0] | 3.0 [1.00, 9.0] | 2.0 [1.00, 5.0] | .24d |
| Fasting glucose (mg/dL) | 82.0 [76.0, 88.0] | 79.0 [76.0, 85.0] | 85.0 [80.0, 90.0] | .012d |
| Fasting insulin (mU/mL) | 24.0 [17.4, 35.6] | 22.0 [15.2, 29.0] | 29.0 [18.1, 43.9] | .065d |
| HbA1c (%) | 5.6 [5.3, 5.7] | 5.6 [5.3, 5.7] | 5.6 [5.3, 5.8] | .56d |
| HOMA-IR | 4.2 [3.0, 6.4] | 3.8 [2.7, 5.3] | 5.0 [3.3, 8.5] | .034d |
Data are presented as mean ± SD, median [interquartile range], or n (%) as appropriate. P values: at test, bPearson's χ2 test, cFisher's exact test, and dKruskal-Wallis test. ADHD = attention deficit hyperactivity disorder, AHI = apnea-hypopnea index, BMI = body mass index, GERD = gastroesophageal reflux disease, HOMA-IR = homeostatic model assessment-insulin resistance, NAFLD = nonalcoholic fatty liver disease, ODI = oxygen desaturation index, OSA = obstructive sleep apnea, SpO2 = peripheral capillary oxygen saturation.
In unadjusted analyses, linear regression models showed that OSA (defined as oAHI ≥ 5 events/h) was associated with 40% greater HOMA-IR levels compared with the non-OSA group (oAHI < 5 events/h; β coefficient = 0.40; 95% CI, 0.06, 0.86). After adjustment of sex, race, and BMI z-score, the association between HOMA-IR and oAHI ≥ 5 events/h remained significant (β coefficient = 0.38; 95% CI, 0.01, 0.87), but this association was mitigated and no longer significant in the WC z-score and WHR models (Table 2).
Table 2.
Linear model of obstructive sleep apnea defined by the obstructive apnea-hypopnea index relative to homeostatic model assessment-insulin resistance.
| Predictor | Coefficient (95% CI) | P | |
|---|---|---|---|
| Model 1 (unadjusted) | oAHI ≥ 5 events/h | 0.40 (0.06, 0.86) | .020 |
| Model 2 | oAHI ≥ 5 events/h | 0.38 (0.01, 0.87) | .021 |
| Sex | −0.12 (−0.35, 0.19) | .41 | |
| Race | 0.02 (−0.25, 0.38) | .89 | |
| BMI z-score | 0.14 (−0.16, 0.54) | .40 | |
| Model 3 | oAHI ≥ 5 events/h | 0.23 (−0.11, 0.70) | .20 |
| Sex | −0.10 (−0.35, 0.25) | .52 | |
| Race | −0.03 (−0.31, 0.34) | .83 | |
| WC z-score | 0.08 (−0.21, 0.48) | .63 | |
| Model 4 | oAHI ≥ 5 events/h | 0.08 (−0.22, 0.50) | .64 |
| Sex | −0.12 (−0.36, 0.20) | .40 | |
| Race | −0.02 (−0.28, 0.34) | .91 | |
| WHR (per 0.1 increase) | 0.21 (0.01, 0.44) | .039 |
BMI = body mass index, CI = confidence interval, oAHI = obstructive apnea-hypopnea index, WC = waist circumference, WHR = waist-height ratio.
Table 3 shows unadjusted and adjusted models of the association of HOMA-IR with the primary predictors, continuous oAHI and ODI, including the interaction of age group. For every 5-unit increase in oAHI, there was a 7% increase in HOMA-IR (β coefficient = 0.07, 95% CI, 0.01, 0.13). Similarly, when patients had a 5-unit increase in ODI, HOMA-IR would increase by 7% (β coefficient = 0.07; 95% CI, 0.005, 0.13; P = .034). After adjustment of sex, race, and BMI z-score, the association between HOMA-IR and oAHI remained significant (β coefficient = 0.06; 95% CI, 0.003, 0.13). After these same adjustments, the association between HOMA-IR and ODI was no longer significant (β coefficient = 0.06; 95% C, −0.01, −0.13). After taking into account sex, race, and WC z-score, and WHR, the relationships between HOMA-IR and oAHI, as well as HOMA-IR and ODI, were no longer statistically significant. Age group was assessed to have a modification effect in the relationship of both oAHI and ODI with respect to HOMA-IR. When WC z-score and WHR were included in the model, older and younger groups had statistically significant differential relationships of OSA indices and HOMA-IR (statistical interaction term for WC z-score: P = .020 and .029 for oAHI and ODI, respectively; for WHR: P = .034 and .040 for oAHI and ODI, respectively). However, when adjusting for BMI z-score, findings were no longer statistically significant (Figure 1 and Figure 2).
