Abstract
Background:
The estimated glomerular filtration rate (eGFR) is frequently used to monitor progression of kidney disease. Multiple values have to be obtained, sometimes over years to determine the rate of decline in kidney function. Recent data suggest that functional MRI (fMRI) methods may be able to predict loss of eGFR. In a prior study, baseline data with multi-parametric MRI in individuals with diabetes and moderate chronic kidney disease (CKD) was reported. This report extends our prior observations in order to evaluate the temporal variability of the fMRI measurements over 36 months and their association with annual change in eGFR.
Methods:
Twenty-four subjects with moderate CKD completed three sets of MRI scans over a 36 month period. Blood oxygenation level dependent (BOLD), arterial spin labeling perfusion and diffusion MRI images were acquired using a 3T scanner. Coefficients of variation was used to evaluate variability between subjects at each time point and temporal variability within each subject. We have conducted mixed effects models to examine the trajectory change in GFR over time using time and MRI variables as fixed effects and baseline intercept as random effect. Associations of MRI image markers with annual change in eGFR were evaluated.
Results:
Multi-parametric functional renal MRI techniques in individuals with moderate CKD showed higher temporal variability in R2* of medulla compared to healthy individuals. This was consistent with the significant lower R2* in medulla observed at 36 months compared to baseline values. The results of liner mixed model showing that R2*_Medulla was the only predictor associated with change in eGFR over time. Furthermore, a significant association of medullary R2* with annual loss of eGFR was observed at all the three time-points.
Conclusions:
The lower R2* values and the higher temporal variability in the renal medulla over time suggest the ability to monitor progressive CKD. These were confirmed by the fact that reduced medullary R2* was associated with higher annual loss in eGFR. These data collectively emphasize the need for inclusion of medulla in the analysis of renal BOLD MRI studies.
Keywords: chronic kidney disease, MRI, BOLD, perfusion, diffusion, longitudinal study
Introduction
Chronic kidney disease (CKD) affects about 37 million people in the United States [1]. However, the rate of progression of disease is variable and only a small fraction progress to end stage kidney disease (ESKD) when dialysis and/or kidney transplantation become the only treatment options [2]. Without the ability to distinguish those with progressive CKD, management cannot be individualized to those at high risk of progressive disease. Much of clinical management is based on renal function estimated by the use of serum creatinine measurement. While a single value is a convenient non-invasive estimate of glomerular filtration rate (GFR), it does not predict risk of progression.
Pathophysiology of progressive CKD is thought to involve the interplay of decreased blood flow, renal hypoxia and fibrosis [3, 4]. Over the last two decades, several studies have indicated the feasibility of using noninvasive MRI to evaluate blood flow [5, 6], kidney oxygen availability [7–9], and fibrosis [10–13] in the evaluation of CKD. Blood oxygen level-dependent (BOLD) MRI is sensitive to intrarenal oxygen availability. Arterial spin labeling (ASL) is able to quantify renal tissue perfusion. Apparent diffusion coefficient (ADC) is a quantitative parameter calculated from diffusion weighted MR imaging (DWI) to probe renal fibrosis. Use of multi-parametric approach in the evaluation of CKD has been shown to be feasible [14, 15] and is promoted by an international consortium [16]. The level of reproducibility for these multiple MRI parameters have been previously reported in healthy individuals [17].
The purpose of this study is first to examine longitudinal functional MRI measurements in individuals with moderate CKD over 36 months to see if they vary over time, and to compare the level of variability with prior estimates in healthy individuals [17]. In addition, we evaluated whether any of the MRI parameters demonstrate associations at any of the three time points with annual change in eGFR.
Materials and Methods
Subjects
All procedures were approved by local institutional review board and a written consent was obtained from each participant prior to study procedures. Inclusion criteria included diabetes (type 1 and 2), ability and willingness of the subject to cooperate with the study protocol and absence of contraindications for MR study (claustrophobia, pacemaker, intra-cranial clips, intraocular debris). Exclusion criteria included 1) Significant co-morbid conditions that lead the investigator to conclude that life expectancy is less than 1 year. 2) Expected to undergo living related transplant or initiation of dialysis in next 24 month. 3) Pregnant or nursing. 4) Involved in any other interventional research protocol. 5) Decompensated heart failure. 6) Previous diagnosis for renal artery stenosis or ureteral obstruction. 7) CKD of other etiologies such as glomerular disease, interstitial disease and polycystic kidney disease 8) Use of nonsteroidal anti-inflammatory agents 9) patients treated for anemia with ferumoxytol (feraheme®). The study included 41 individuals with diabetes and stage 3 CKD and the baseline data was previously reported [18]. Among these, 32 returned for an 18 month scan and 24 participated in a 36 month scan. Here, we report data on the 24 participants that completed all the three study time points.
Baseline characteristics of the study participants are summarized in Table 1. Participants were instructed not to take non-steroidal anti-inflammatory drugs (NSAIDs) for 3 days prior and angiotensin converting enzyme inhibitor or angiotensin receptor blockers (ACEi/ARBs) for 1 day prior to MRI. Individuals using insulin were asked to take half of their dose on the day of the MRI. During each scan visit, eGFR was calculated based on serum creatinine concentration using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [19]. Serum glucose, glycated hemoglobin (HbA1c) concentrations and data to estimate urine albumin creatinine ration (ACR) measurements were obtained from the electronic medical records (EMR) close to each scan time for each patient. Additionally, eGFR data from EMR was extracted from up to two years prior to enrollment and till the end of the study in order to estimate annual change in renal function (eGFR_slope).
