Abstract
Glioblastoma (GBM), the most aggressive primary brain tumor, has a dismal prognosis. Despite our growing knowledge of genomic and epigenomic alterations in GBM, standard therapies and outcomes have not changed significantly in the past two decades. There is therefore an urgent unmet need to develop novel therapies for GBM. The inter- and intratumoral heterogeneity of GBM, inadequate drug concentrations in the tumor owing to the blood–brain barrier, redundant signaling pathways contributing to resistance to conventional therapies, and an immunosuppressive tumor microenvironment, have all hindered the development of novel therapies for GBM. Given the high frequency of DNA damage pathway alterations in GBM, researchers have focused their efforts on pharmacologically targeting key enzymes, including poly(ADP-ribose) polymerase (PARP), DNA-dependent protein kinase, ataxia telangiectasia-mutated, and ataxia telangiectasia and Rad3-related. The mainstays of GBM treatment, ionizing radiation and alkylating chemotherapy, generate DNA damage that is repaired through the upregulation and activation of DNA damage response (DDR) enzymes. Therefore, the use of PARP and other DDR inhibitors to render GBM cells more vulnerable to conventional treatments is an area of intense investigation. In this review, we highlight the growing body of data behind DDR inhibitors in GBM, with a focus on putative predictive biomarkers of response. We also discuss the challenges involved in the successful development of DDR inhibitors for GBM, including the intracranial location and predicted overlapping toxicities of DDR agents with current standards of care, and propose promising strategies to overcome these hurdles.
Keywords: DDR inhibitors, glioblastoma, MGMT methylation, radiation, temozolomide
Key Points.
Genomic analyses have uncovered frequent alterations in DNA damage pathways in glioblastoma.
Radiotherapy and temozolomide activate DNA damage response pathways in glioblastoma.
DNA damage response inhibitors are a novel and promising group of drugs for the treatment of glioblastoma.
The current standard treatment for glioblastoma (GBM) includes maximal safe resection, radiotherapy (RT), and chemotherapy with the alkylating agent temozolomide (TMZ).1 Recently, tumor treating fields have evolved as the fourth modality for the treatment of GBM.2 Despite such multimodality treatment, GBM invariably recurs and ultimately leads to death. Thus, there is an urgent unmet need to develop novel therapeutic approaches for GBM.
DNA repair pathways are among the most important key players of oncogenic mutations associated with resistance to both chemotherapy and radiation in GBM.3 The most common DNA repair pathway alterations in GBM are downregulation of p53 signaling pathways, downregulation of retinoblastoma signaling pathways, and methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter.4 In addition, alteration of PTEN and upregulation of EGFR/PI3K, found in approximately one third of GBM, are thought to augment DNA damage response pathways in GBM.5,6 The high frequency of these alterations in GBM suggests that DNA repair pathways play an important role in the pathogenesis of GBM and that the pharmacological modulation of these pathways can provide a therapeutic benefit in GBM patients.
Ionizing RT, the cornerstone of GBM treatment, generates DNA single-strand breaks (SSBs) and double-strand breaks (DSBs).7 SSBs are repaired through the base excision repair (BER) pathway through poly(ADP-ribose) polymerase (PARP) activation.8 DSBs are repaired through the DNA damage response (DDR) kinases DNA-dependent protein kinase (DNA-PK), ataxia telangiectasia-mutated (ATM), and ataxia telangiectasia and Rad3-related (ATR).9 TMZ when given with radiation and in the adjuvant setting is the only FDA-approved drug that prolongs the survival of newly diagnosed GBM patients. The methylation status of MGMT promoter determines response to TMZ. Patients with GBM tumors containing a methylated MGMT promoter benefit from TMZ, whereas those with unmethylated MGMT promoter do not.10 TMZ causes cell death by inducing the formation of DNA adducts. The major TMZ-induced DNA adduct is O6-methylguanine; in GBM with high MGMT expression (MGMT promoter unmethylated), O6-methylguanine is removed by MGMT, thereby reversing the DNA damage caused by TMZ. However, unrepaired O6-methylguanine causes O6-methylguanine:T mismatches, which are detected and processed by mismatch repair (MMR) enzymes, which in turn signal to activate DDR enzymes, leading to DNA repair and resistance to TMZ.11,12 Attempts at modulating MGMT via its pharmacological inhibition (O6-benzylguanine and PaTrim-2 (Lomeguatrib)) were made in human clinical trials. MGMT inhibitors did not improve the response rate to TMZ and resulted in increased hematological toxicities,13,14 therefore alternative approaches to increase sensitivity of GBM to TMZ are needed.