Table 3.
Unadjusted and adjusted models of the association of homeostatic model assessment-insulin resistance with primary predictors: continuous oAHI and ODI.
| Predictor | oAHI | ODI | |||
|---|---|---|---|---|---|
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | ||
| Model 1 (unadjusted) | oAHI or ODI (per 5 units) | 0.07 (0.01, 0.13) | .015 | 0.07 (0.005, 0.13) | .034 |
| Model 2 | oAHI or ODI (per 5 units) | 0.06 (0.003, 0.13) | .041 | 0.06 (−0.01, 0.13) | .082 |
| Sex | −0.09 (−0.33, 0.23) | .53 | −0.10 (−0.35, 0.23 | .48 | |
| Race | 0.008 (−0.26, 0.36) | .99 | 0.01 (−0.26, 0.39) | .94 | |
| BMI z-score | 0.11 (−0.19, 0.51) | .51 | 0.10 (−0.20, 0.53) | .54 | |
| Model 3 | oAHI or ODI (per 5 units) | 0.04 (−0.02, 0.11) | .21 | 0.04 (−0.04, 0.11) | .34 |
| Sex | −0.09 (−0.35, 0.26) | .55 | −0.10 (−0.36, 0.26) | .53 | |
| Race | −0.04 (−0.31, 0.34) | .81 | −0.03 (−0.31, 0.37) | .86 | |
| WC z-score | 0.05 (−0.24, 0.45) | .77 | 0.05 (−0.25, 0.47) | .77 | |
| Model 4 | oAHI or ODI (per 5 units) | 0.02 (−0.04, 0.08) | .56 | 0.01 (−0.06, 0.09) | .82 |
| Sex | −0.12 (−0.35, 0.20) | .42 | −0.12 (−0.36, 0.22) | .43 | |
| Race | −0.02 (−0.29, 0.34) | .88 | −0.01 (−0.29, 0.37) | .93 | |
| WHR (per 0.1 increase) | 0.20 (0.01, 0.44) | .043 | 0.22 (0.01, 0.47) | .036 | |
| Model 5a | oAHI or ODI (per 5 units); age < 12 years | 0.01 (−0.09, 0.12) | .81 | −0.004 (−0.12, 0.13) | .95 |
| oAHI or ODI (per 5 units); age ≥ 12 years | 0.07 (−0.01, 0.14) | .071 | 0.06 (−0.02, 0.14) | .15 | |
| Sex | −0.13 (−0.36, 0.17) | .35 | −0.14 (−0.37, 0.18) | .36 | |
| Race | −0.05 (−0.30, 0.29) | .72 | −0.03 (−0.29, 0.33) | .83 | |
| BMI z-score | 0.24 (−0.10, 0.72) | .18 | 0.27 (−0.10, 0.78) | .17 | |
| Age ≥ 12 vs < 12 years | 0.26 (−0.12, 0.81) | .21 | 0.28 (−0.13, 0.87) | .20 | |
| Model 6a | oAHI or ODI (per 5 units); age ≥ 12 years | −0.10 (−0.20, 0.02) | .093 | −0.12 (−0.24, 0.01) | .078 |
| oAHI or ODI (per 5 units); age ≥ 12 years | 0.06 (−0.01, 0.14) | .088 | 0.05 (−0.03, 0.14) | .20 | |
| Sex | −0.13 (−0.36, 0.18) | .37 | −0.11 (−0.36, 0.22) | .46 | |
| Race | −0.11 (−0.35, 0.22) | .46 | −0.10 (−0.35, 0.25) | .53 | |
| WC z-score | 0.37 (−0.03, 0.93) | .073 | 0.38 (−0.03, 0.97) | .076 | |
| Age ≥ 12 vs < 12 years | 0.23 (−0.16, 0.81) | .28 | 0.25 (−0.17, 0.88) | .28 | |
| Model 7a | oAHI or ODI (per 5 units); age ≥ 12 years | −0.09 (−0.19, 0.02) | .097 | −0.12 (−0.23, 0.01) | .066 |
| oAHI or ODI (per 5 units); age ≥ 12 years | 0.05 (−0.02, 0.12) | .20 | 0.03 (−0.04, 0.12) | .39 | |
| Sex | −0.16 (−0.37, 0.13) | .26 | −0.14 (−0.37, 0.17) | .33 | |
| Race | −0.09 (−0.33, 0.23) | .52 | −0.08 (−0.33, 0.25) | .58 | |
| WHR (per 0.1 increase) | 0.22 (0.03, 0.45) | .022 | 0.24 (0.04, 0.48) | .015 | |
| Age ≥ 12 vs < 12 years | 0.13 (−0.21, 0.60) | .50 | 0.14 (−0.22, 0.65) | .50 | |
Models 2 and 5 adjusted for BMI z-score; models 3 and 6 adjusted for WC z-score; models 4 and 7 adjusted for WHR; and models 5, 6, and 7 include the interaction of age groups. aModel 5, P = .41; model 6, P = .020 for oAHI and P = .029 for ODI; model 7, P = .034 for oAHI and P = .040 for ODI. BMI = body mass index, oAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index, WC = waist circumference, WHR = waist-height ratio.