Table 1.
Baseline Characteristics of study population
| Variable | CKD (n=24) |
|---|---|
| Age, years | 64.6 ± 8.9 |
| Gender, male, n(%) | 12 (50) |
| Race, n(%) | |
| White | 17 (71) |
| African-American | 6 (25) |
| Other | 1 (4) |
| BMI, kg/m2 | 31.0 ± 6.7 |
| Diabetes, n(%) | |
| Type 2 | 19 (79) |
| Type 1 | 5 (21) |
| Systolic BP, mm Hg | 134.4 ± 21.4 |
| Serum glucose, mg/dL | 160.7 ± 80.5 |
| Urine Albumin/Creatinine (ACR) | 0.13 (0.03 ∼0.69)* |
| HbA1c (%) | 7.41 ± 0.93 |
| eGFR (mL/min/1.73m2) | 52.5 ± 13.4 |
Shown as median (interquartile interval)
MRI image acquisition
Participants were instructed to fast from midnight prior to the MRI scan performed in the morning. Experiments were performed on a 3 Tesla scanner (Siemens Healthcare, Erlangen, Germany). Individuals were positioned feet first in supine position. Body coil was used as the transmitter, and the combination of spine and body array coils was used as the receiver.
BOLD MRI scans were performed using a multiple gradient-recalled-echo (mGRE) sequence [20] to acquire images in the coronal plane during a 13 seconds breath-hold at the end-expiration. Following were the acquisition parameters: Field of view (FOV) = 380 – 400, number of slices = 5, slice thickness = 5.0mm, matrix = 256 × 256, repletion time (TR) = 51ms, number of echoes = 8 and equally spaced (3.1–30.5ms), # averages = 1 and bandwidth = 260 Hz/pixel.
Perfusion MRI images were acquired using a 2D navigator-gated flow-sensitive alternating inversion recovery (FAIR) True-FISP sequence [21] with the following parameters: FOV = 380 mm, slice thickness = 8 mm, TR = 3s; echo spacing/TE = 4.0/2.2 ms, flip angle = 60°, 128 × 128 matrix, bandwidth = 501 Hz/pixel, number of measurements = 100, and inversion delay = 2,000 ms. An oblique-coronal orientation was prescribed on the scout image so as to avoid major vessels. A 30 mm slice selective inversion band was placed over the ASL image slice. A 10.24 ms adiabatic frequency offset correction inversion (FOCI) pulse (μ=6, β=1078) was applied for selective inversion [22]. A 2D low resolution navigator image was acquired (TR / TE = 2.2 / 1.2 ms; FA = 5o; FOV = 400 × 400 mm2; matrix = 96 × 96; BW = 1000 Hz / pixel; GRAPPA factor = 2) immediately following the True-FISP image acquisition. Proton density images with no inversion were acquired to measure M0, necessary to obtain absolute quantitative blood flow.
Diffusion MRI was acquired using spin echo planar imaging (EPI) in matched slice positions as the BOLD image acquisition. The diffusion sensitizing gradients were applied along three different directions in order to calculate diffusion trace. Following were the MRI parameters: TE = 78 ms; FOV = 380–400mm; TR = 3,000 ms; bandwidth = 1,628 Hz/pixel; matrix = 192×154; #slices = 5; slice thickness = 5 mm; five different b values (200, 300, 500, 700 and 1,000 s/mm2). Five acquisitions were averaged to improve signal to noise ratio and to minimize motion artifacts.
After acquiring baseline data, each participant received 20 mg of furosemide iv (Hospira, Lake Forest, IL, USA) and BOLD MRI acquisitions were repeated for 20 minutes post administration.