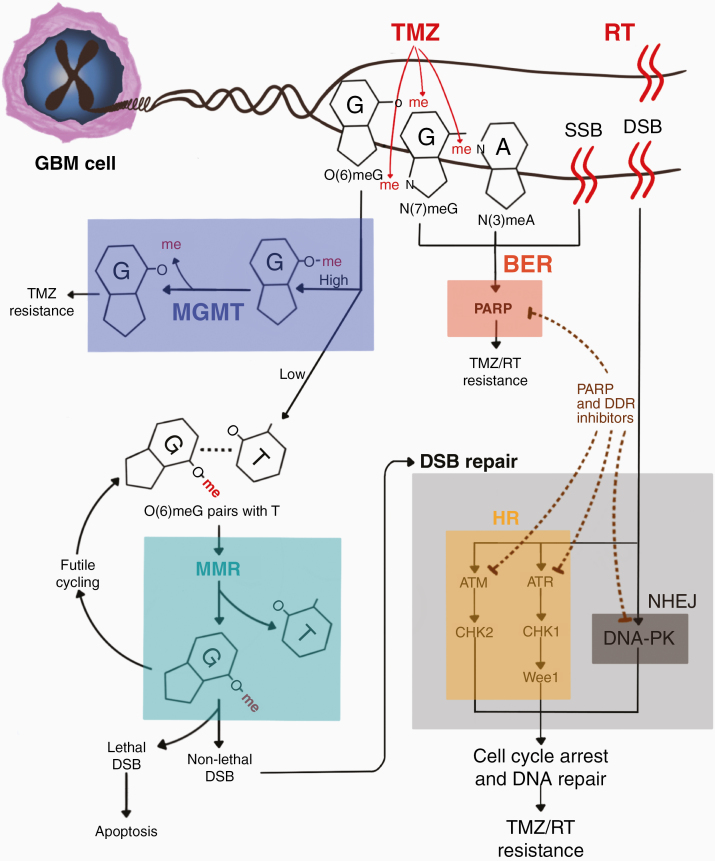
There is strong rationale to leverage mechanisms of action against the DDR to augment the antitumor activity of RT and TMZ in GBM, as both these interventions kill tumor cells by inducing DNA damage that can be repaired through DDR enzymes. Figure 1 depicts mechanisms of DNA damage response pathway activation with GBM therapies.
Figure 1.
Mechanisms of DNA damage response pathways in GBM. ATM, ataxia telangiectasia-mutated; ATR, ataxia telangiectasia and Rad3-related; BER, base excision repair; DNA-PK, DNA-dependent protein kinase; DSB, double-stranded break; MGMT, O6-methylguanine-DNA methyltransferase; HR, homologous recombination; MMR, mismatch repair; NHEJ, non-homologous end joining; PARP, poly(ADP-ribose) polymerase; RT, radiation therapy; SSB, single-stranded break; TMZ, temozolomide.
Herein, we review our current knowledge of preclinical and early clinical studies of DDR inhibitors in GBM, discuss barriers to the successful clinical implementation of these inhibitors, and recommend strategies to overcome these obstacles.
DDR Enzymes
DDR enzymes are attractive targets in cancer therapy because they promote unwanted DNA repair and render cancer cells less vulnerable to DNA-damaging treatments such as chemotherapy and RT. Predominant DNA damage mechanisms include SSBs and DSBs. DNA SSBs are primarily repaired through the recruitment and ribosylation of PARP1, which leads to the activation of effector DNA repair proteins.8 DNA DSBs are mainly repaired through non-homologous end joining (NHEJ) and homologous recombination (HR).