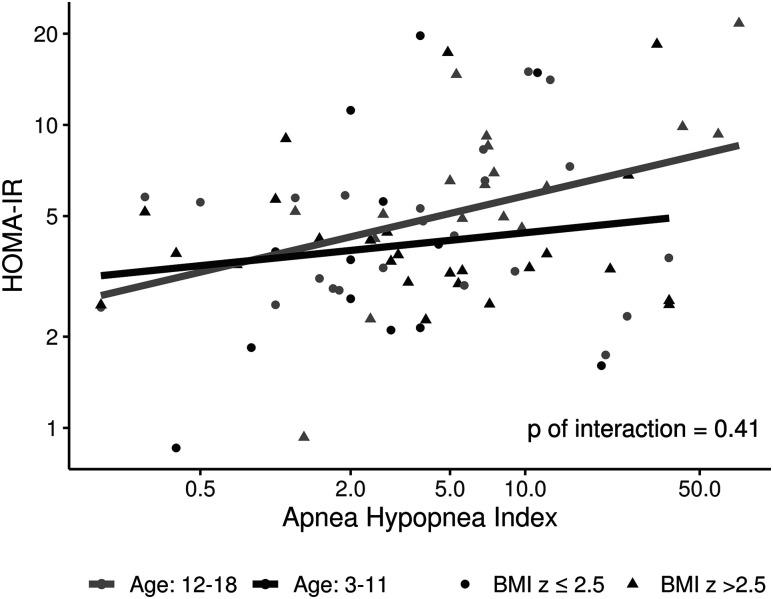
Figure 1. Scattered plots showing the relationship of HOMA-IR and oAHI along with interactions of age and BMI z-score.
The relationship between HOMA-IR and oAHI had no significant difference (P = .41) among age groups when using the interaction of BMI z-score. BMI = body mass index, HOMA-IR = homeostatic model assessment of insulin resistance, oAHI = obstructive apnea-hypopnea index.
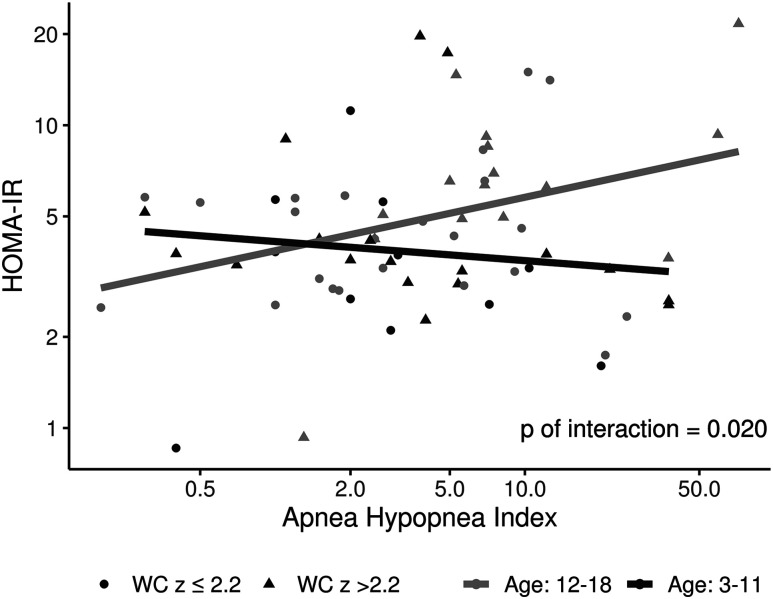
Figure 2. Scatter plots showing the relationship of HOMA-IR and oAHI along with interactions of age and waist circumference z-score.
The relationship between HOMA-IR and ODI was significant (P = .029), when factoring waist circumference z-score, where HOMA-IR increased in the older group but decreased in the younger group when oAHI increased. BMI = body mass index, HOMA-IR = homeostatic model assessment of insulin resistance, oAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index.