MRI analysis methods
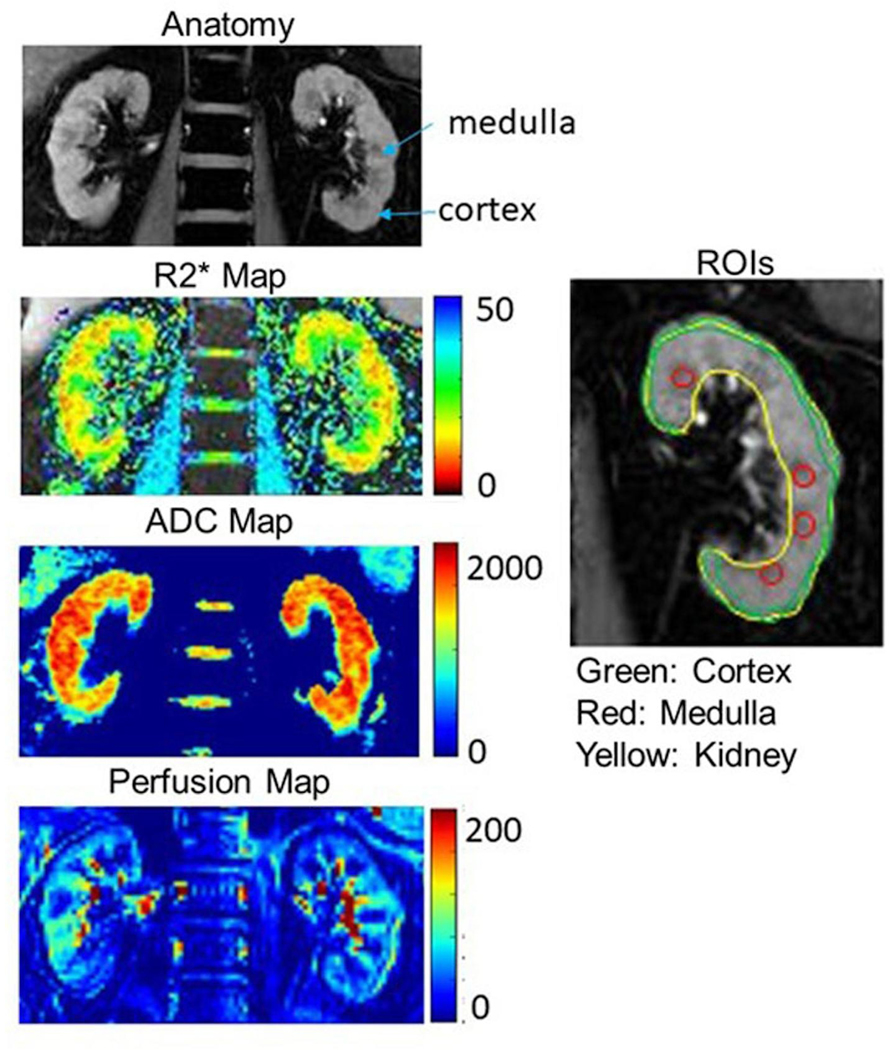
R2* and ASL perfusion maps were generated with a customized Python and Matlab code respectively [21, 23]. A Python [24] based toolbox was also used to perform regions of interest (ROIs) analysis for all parametric maps, i.e. ADC, perfusion and R2* maps. ROIs were manually defined to include cortex, medulla or whole kidney by experienced researchers. A ring shape ROI encompassed entire cortical tissue, multiple small ROIs in medulla, and entire renal parenchyma (representing whole kidney) ROI were defined on the anatomic images [Figure 1]. Once the ROIs are defined, the R2* maps were calculated and the ROIs applied to estimate regional R2* values. Cortical ROIs were defined directly on ASL and ADC maps. Mean values from all individual ROIs from the 5 slices for BOLD and diffusion MRI from both left and right kidneys were averaged to calculate representative MRI parameters (R2*_Cortex, R2*_Kidney, R2*_Medulla, and ADC_Cortex from DWI scan). Representative perfusion in cortex (Perfusion_Cortex) was averaged from both left and right kidney from one slice of the ASL scan.
Figure 1.
Parametric (R2*, ADC and ASL perfusion) maps in a representative participant. The color bars for each of the parameters are included. Also included are anatomic MR images (short TE images from BOLD MRI acquisition) as a reference and an illustration of ROI definitions in cortex, medulla and kidney.
For the estimation of eGFR_slope, we included additional eGFR measurements available from EMR. The average time range for eGFR readings was 2.8 –7.6 years with an average of 4.8 years.
Statistical analysis
Mean and standard deviation values are reported for each measurement. Non-parametric one way ANOVA (Kruskal-Wallis analysis) was used to evaluate differences between the three time points for all the MRI parameters. Post-hoc pairwise comparisons were conducted to determine which scan pairs are significantly different with each other.
Inter- and intra-subject coefficients of variation (CV) were calculated as a measure of within subject variability at each time point, and temporal variability from baseline to 18 and 36 month data, respectively. Calculations of Inter- and intra-CV (SD/mean, expressed as %) were based on definitions previously described [25].
We conducted mixed effects models to examine the trajectory change in GFR over time using time and MRI variables as fixed effects and baseline intercept as random effect. In addition, associations of MRI parameters with eGFR_slope (as indicator of progressive CKD) was conducted using non-parametric Spearman correlation analysis at each of the three time points. If significant correlation was observed with any of the MRI parameters, further linear regression analysis was performed to evaluate any potential confounding factors (e.g. age, sex, BMI, serum glucose, ACR and eGFR). Given the skewed distribution of ACR values, Ln(ACR) was used.
All of the statistical analyses were carried out using IBM SPSS Statistics 22 (IBM Corporation, Armonk, NY, USA), and p value < 0.05 was regarded as statistically significant.
Results
The average interval between baseline and 18 month scan was 19.2 ± 1.5 months (574.5 ± 44.6 days). The average interval between 18 and 36 month scans was 18.7 ± 2.0 months (559.7 ± 60.5 days), and interval between baseline and 36 month was 38.0 ± 2.2 months (1139.8 ± 67.1 days).
Figure 1 shows an anatomic MRI image of the kidney from a representative individual and the related functional maps for BOLD, ADC and ASL. Included is an illustration of the ROIs defined.