NHEJ, the predominant DSB repair pathway in humans, is active throughout the cell cycle and is mainly regulated by DNA-PK.15 DNA-PK is composed of DNA binding domains Ku70 and Ku80 and the catalytic subunit, DNA-PKcs. DNA-PK functional complex binds and protects DSB from degradation by DNA nucleases and also activates downstream components of NHEJ pathway such as Ku, XRCC4, XLF, Artemis, and DNA-PKcs itself.16 HR is restricted to late-S/G2 phases of the cell cycle and is operated by ATM and ATR. ATM is recruited to DSBs by the MRN complex and phosphorylates the histone variant γ-H2AX, which results in phosphorylation of CHK2, p53 and MDM leading to G1/S arrest.17,18 Replication protein A binds to and stabilizes the ssDNA overhang, leading to ATR activation and its recruitment to DSB sites with its binding partner, ATR-interacting protein (ATRIP).19 ATR activation leads to downstream signaling events most notably CHK1 phosphorylation.20 CHK1 phosphorylates and inactivates CDC25 isoforms leading to a decrease in CDK2 activity in S-phase and abolishing activity of CDK1/cylcin B kinase resulting in G2/M arrest.19
Overall, DNA-PK, ATM, and ATR coordinate DSB signaling events and trigger various post-translational modifications and protein complex assemblies, resulting in the amplification of the DNA damage signal within the cell and the activation of cellular senescence or apoptosis.21 Several inhibitors of these DDR enzymes have had remarkable antitumor activity in combination with DNA-damaging treatments, such as RT and chemotherapy in preclinical studies and are now in varying stages of clinical development. In this review, we will focus on preclinical and clinical development of DDR inhibitors in GBM. A growing body of literature has shed light on the role of PARP inhibitor combinations with RT and TMZ in GBM and investigates the role of IDH1/2 mutation in conferring a homologous recombination deficiency phenotype and increased susceptibly to PARP inhibitors in low-grade gliomas. A comprehensive review of PARP inhibitors in gliomas was recently published.22
DNA-PK Inhibitors
DNA-PK inhibitors are the most clinically advanced inhibitors of NHEJ proteins.23 DNA-PK, a PI3K-related kinase, recruits repair proteins and activates checkpoints by phosphorylating its substrates.24 DNA-PK inhibitors are postulated to either sensitize cancer cells to DNA-damaging treatments that cause DNA DSBs, such as RT and chemotherapy, or have single-agent activity in tumors with relevant dysfunctional DNA repair pathways.25,26
Phospho-DNA-PK expression is significantly higher in human glioma than their respective adjacent non-tumorous tissue and correlates with malignant development and poor prognosis in glioma patients.27 A recent transcriptome analysis identified DNA-PK as a predominant DNA repair enzyme in glioma stem cells (GSCs), which drives radiation resistance in GBM.28
Preclinical studies of DNA-PK inhibition in GBM have yielded promising results. Lan et al. demonstrated that both the siRNA knockdown of DNA-PK and its pharmacological inhibition with KU0060648 reduces glioma cell proliferation in vitro and reduces xenograft tumor volume in vivo.27 The antiproliferative effect of DNA-PK inhibition monotherapy could be due to genomic instability and intrinsic levels of DNA damage in GBM cell lines used. The authors also showed that the antitumor effect of KU0060648 was enhanced in TMZ-treated cells and animals. Timmer et al., testing the combination of RT and the DNA-PK inhibitor VX-984 in U251 cells and GSCs, demonstrated that VX-984 inhibited RT-induced DNA-PK phosphorylation in vitro and in orthotopic brain tumor xenografts in a concentration-dependent manner. The survival duration of mice receiving VX-984 plus RT was significantly longer than that of mice receiving RT or VX-984 alone.29
Developing biomarkers of response is crucial to optimizing the efficacy of individual DDR inhibitors. Sun et al. identified p53 as a potential predictive biomarker of response to the combination of DNA-PK inhibition and RT.30 They demonstrated that the DNA-PK inhibitor M3814 blocks the repair of radiation-induced DSBs and enhances p53 activation. In p53-wildtype cell lines, M3814 combined with RT leads to the activation of the ATM-CHK2 pathway and cellular senescence. In contrast, in cells with dysfunctional p53, cell cycle progression is not arrested, leading to mitotic catastrophe and apoptosis. Therefore, P53 can be a potential biomarker of response to DNA-PK inhibition given the high frequency of inactivating mutations of p53 in gliomas.31
DNA-PK inhibitors currently in clinical development in combination with chemotherapy in patients with advanced solid cancers include VX-984 (NCT02644278), M3814 (NCT02316197), AZD7648 (NCT03907969), and CC-115 (NCT02977780). A phase I window-of-opportunity trial of M3814 in combination with RT is currently accruing newly diagnosed GBM patients with unmethylated MGMT status (NCT04555577). To our knowledge, this is the first clinical trial of a DNA-PK inhibitor in GBM patients. CC-115, a dual mTOR kinase and DNA-PK inhibitor, was tested in a phase I study as monotherapy in patients with advanced solid or hematological malignancies, including 14 GBM patients in a cohort expansion. GBM patients were enrolled only if salvage tumor resection was planned approximately 2 weeks into therapy. CC-115 was shown to penetrate GBM tumor tissue and had a mean tumor-to-plasma ratio of 0.73.32 In this study, CC-115 was well tolerated with toxicity consistent with mTOR inhibitors including fatigue, nausea and decreased appetite. The combination of CC-115 and RT is currently being studied in the Individualized Screening Trial of Innovative Glioblastoma Therapy trial in newly diagnosed MGMT unmethylated GBM patients (NCT02977780). The rational for the use of a dual kinase inhibitor is the lack of success with several mTOR inhibitors as monotherapy or in combination with conventional treatments, despite mTOR pathway being commonly activated in GBM.33–35 The ineffectiveness of mTOR inhibitors maybe due to several feedback loops that promote tumor growth. Whether a dual mTOR/DNA-PK inhibitor would have an advantage over mTOR inhibition or DNA-PK inhibition alone is yet to be determined.