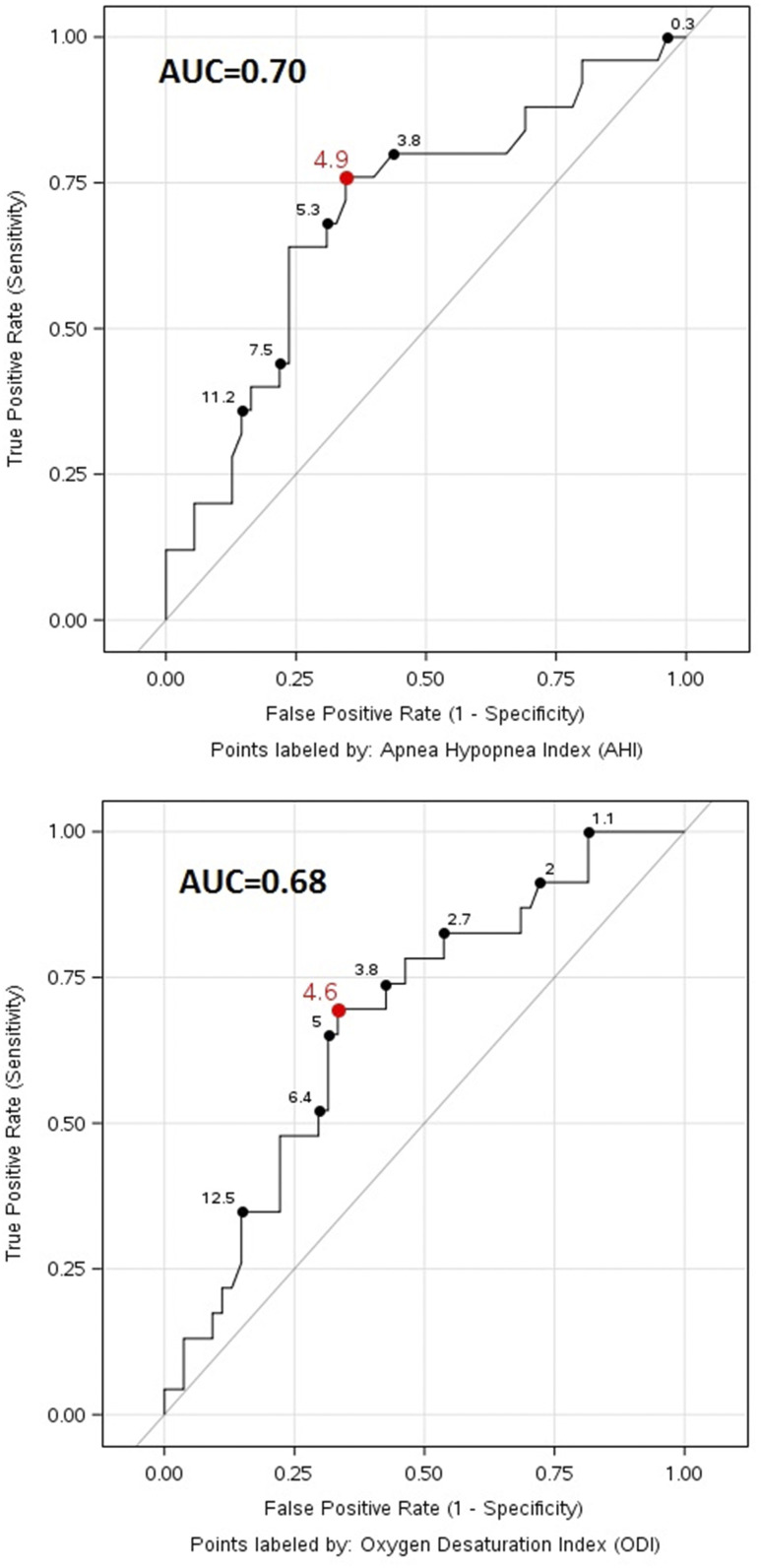
Additional secondary analyses were focused on the evaluation of oAHI and ODI cutoffs by assessment of ROC curves. ROC curves identified the oAHI cut-point of ≥4.9 as predicting HOMA-IR ≥ 3 with a sensitivity 0.76, specificity of 0.66, and AUC of 0.70 (95% CI, 0.57, 0.83; P = .028). The ODI cut-point of ≥4.6 showed a sensitivity of 0.70, specificity of 0.67, and AUC of 0.68 (95% CI, 0.55, 0.80) for predicting HOMA-IR ≥ 3, without significant associations between ODI and high HOMA-IR (P = .11) (Figure 3).
Figure 3. Receiver operating characteristic curve (ROC) of sleep indices on HOMA-IR ≥ 3.