Table 2 summarizes all the MRI measurements at the three time points. Among the 24 subjects, ASL scan was not performed in 5 individuals at baseline and 18 month due to the custom sequence not being available either due to delay in implementation initially at baseline and/or due to a system upgrade during the 18 month scan. Significant differences were observed for R2*_Kidney, R2*_Medulla, ΔR2*_Medulla and ADC-Cortex over the 3 time points by ANOVA analysis. Post hoc pairwise comparisons further revealed that the significant difference existed between baseline and the 36 month time points for R2* in whole kidney, medulla and response to furosemide in medulla. Post-hoc analysis did not report significant differences between ADC_Cortex at any of the two time points. Rapid progressors had significantly lower medullary R2* (20.21 ± 3.45 s−1) compared to the slower progressors (29.79 ± 4.85 s−1, p=0.023) at baseline.
Table 2.
Summary of Kruska-Wallis analysis for all measurements and Post-Hoc Pairwise Comparison
| Time point | N | Mean | Std. Deviation | p value | Post-hoc analysis |
||
|---|---|---|---|---|---|---|---|
| Pairs | p value | ||||||
| R2*_Cortex (s−1) | 1 | 24 | 20.63 | 4.19 | 0.287 | ||
| 2 | 24 | 20.35 | 3.20 | ||||
| 3 | 24 | 19.51 | 3.73 | ||||
| R2*_Kidney (s−1) | 1 | 24 | 23.85 | 4.11 | 0.009 | 1–2 | 1.000 |
| 2 | 24 | 22.56 | 2.65 | 1–3 | 0.009 | ||
| 3 | 24 | 21.22 | 3.38 | 2–3 | 0.089 | ||
| R2*_Medulla (s−1) | 1 | 24 | 28.59 | 5.66 | 0.023 | 1–2 | 0.636 |
| 2 | 24 | 26.57 | 4.39 | 1–3 | 0.018 | ||
| 3 | 24 | 24.72 | 3.82 | 2–3 | 0.404 | ||
| ΔR2*_Cortex (s−1) | 1 | 24 | 1.02 | 2.17 | 0.076 | ||
| 2 | 24 | 1.57 | 1.26 | ||||
| 3 | 24 | 0.75 | 0.80 | ||||
| ΔR2*_Kidney (s−1) | 1 | 24 | 2.05 | 1.38 | 0.241 | ||
| 2 | 24 | 2.17 | 1.31 | ||||
| 3 | 24 | 1.49 | 1.26 | ||||
| ΔR2*_Medulla (s−1) | 1 | 24 | 5.65 | 3.64 | 0.028 | 1–2 | 0.992 |
| 2 | 24 | 4.48 | 3.63 | 1–3 | 0.024 | ||
| 3 | 24 | 2.97 | 2.71 | 2–3 | 0.281 | ||
| Perfusion_Cortex (mL/100g/min) | 1 | 21 | 112.79 | 2.71 | 0.435 | ||
| 2 | 22 | 110.08 | 37.33 | ||||
| 3 | 24 | 104.32 | 51.89 | ||||
| ADC_Cortex (mm2/s) | 1 | 22 | 1603.99 | 195.33 | 0.045 | 1–2 | 0.096 |
| 2 | 24 | 1515.01 | 173.48 | 1–3 | 0.085 | ||
| 3 | 24 | 1528.30 | 149.12 | 2–3 | 1.000 | ||
Note: time point: 1=baseline; 2=18 month; 3=36 month.
Post hoc pairwise p values only provided for variables shows significant difference by Kruska-Wallis teset.
p < 0.05 is statistically significant.
Table 3 is the summary of coefficient of variation for all MRI measurements. Inter_CV was higher than intra_CV for all variables. The CV from a group of healthy controls previously published [17] is also listed (in parenthesis) for comparison purpose. Intra subject CV for 36 month and baseline is higher than that for 18 month and baseline except for R2*_Cortex and ADC measurements. Intra_CVs in CKD are generally higher than that in control as one would suspect, except in the case of Perfusion_Cortex between 18 month and baseline.
Table 3.
Summary of Coefficient of Variation (%) in moderate CKD
| Inter-CV (control) | Intra-CV compared to baseline (control) | ||||
|---|---|---|---|---|---|
| Variable | Baseline | 18 Month | 36 Month | 18 Month | 36 Month |
| R2*_Cortex (s−1) | 20.3 (8.1) | 15.7 (10.3) | 19.1 | 9.9 (9.0) | 9.6 |
| R2*_Kidney (s−1) | 17.2 (6.2) | 11.7 (8.4) | 15.9 | 9.1 (5.3) | 12.0 |
| R2*_Medulla (s−1) | 19.8 (11.0) | 16.5 (5.1) | 15.5 | 9.5 (8.5) | 14.2 |
| Perfusion_Cortex (mL/100g/min) | 34.3 (21.2) | 33.9 (17.4) | 49.7 | 14.4 (17.4) | 23.1 |
| ADC_Cortex (mm2/s) | 11.6 (11.3) | 11.5 (15.5) | 9.8 | 7.4 (6.1) | 6.6 |
Note: CKD group: N=24 for R2* and ADC at all 3 time points; N=21, 22 and 24 for Perfusion_Cortex at baseline, 18 month and 36 month respectively. Control group: N=10 for all MRI data.
The results of linear mixed model showed that R2*_Medulla was the only MRI predictor associated with change in eGFR over time (Table 4). Table 5.a summarizes the correlations of MRI measurements with annual loss of eGFR (eGFR_slope). The eGFR_slope data used for this analysis was the same for all three time points. The most striking observation was that medullary R2* was consistently associated with annual loss of eGFR at all three time points. Furthermore, regression analysis (Table 6) also demonstrated that a linear relationship between R2*_Medulla and annual loss of eGFR at all time points. When adjusted for the eGFR, ACR, age, sex, BMI, blood glucose, systolic blood pressure, and use of furosemide and ACE inhibitors, the linear relationship remained significant for baseline. Perfusion showed significant linear relationship with eGFR_slope at baseline and 18 months, but not after adjusting for potential confounders. Other parameters evaluated did not show a linear relationship with eGFR_slope, except for ΔR2*_Kidney and ΔR2*_Medulla at baseline but only after adjusting for potential confounders. Table 5.b shows the correlation of BOLD MRI measurements analyzed by the twelve layer concentric objects (TLCO) method [7] with eGFR_slope. Note that data presented in Table 5.b is from a subset of the participants included in our prior report [7], matching the cohort in the current study.
Table 4 -.
Liner Mixed Model of eGFR over timeb
| Variablesa | β | SE | p value |
|---|---|---|---|
| R2*_Medulla | 4.73 | 2.05 | 0.0266 |
| ΔR2*_Cortex | 0.02 | 1.63 | 0.9884 |
| ΔR2*_Kidney | 0.81 | 0.83 | 0.3394 |
| ADC | 0.02 | 0.01 | 0.1455 |
| Time 1 (baseline) | 40.3 | 26.30 | 0.0339 |
| Time 2 (18 months) | 18.1 | 28.30 | 0.0976 |
| Time 3 (36 months) | Ref | - | - |
| R2*_Medulla X Time 1 | −1.76 | 1.00 | 0.0492 |
| R2*_Medulla X Time 2 | −0.87 | 1.10 | 0.1495 |
| R2*_Medulla X Time 3 | Ref | - | - |
Only MRI variables with p<0.1 in the univariate analysis were included in the multivariable mixed model
Model adjusted for eGFR, Ln(ACR), age, BMI, sex, systolic blood pressure, blood glucose, use of furosemide and ACE inhibitors.
Table 5. a.
Summary of Spearman’s Correlation of MRI parameters with progression index, eGFR_slope
| Baseline | 18 Months | 36 Months | ||
|---|---|---|---|---|
| R2*_Cortex | ρ | 0.130 | 0.047 | 0.333 |
| Sig. | 0.546 | 0.829 | 0.112 | |
| N | 24 | 24 | 24 | |
| R2*_Kidney | ρ | 0.385 | 0.311 | 0.504* |
| Sig. | 0.063 | 0.139 | 0.012 | |
| N | 24 | 24 | 24 | |
| R2*_Medulla | ρ | 0.506* | 0.507* | 0.652** |
| Sig. | 0.012 | 0.012 | 0.001 | |
| N | 24 | 24 | 24 | |
| ΔR2*_Cortex | ρ | 0.331 | 0.202 | 0.304 |
| Sig. | 0.114 | 0.344 | 0.149 | |
| N | 24 | 24 | 24 | |
| ΔR2*_Kidney | ρ | 0.597** | 0.372 | 0.180 |
| Sig. | 0.002 | 0.074 | 0.400 | |
| N | 24 | 24 | 24 | |
| ΔR2*_Medulla | ρ | 0.438* | 0.287 | 0.143 |
| Sig. | 0.032 | 0.173 | 0.506 | |
| N | 24 | 24 | 24 | |
| Perfusion_Cortex | ρ | 0.466* | 0.534* | 0.226 |
| Sig. | 0.033 | 0.011 | 0.288 | |
| N | 21 | 22 | 24 | |
| ADC_Cortex | ρ | 0.117 | 0.307 | 0.197 |
| Sig. | 0.603 | 0.144 | 0.355 | |
| N | 22 | 24 | 24 | |
| eGFR | ρ | 0.065 | 0.391 | 0.363 |
| Sig. | 0.762 | 0.059 | 0.081 | |
| N | 24 | 24 | 24 | |
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
Table 6.a:
Linear Regression of MRI parameters with eGFR_slope in CKD at baseline
| Adjusted* | |||||||
|---|---|---|---|---|---|---|---|
| Dependent variable | Predictor | β | SE | p | β | SE | p |
| eGFR_slope | R2*_Medulla | 0.28 | 0.104 | 0.014 | 0.399 | 0.112 | 0.005 |
| ΔR2*_Kidney | 0.857 | 0.461 | 0.079 | 1.588 | 0.542 | 0.015 | |
| ΔR2*_Medulla | 0.316 | 0.173 | 0.084 | 0.538 | 0.215 | 0.031 | |
| Perfusion_Cortex | 0.044 | 0.018 | 0.028 | 0.016 | 0.037 | 0.680 | |
Table 5.b.
Spearman’s Correlation of TLCO measurements with eGFR_slope
| Variables | eGFR_slope | |
|---|---|---|
| R2*_inner_TLCO | ρ | 0.430* |
| Sig. | 0.046 | |
| N | 22 | |
| R2*_outer_TLCO | ρ | 0.158 |
| Sig. | 0.482 | |
| N | 22 | |
| R2*_slope_TLCO | ρ | 0.311 |
| Sig. | 0.159 | |
| N | 22 | |
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
In the interest of the larger renal MRI community and to improve awareness, we have included tables as supplementary material showing the level of consistency of our acquisition and analysis protocols with those recently recommended by the international consortium PARENCHIMA [13, 26, 27]. It should be emphasized that our study was conducted much earlier than the development of the consensus based recommendations.