Top: ROC of oAHI on HOMA-IR ≥ 3. The association of oAHI and HOMA-IR ≥ 3 was significant at P = .028. The oAHI cutoff value was 4.9 (sensitivity, 0.76; specificity, 0.66) and AUC was 0.70 (95% confidence interval, 0.57–0.83). Bottom: The association of ODI and HOMA-IR ≥ 3 was not significant (P = .11). The ODI cutoff value was 4.6 (sensitivity, 0.70; specificity, 0.67, and AUC was 0.68 (95% confidence interval, 0.55–0.80). AUC = area under the receiver operating characteristic curve, HOMA-IR = homeostatic model assessment of insulin resistance, oAHI = obstructive apnea-hypopnea index, ODI = oxygen desaturation index.
DISCUSSION
In this study of patients presenting to a pediatric obesity clinic (median BMI = 34.6 kg/m2), we identified a significant association of OSA (both defined by oAHI dichotomized at 5 events/h and also as a continuous measure) and HOMA-IR even after adjustment for obesity. This study extends current knowledge of OSA and association with insulin resistance in pediatric obesity to a clinical cohort with sex- and race-based diversity, as well as careful consideration of anthropometric measures. When considering a 5-unit increase in oAHI or ODI as a continuous predictors, a 7% increase in HOMA-IR was observed even after accounting for obesity (based on BMI z-score). The statistical interaction of both oAHI and ODI with respect to age on HOMA-IR was significant even after adjustment for waist z-circumference.
Our study contrasts with a similar study investigating children without obesity with habitual snoring, in which BMI was a significant predictor of fasting insulin and HOMA-IR and not the severity of sleep-disordered breathing.40 Because BMI may not precisely reflect adiposity and obesity in children, we chose to examine associations with BMI z-score, which may allow for more accurate discernment of influence of obesity and adiposity. In a study of snoring in children with and without obesity, obesity was defined as the principal determinant of IR, whereas snoring appeared to have a lesser role.25 In both these studies, snoring, and not PSG measuring oAHI, was used. Snoring alone is on the spectrum of sleep-disordered breathing and may by itself not be associated with IR. Our results indicate that OSA, as measured by the gold standard PSG, is associated with IR in an obese pediatric population beyond the influence of weight or BMI alone.
We also examined adiposity in the relationship between IR and OSA in children because of previous findings that adiposity may be the predominant determinant of IR compared with OSA or sleep architecture.41 We examined adiposity by including both the BMI z-score and WC z-score to measure clinically derived measures, with the latter being more strongly associated with cardiometabolic risks because of the relationship with central adiposity.35 When taking waist circumference z-score into account, oAHI and ODI were no longer significantly associated with HOMA-IR. Similarly, in our secondary analysis, we examined WHR in the relationship with IR and OSA. When taking WHR into account, oAHI and ODI were no longer associated with HOMA-IR. In fact, WHR appeared more sensitive to IR than BMI z-score and WC z-score in the different models (Table 3). With every 0.1-unit increase in WHR, HOMA-IR would increase 22% (95% CI, 0.036, 0.44; P = .018).
However, we demonstrated an important association of OSA and IR in children with obesity, particularly using WC z-score and WHR when we examined age groups. In the older pediatric population (age ≥ 12 years), increasing AHI conferred a risk of increasing HOMA-IR compared with the younger group (age < 12 years). There are several potential explanations for our findings. Older children tend to have more arousals/sleep disruption from OSA compared with younger children, who tend to preserve their sleep architecture, which may play some role in these age-related findings.42 In fact, sleep-specific predictors of IR children with and without obesity undergoing sleep testing include elevated arousal index and short sleep duration.41,43 However, in our study, these parameters were not predictive of IR. As anticipated, arousals and sleep fragmentation were more likely to occur in the sleep apnea group but did not incur an independent risk of IR apart from OSA. Another factor that may play some role in our age-related findings may be attributable to the use of WCs in the younger population. Although WC may serve as an indirect measure of adiposity in the older age group in which adiposity prevalence is higher, utility in the younger group with lower degrees of adiposity may not be comparable, because WC reflects the visceral fat deposition over time, which accumulates with age. In total, these data may support a dichotomy of the primary influence of adiposity on IR in the younger population, whereas the older population may have associations with both adiposity and OSA on the risk for IR. Although beyond the scope of this study, duration of obstructive breathing may explain why OSA is associated with increased IR in the older group. Further studies are needed to corroborate these findings.
Other studies may support the pathophysiologic influences of OSA in the obese population. In studies examining IR, OSA severity was associated with fasting insulin and other metabolic derangements.22 Another study demonstrated that fasting insulin and HOMA-IR predicted severe OSA independent of age, sex, and BMI z-score.22 Our findings concur with these studies. The physiologic mechanism of OSA associations with IR have been elucidated in several experimental studies. Intermittent nocturnal hypoxemia is an important mechanism for increased sympathetic activity and upregulation of systemic inflammation that can lead to metabolic alterations.44 One recent study demonstrated intermittent hypoxia induction of a proinflammatory phenotype of the adipose tissue linked potential associations between OSA and the development of insulin resistance.45 Thus, taken together, OSA likely contributes in part to IR in children with obesity.
Furthermore, we evaluated the role of oAHI and ODI values in the relationship with IR. PSG data using a cutoff of oAHI ≥ 4.9 events/h may offer a way to predict patients with obesity at risk of IR with a sensitivity and specificity of 76% and 66%, respectively. Our results are comparable to another study that showed adolescents with oAHI ≥ 5 events/h had a high odds of metabolic syndrome compared with those with oAHI < 5 events/h (odds ratio, 7.74; 95% CI, 3.10, 19.35).21 Taken together, the metabolic syndrome (dyslipidemia, hypertension, and IR) in children with obesity is associated with cardiovascular disease later in adulthood. Therefore, identification of those children at risk for development of metabolic alterations, whether related to their level of sleep disturbance, BMI, or both, continues to be an important area of study.
Early recognition and treatment of OSA in children with obesity with IR may lead to improved outcomes. Few studies examined the effects of OSA treatment either by the surgery or application of continuous positive airway pressure. Some show a potential reduction in IR in children with obesity with OSA.23,24,46 Alternatively, some studies showed continuous positive airway pressure treatment fails to improve IR in obese adolescents.26 Future studies are needed to investigate the impact of OSA and sleep disorders on IR in children with obesity. Given the impact of BMI and adiposity on IR in children, weight loss should be encouraged aggressively as part of the management of OSA in children with obesity.
Strengths of the study included OSA diagnosis and measurement of severity by the historically considered gold standard in-laboratory PSG. Additionally, we examined known factors that may relate to the relationship between sleep and metabolic factors, such as habitual sleep duration and arousal index. Our population was an at-risk population of children with obesity, and we controlled for factors that impact BMI, sleep, and IR. We also examined different measures of weight and adiposity using clinical measurements: BMI z-score, WC z-score, and WHR. We report the first findings to include all these measurements to examine the risk of IR in OSA in a pediatric obese cohort. Our population was rich with diversity of race, age, and comorbidity in an obese high-risk population. We excluded patients on medications that effected IR, such as metformin. In our study, we used a cutoff value based on prior work, which has shown the mean value for 95th percentile in a study of healthy children with a similar age range that therefore provides tenable rationale.
The retrospective examination of prospectively collected clinic-based data is a limitation; however, overall, our cohort had a high level of completeness of data. Our results are based on a clinic-based cohort of children with obesity and therefore may not be generalizable to children who are overweight or normal weight. Although metabolic measures were not performed on the same day as the PSG, there was a relatively narrow time frame of 2 months (median) between the timing of the PSG and fasting blood work. The study was limited by a small sample size but was comparable to similar studies and sufficiently powered to conduct an analysis of children with obesity with and without significant OSA. Inclusion criteria were those who were referred for PSG, which may incur a possible referral bias. We aimed to address the effects of age but not pubertal status because of the lack of Tanner staging data. Because our study modeled a clinical cohort, our study did not directly measure adiposity or visceral fat distribution, which has previously been shown to be independently predictive of OSA severity in children with obesity.47,48 Our study used anthropometric measurements that would be clinically relevant, such as BMI, neck circumference, and WC. The clinical cohort cannot determine the length of duration of obstructive breathing or OSA, and thus exposure effects are limited. Our analysis used pediatric criteria of scoring respiratory events, with variability of event duration. However, our results are reflective of a clinically based sample. Moreover, cutoff values of HOMA-IR in children are not well established. Evidence shows HOMA-IR cutoff values differ by race, age, sex, BMI, and individuals with different diseases.49–51
CONCLUSIONS
Our findings support the association of OSA and IR in children with obesity. However, in younger children, after adjusting for adiposity using WC z-score and WHR, the relationship does not appear to be statistically significant. Obesity may be the primary determinant of IR in some children. There remains some impact of OSA on IR in this high-risk pediatric population, such that those with a cutoff oAHI of ≥4.9 events/h appear to be at higher risk of IR. This cutoff may be used to determine risk and treatment of OSA discussions clinically. Future studies are needed to verify these findings and investigate the impact of treatment on OSA and metabolic outcomes in a larger-scale pediatric obesity studies.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Cleveland Clinic Foundation. Conflict of interest: R.M. reports receiving National Institutes of Health funding support from the National Heart, Lung, and Blood Institute [U01HL125177, UG3HL140144] and the American Heart Association. R.M. has received funds for service on the American Board of Internal Medicine Sleep Medicine Exam test writing committee and received royalties from UpToDate. All other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff of the Sleep Disorders Center, Cleveland Clinic, who participated in this study and the pediatric Be-well obesity management program, Cleveland Clinic.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BMI