Discussion / Conclusion
Few recent reports have shown that reduced cortical R2* in individuals with CKD is associated with annual loss of renal function [7–9]. While the study cohorts included individuals with diabetes, there was no data to indicate that it had any effect of their observations in multivariate analysis. Only one of these three studies reported on medullary R2* and showed higher values compared to healthy controls, but did not find an association with annual loss of renal function. In our own prior report on the baseline analysis from the present study we had reported cortical perfusion and response to furosemide on renal medullary R2* to be associated with annual loss of renal function.
The result of this longitudinal study in small number of individuals with moderate CKD showed lower R2* and higher temporal variability in medullary R2* over time. These observations suggest that BOLD MRI can be used to monitor progression in CKD. The most novel finding from this study was that R2* in the renal medulla showed a significant association with annual change of eGFR (eGFR_slope) at all 3 time points. These observations suggest that a decreased medullary R2* can serve as a marker for progressive CKD.
Inter-subject CV (i.e. between individual variation) was about twice the values in CKD compared to healthy volunteers previously reported [17], implying that MRI measurements are sensitive to the differences based on disease burden. The intra-CV (i.e. temporal variation in each subject) was higher at both time points for R2*_Medulla and R2*_Kidney, probably indicating sensitivity to progressive changes in disease burden. Furthermore, ANOVA analysis indicated that R2* in medulla and whole kidney ROIs were significantly lower at 36 months compared to baseline. The baseline R2*_Medulla and R2*_Kidney values in this study are comparable to those in healthy controls we had reported before [18]. However, our prior data in advanced CKD [10] showed lower R2* in the medulla and is also consistent with Pruijm et al. [7] using twelve layer concentric objects (TLCO) analysis indicating lower R2*_slope in CKD (probably due to increased outer (corresponding to cortex) and decreased inner (i.e. medulla) R2* values). The trend of decreasing R2* in medulla even when R2* in cortex was increasing have been reported in diabetic nephropathy [28]. Based on these earlier observations, the lower R2* at 36 months suggest that these changes may be related to CKD progression.
Linear mixed effect model showed that lower R2*_Medulla and higher decline in R2*_Medulla predict loss of eGFR. Note this analysis only used the study realted eGFR measurements at the three time points. In order to further verify if the observed trends in BOLD MRI index was related to progressive disease, the association of MRI parameters with annual loss of renal function (estimated based on all eGFR measurements available from EMR) was evaluated at all three time points. The baseline data is consistent with a prior report [18] that had indicated significant association of cortical perfusion and response to furosemide in the medulla and whole kidney ROIs. In addition, in the subset of individuals who participated at all three time points, R2*_Medulla also showed a significant association with annual loss in renal function. More interestingly, this association in renal medulla was also observed at the two follow-up visits. Prior reports on BOLD MRI in CKD demonstrating associations with annual loss of eGFR have ignored medullary R2* measurements because of the potential concern regarding subjectivity of defining medullary ROIs [8, 9]. However, we recently demonstrated that the baseline medullary data previously published from the same cohort as in the current study [18] was consistent with the more objective TLCO method [29]. When using R2*_inner (corresponding to R2*_Medulla) by TLCO in the subset corresponding to the cohort used in the current study, we observed a significant association with annual loss of eGFR (Table 5.b). Combined with our prior evidence that medullary R2* values are lower in advanced CKD [10], the current data suggest that it may be a sensitive and more consistent marker of progressive CKD [7, 18].
Lower R2* values (suggesting higher oxygen availability) may be in an apparent contradiction to the chronic hypoxia hypothesis [4, 30]. Whether the observed lower R2* values necessarily imply better oxygen availability is not yet clear, since it may be at least in part due to concomitant change in regional blood volume, hematocrit and/or Hb-O2 desaturation curve [31]. This needs further investigation. There is data available to support lower GFR to be associated with lower blood volume [32].
There are a few limitations in this study. The overall number of subjects (n=24) is small and the individuals had only moderate level of CKD. However, it is interesting that R2*_Medulla consistently showed the relationship with eGFR_slope at all the three time points. Due to the rate of recruitment of individuals for the study, we had to use the same eGFR_slope for all time points. Furthermore, eGFR data was extended two years prior to recruitment so as to allow for a sufficient duration to estimate eGFR_slope in all individuals. We interpret the negative eGFR_slope as an indicator of progressive CKD. The use of same dose of furosemide (20 mg) in all individuals may not be ideal. Dosing based on body weight and eGFR may be more appropriate for future studies.
In summary, longitudinal study with multi-parametric functional renal MRI techniques demonstrate sensitivity to progressive changes at least with medullary R2*. These observations were further strengthened by the associations observed for R2*_Medulla with annual loss of eGFR (a measure of progressive CKD). This was true at all time points by both Spearman correlation and linear regression. This is the first report to illustrate the decrease in medullary R2* as a marker for progression in CKD and further supports the need to include the medulla in the analysis of BOLD MRI in the evaluation of CKD. Whether the reduced R2* is a true reflection of improved oxygen availability or related to potential confounding factors such as reduced blood volume and/or hematocrit needs further investigation.