body mass index
- HOMA-IR
homeostatic model assessment of insulin resistance
- IR
insulin resistance
- oAHI
obstructive apnea-hypopnea index
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- ROC
receiving operator curve
- WC
waist circumference
- WHR
waist-height ratio
REFERENCES
- 1.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012;345:e4759. 10.1136/bmj.e4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(23):2417–2426. 10.1001/jama.2017.6803 [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346(11):802–810. 10.1056/NEJMoa012578 [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes. 2002;51(1):204–209. 10.2337/diabetes.51.1.204 [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164(1):16–30. 10.1164/ajrccm.164.1.2008171 [DOI] [PubMed] [Google Scholar]
- 6.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol. 1993;16(2):124–129. 10.1002/ppul.1950160208 [DOI] [PubMed] [Google Scholar]
- 7.Verhulst SL, Van Gaal L, De Backer W, Desager K. The prevalence, anatomical correlates and treatment of sleep-disordered breathing in obese children and adolescents. Sleep Med Rev. 2008;12(5):339–346. 10.1016/j.smrv.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Seif F, Patel SR, Walia HK, et al. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens. 2014;32(2):267–275. 10.1097/HJH.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18(2):179–189. 10.1016/j.smrv.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Chan JY, Li AM, Au CT, et al. Cardiac remodelling and dysfunction in children with obstructive sleep apnoea: a community based study. Thorax. 2009;64(3):233–239. 10.1136/thx.2007.094904 [DOI] [PubMed] [Google Scholar]
- 11.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128(1):e85–e92. 10.1542/peds.2010-3431 [DOI] [PubMed] [Google Scholar]
- 12.Monneret D, Tamisier R, Ducros V, et al. Glucose tolerance and cardiovascular risk biomarkers in non-diabetic non-obese obstructive sleep apnea patients: effects of long-term continuous positive airway pressure. Respir Med. 2016;112:119–125. 10.1016/j.rmed.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 13.Karney A, Bragoszewska H, Soluch L, Oltarzewski M.. [Risk factors for atherosclerosis in obese children aged 6-12 years]. Dev Period Med. 2017;21(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kheirandish-Gozal L, Philby MF, Qiao Z, Khalyfa A, Gozal D. Endothelial dysfunction in children with obstructive sleep apnea is associated with elevated lipoprotein-associated phospholipase A2 plasma activity levels. J Am Heart Assoc. 2017;6(2. 10.1161/JAHA.116.004923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehra R, Storfer-Isser A, Kirchner HL, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166(16):1725–1731. 10.1001/archinte.166.16.1725 [DOI] [PubMed] [Google Scholar]
- 16.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. 10.1093/aje/kwh261 [DOI] [PubMed] [Google Scholar]
- 17.Tahrani AA, Ali A, Stevens MJ. Obstructive sleep apnoea and diabetes: an update. Curr Opin Pulm Med. 2013;19(6):631–638. 10.1097/MCP.0b013e3283659da5 [DOI] [PubMed] [Google Scholar]
- 18.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155(5):387–393. 10.1093/aje/155.5.387 [DOI] [PubMed] [Google Scholar]
- 19.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34(11):2442–2447. 10.2337/dc11-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koren D, O’Sullivan KL, Mokhlesi B. Metabolic and glycemic sequelae of sleep disturbances in children and adults. Curr Diab Rep. 2015;15(1):562. 10.1007/s11892-014-0562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401–408. 10.1164/rccm.200703-375OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhushan B, Maddalozzo J, Sheldon SH, et al. Metabolic alterations in children with obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2014;78(5):854–859. 10.1016/j.ijporl.2014.02.028 [DOI] [PubMed] [Google Scholar]
- 23.Koren D, Gozal D, Bhattacharjee R, Philby MF, Kheirandish-Gozal L. Impact of adenotonsillectomy on insulin resistance and lipoprotein profile in nonobese and obese children. Chest. 2016;149(4):999–1010. 10.1378/chest.15-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz SL, MacLean JE, Hoey L, et al. Insulin resistance and hypertension in obese youth with sleep-disordered breathing treated with positive airway pressure: a prospective multicenter study. J Clin Sleep Med. 2017;13(9):1039–1047. 10.5664/jcsm.6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tauman R, O’Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116(1):e66–e73. 10.1542/peds.2004-2527 [DOI] [PubMed] [Google Scholar]