Supplementary Material
Table 6.b:
Linear Regression of MRI parameters with eGFR_slope in CKD at 18 months
| Adjusted* | |||||||
|---|---|---|---|---|---|---|---|
| Dependent variable | Predictor | β | SE | p | β | SE | p |
| eGFR_slope | R2*_Medulla | 0.368 | 0.126 | 0.008 | 0.188 | 0.135 | 0.192 |
| Perfusion_Cortex | 0.043 | 0.017 | 0.022 | 0.010 | 0.012 | 0.421 | |
Table 6.c:
Linear Regression of MRI parameters with eGFR_slope in CKD at 36 months
| Adjusted* | |||||||
|---|---|---|---|---|---|---|---|
| Dependent variable | Predictor | β | SE | p | β | SE | p |
| eGFR_slope | R2*_Medulla | 0.277 | 0.081 | 0.003 | 0.156 | 0.124 | 0.239 |
| R2*_Kidney | 0.18 | 0.099 | 0.087 | 0.137 | 0.126 | 0.306 | |
Adjusted for eGFR, Ln(ACR), age, BMI, sex, systolic blood pressure, and blood glucose, use of furosemide and ACE inhibitors. p<0.05 are shown in Bold
Acknowledgement
The authors thank Dr. Ivana Lazich, Ms. Shoshana Fettman, Ms. Dana Factor, Ms. Sharon Trevino, and Ms. Claire Feczko for their technical assistance during the study. Thanks also to Ms. Sally Gartman for editorial assistance with the manuscript.
Funding Sources
Work supported in part by grants from the National Institute of Diabetes Digestive and Kidney Diseases, R21-DK079080 (P.V.P.) and R01-DK093793 (P.V.P.) and National Heart Lung and Blood Institute F31HL123360 (J.M.T.).
Footnotes
Statement of Ethics
All subjects have given their written informed consent. The study protocol has been approved by the institutional committees on human research.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References:
- 1.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3 Suppl 1):A7–A8. Epub 2019/02/26. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. Chronic Kidney Disease in the United States, 2019: U.S. Department of Health & Human Services; 2020. [updated March 05, 2019; cited 2020]. Available from: https://www.cdc.gov/kidneydisease/publications-resources/2019-nationalfacts.html#:~:text=CKD%20Is%20Common%20Among%20US%20Adults&text=CKD%20is%20more%20common%20in,%2DHispanic%20Asians%20(12%25).
- 3.Nangaku M Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17(1):17–25. Epub 2005/11/18. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 4.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–8. Epub 1998/04/29. [PubMed] [Google Scholar]
- 5.Li LP, Tan H, Thacker JM, Li W, Zhou Y, Kohn O, et al. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion Magnetic Resonance Imaging. Kidney Int Rep. 2017;2(1):36–43. Epub 2017/09/05. doi: 10.1016/j.ekir.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora-Gutierrez JM, Garcia-Fernandez N, Slon Roblero MF, Paramo JA, Escalada FJ, Wang DJ, et al. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J Magn Reson Imaging. 2017;46(6):1810–7. Epub 2017/04/07. doi: 10.1002/jmri.25717. [DOI] [PubMed] [Google Scholar]
- 7.Pruijm M, Milani B, Pivin E, Podhajska A, Vogt B, Stuber M, et al. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney international. 2018;93(4):932–40. doi: 10.1016/j.kint.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama K, Inoue T, Kozawa E, Ishikawa M, Shimada A, Kobayashi N, et al. Reduced oxygenation but not fibrosis defined by functional magnetic resonance imaging predicts the long-term progression of chronic kidney disease. Nephrol Dial Transplant. 2020;35(6):964–70. Epub 2018/11/13. doi: 10.1093/ndt/gfy324. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Yang M, Jiang Z, Ding J, Di J, Cui L. Renal Hypoxia: An Important Prognostic Marker in Patients with Chronic Kidney Disease. Am J Nephrol. 2018;48(1):46–55. Epub 2018/07/27. doi: 10.1159/000491551. [DOI] [PubMed] [Google Scholar]
- 10.Prasad PV, Li W, Raj DS, Carr J, Carr M, Thacker J, et al. Multicenter Study Evaluating Intrarenal Oxygenation and Fibrosis Using Magnetic Resonance Imaging in Individuals With Advanced CKD. Kidney Int Rep. 2018;3(6):1467–72. Epub 2018/11/20. doi: 10.1016/j.ekir.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Cai X, Lee J, Li W, Larive B, Kendrick C, et al. Kidney Functional Magnetic Resonance Imaging and Change in eGFR in Individuals with CKD. Clin J Am Soc Nephrol. 2020. Epub 2020/04/30. doi: 10.2215/CJN.13201019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung G, Kirpalani A, Szeto SG, Deeb M, Foltz W, Simmons CA, et al. Could MRI Be Used To Image Kidney Fibrosis? A Review of Recent Advances and Remaining Barriers. Clin J Am Soc Nephrol. 2017;12(6):1019–28. Epub 2017/03/17. doi: 10.2215/CJN.07900716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljimani A, Caroli A, Laustsen C, Francis S, Mendichovszky IA, Bane O, et al. Consensus-based technical recommendations for clinical translation of renal diffusion-weighted MRI. MAGMA. 