- 26.Nakra N, Bhargava S, Dzuira J, Caprio S, Bazzy-Asaad A. Sleep-disordered breathing in children with metabolic syndrome: the role of leptin and sympathetic nervous system activity and the effect of continuous positive airway pressure. Pediatrics. 2008;122(3):e634–e642. 10.1542/peds.2008-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. 10.1093/clinchem/41.2.264 [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 29.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. 10.2337/diacare.23.1.57 [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther. 2015;9(6):380–385. 10.5582/ddt.2015.01207 [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Li M, Xu L, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol Metab Syndr. 2013;5(1):71. 10.1186/1758-5996-5-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AC, Chou YT, Chang TL. Usefulness of the Mini Nutritional Assessment (MNA) in predicting the nutritional status of people with mental disorders in Taiwan. J Clin Nurs. 2011;20(3-4):341–350. 10.1111/j.1365-2702.2010.03467.x [DOI] [PubMed] [Google Scholar]
- 33. McQuillan GM, McLean JE, Chiappa M, Corporation H, Lukacs SL. National Health and Nutrition Examination Survey Biospecimen Program: NHANES III (1988-1994) and NHANES 1999-2014. Vital Health Stat 2. 2015;170:1-14. [PubMed]
- 34.Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78(6):723–729. 10.1038/pr.2015.160 [DOI] [PubMed] [Google Scholar]
- 35.Sharma AK, Metzger DL, Rodd CJ. Prevalence and severity of high blood pressure among children based on the 2017 American Academy of Pediatrics Guidelines. JAMA Pediatr. 2018;172(6):557–565. 10.1001/jamapediatrics.2018.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry R, Brooks R, Gamaldo C, et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012 [Google Scholar]
- 37.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111(15):1978–1984. 10.1161/01.CIR.0000161819.76138.5E [DOI] [PubMed] [Google Scholar]
- 38.Mitchell RB, Garetz S, Moore RH, et al. The use of clinical parameters to predict obstructive sleep apnea syndrome severity in children: the Childhood Adenotonsillectomy (CHAT) study randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(2):130–136. 10.1001/jamaoto.2014.3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–286. 10.1111/j.1467-789X.2011.00952.x [DOI] [PubMed] [Google Scholar]
- 40.Kaditis AG, Alexopoulos EI, Damani E, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol. 2005;40(6):515–523. 10.1002/ppul.20306 [DOI] [PubMed] [Google Scholar]
- 41.Koren D, Gozal D, Philby MF, Bhattacharjee R, Kheirandish-Gozal L. Impact of obstructive sleep apnoea on insulin resistance in nonobese and obese children. Eur Respir J. 2016;47(4):1152–1161. 10.1183/13993003.01430-2015 [DOI] [PubMed] [Google Scholar]
- 42.Busby KA, Mercier L, Pivik RT. Ontogenetic variations in auditory arousal threshold during sleep. Psychophysiology. 1994;31(2):182–188. 10.1111/j.1469-8986.1994.tb01038.x [DOI] [PubMed] [Google Scholar]
- 43.Lesser DJ, Bhatia R, Tran WH, et al. Sleep fragmentation and intermittent hypoxemia are associated with decreased insulin sensitivity in obese adolescent Latino males. Pediatr Res. 2012;72(3):293–298. 10.1038/pr.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamsuzzaman A, Szczesniak RD, Fenchel MC, Amin RS. Glucose, insulin, and insulin resistance in normal-weight, overweight and obese children with obstructive sleep apnea. Obes Res Clin Pract. 2014;8(6):e584–e591. 10.1016/j.orcp.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy AM, Thomas A, Crinion SJ, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. 2017;49(4):1601731. 10.1183/13993003.01731-2016 [DOI] [PubMed] [Google Scholar]
- 46.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177(10):1142–1149. 10.1164/rccm.200711-1670OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canapari CA, Hoppin AG, Kinane TB, Thomas BJ, Torriani M, Katz ES. Relationship between sleep apnea, fat distribution, and insulin resistance in obese children. J Clin Sleep Med. 2011;7(3):268–273. 10.5664/JCSM.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhatia R, Lesser DJ, Oliveira FG, et al. Body fat composition: a predictive factor for sleep related breathing disorder in obese children. J Clin Sleep Med. 2015;11(9):1039–1045. 10.5664/jcsm.5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakai Y, Nakaishi S, Kishimoto H, et al. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. 2002;19(4):346–347. 10.1046/j.1464-5491.2002.00712_3.x [DOI] [PubMed] [Google Scholar]
- 50.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013;13(1):47. 10.1186/1472-6823-13-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shashaj B, Luciano R, Contoli B, et al. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016;53(2):251–260. 10.1007/s00592-015-0782-4 [DOI] [PubMed] [Google Scholar]