2020;33(1):177–95. Epub 2019/11/05. doi: 10.1007/s10334-019-00790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan CE, Mahmoud H, Cox EF, McCulloch T, Prestwich BL, Taal MW, et al. Quantitative assessment of renal structural and functional changes in chronic kidney disease using multi-parametric magnetic resonance imaging. Nephrol Dial Transplant. 2020;35(6):955–64. Epub 2019/07/02. doi: 10.1093/ndt/gfz129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad PV, Thacker J, Li LP, Haque M, Li W, Koenigs H, et al. Multi-Parametric Evaluation of Chronic Kidney Disease by MRI: A Preliminary Cross-Sectional Study. PLoS One. 2015;10(10):e0139661. Epub 2015/10/03. doi: 10.1371/journal.pone.0139661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby NM, Blankestijn PJ, Boor P, Combe C, Eckardt KU, Eikefjord E, et al. Magnetic resonance imaging biomarkers for chronic kidney disease: a position paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol Dial Transplant. 2018;33(suppl_2):ii4–ii14. Epub 2018/08/24. doi: 10.1093/ndt/gfy152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li LP, Thacker J, Li W, Tan H, Wang C, Kohn O, et al. Consistency of Multiple Renal Functional MRI Measurements Over 18 Months. J Magn Reson Imaging. 2018;48(2):514–21. Epub 2018/03/09. doi: 10.1002/jmri.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad PV, Li LP, Thacker JM, Li W, Hack B, Kohn O, et al. Cortical Perfusion and Tubular Function as Evaluated by Magnetic Resonance Imaging Correlates with Annual Loss in Renal Function in Moderate Chronic Kidney Disease. Am J Nephrol. 2019;49(2):114–24. Epub 2019/01/23. doi: 10.1159/000496161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. Epub 2009/05/06. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad PV, Chen Q, Goldfarb JW, Epstein FH, Edelman RR. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging. 1997;7(6):1163–5. Epub 1997/12/24. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 21.Tan H, Koktzoglou I, Prasad PV. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn Reson Med. 2014;71(2):570–9. Epub 2013/03/01. doi: 10.1002/mrm.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordidge RJ, Wylezinska M, Hugg JW, Butterworth E, Franconi F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn Reson Med. 1996;36(4):562–6. Epub 1996/10/01. doi: 10.1002/mrm.1910360410. [DOI] [PubMed] [Google Scholar]
- 23.Thacker JM, Li LP, Li W, Zhou Y, Sprague SM, Prasad PV. Renal Blood Oxygenation Level-Dependent Magnetic Resonance Imaging: A Sensitive and Objective Analysis. Investigative radiology. 2015;50(12):821–7. doi: 10.1097/RLI.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Python. https://scipy.org/ [August 22, 2019]. Available from: https://scipy.org/.
- 25.Li LP, Storey P, Pierchala L, Li W, Polzin J, Prasad P. Evaluation of the reproducibility of intrarenal R2* and DeltaR2* measurements following administration of furosemide and during waterload. J Magn Reson Imaging. 2004;19(5):610–6. Epub 2004/04/28. doi: 10.1002/jmri.20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bane O, Mendichovszky IA, Milani B, Dekkers IA, Deux JF, Eckerbom P, et al. Consensus-based technical recommendations for clinical translation of renal BOLD MRI. MAGMA. 2020;33(1):199–215. Epub 2019/11/27. doi: 10.1007/s10334-019-00802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nery F, Buchanan CE, Harteveld AA, Odudu A, Bane O, Cox EF, et al. Consensus-based technical recommendations for clinical translation of renal ASL MRI. MAGMA. 2020;33(1):141–61. Epub 2019/12/14. doi: 10.1007/s10334-019-00800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin WJ, Liu F, Li XM, Yang L, Zhao S, Huang ZX, et al. Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol. 2012;81(7):1426–31. Epub 2011/04/08. doi: 10.1016/j.ejrad.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Li LP, Milani B, Pruijm M, Kohn O, Sprague S, Hack B, et al. Renal BOLD MRI in patients with chronic kidney disease: comparison of the semi-automated twelve layer concentric objects (TLCO) and manual ROI methods. MAGMA. 2020;33(1):113–20. Epub 2019/12/12. doi: 10.1007/s10334-019-00808-5. [DOI] [PubMed] [Google Scholar]
- 30.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney international. 2008;74(7):867–72. Epub 2008/07/18. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 31.Prasad PV. Update on renal blood oxygenation level-dependent MRI to assess intrarenal oxygenation in chronic kidney disease. Kidney international. 2018;93(4):778–80. Epub 2018/03/25. doi: 10.1016/j.kint.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita M, Inaba T, Oda Y, Mizukawa N. Renal blood volume vs. glomerular filtration rate: evaluation with C15O and 88Ga-ethylenediaminetetraacetic acid (EDTA) study. Radioisotopes. 1989;38(9):373–6. Epub 1989/09/01. doi: 10.3769/radioisotopes.38.9_373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.