ATM Inhibitors
ATM, an essential kinase that regulates HR, is ubiquitously expressed in cancer cell lines. ATM is a promising therapeutic target, as its inhibition likely sensitizes tumors to the DNA-damaging effects of RT and chemotherapy.
MMR converts TMZ-induced DNA adducts into secondary lesions that block the replication fork, thereby resulting in DSBs and the activation of DDR enzymes. TMZ has been shown to activate ATM- and ATR-dependent signaling pathways.12 Caporali et al. demonstrated that in MMR-proficient human B-cell lymphoblasts, exposure to low-dose TMZ activates ATR and then ATM and results in CHK1, CHK2, and p53 phosphorylation and G2/M arrest. Similarly, Eich et al. showed that ATM- and ATR-mutant fibroblasts are hypersensitive to TMZ and that knockdown of ATM and ATR enhances LN229 glioma cell death.36 Both groups suggested that ATR deficiency has a stronger effect than ATM deficiency in sensitizing glioma cells to TMZ. However, ATM inhibition is hypothesized to be less toxic than ATR inhibition to noncancerous cells. Null mutations of ATR in mice are embryonic lethal, but knockout of ATM results in viable mice that display a phenotype similar to that of ataxia telangiectasia patients, with growth retardation and sensitivity to RT.37 ATM inhibition may result in less toxicity to normal brain tissue concurrently exposed to RT.
As with DNA-PK inhibition, preclinical work suggests a role for p53 as a biomarker of response to ATM inhibition in GBM. Blake et al. showed that genetic inactivation of the ATM cofactor (ATMIN) suppressed GBM formation in a TP53-deficient mouse model of GBM. ATMIN binds ATM in normal conditions and dissociates from ATM in response to DNA damage to allow ATM to be recruited to DSB sites.38 ATMIN deletion in the TP53-deficient background normalized the expression of several GBM-associated genes, including PDGFRA. In addition, the combination of ATM inhibitors and PDGFRA inhibitors reduced the survival of TP53-mutant primary human GBM cells, indicating a role for ATM inhibitors in the treatment of GBM patients with p53 mutations. Similarly, treatment with KU60019, a second-generation ATM inhibitor analogue, resulted in more pronounced radiosensitization in p53-mutant U87 glioma cells than in genetically matched wildtype cells.39 In addition, KU60019’s radiosensitization was associated with the inhibited phosphorylation of the major DNA damage effectors p53, H2AX, KAP1, and AKT. In an orthotopic U1242 xenograft model, the combination of KU60019 and RT resulted in a longer survival duration than either RT or KU60019 alone did.40
KU60019 is remarkably stable41 but cannot cross the blood–brain barrier (BBB). Newer-generation ATM inhibitors such as AZ32 and AZD1390 (AstraZeneca) are specifically designed to cross the BBB. AZ32, which has a brain:plasma area under the curve ratio of 0.26, sensitizes a wide range of glioma cell lines, as well as orthotopic intracranial glioma models, to radiation.42 Similarly, AZD1390 effectively crosses the BBB in mice, rats, and monkeys. Compared with RT alone, the combination of AZD1390 and RT induces tumor regression and increases survival in syngeneic and patient-derived glioma models as well as in orthotopic models of lung cancer brain metastases.43
AZD1390, the most clinically advanced ATM inhibitor for the treatment of brain tumors, is being tested in a phase I trial in GBM patients and brain metastases (NCT03423628). The trial is evaluating the safety and tolerability of AZD1390 in combination with intensity-modulated RT in patients with recurrent GBM (35 Gy over 2 weeks) or newly diagnosed GBM (60 Gy over 6 weeks) and in combination with whole or partial brain RT (30 Gy over 2 weeks) in patients with brain metastases.
ATR Inhibitors
The ATR-CHK1 pathway, the principal effector of replication checkpoints and DNA damage, prevents cells with damaged DNA from entering mitosis. ATR is of particular interest in GBM, as it plays a dominant role in protecting GBM cells against TMZ. As with other DDR inhibitors, there is some concern regarding the toxicity of ATR inhibitors to noncancerous cells, as ATR is essential to the survival of many cell types.
ATR knockdown augments TMZ-induced apoptosis in GBM cell lines.36 In addition to inducing apoptosis, TMZ also activates survival pathways, such as senescence.44 The hallmarks of cellular senescence are DDR activation and cell cycle arrest, which enables the cells to survive without proliferating and contributes to recurrence. TMZ-induced senescence in GBM cells depends on the activation of the ATR-CHK1 pathway.45 Jackson et al. demonstrated that TMZ activated ATR in an MGMT-dependent manner and that treatment of MGMT-deficient cells with TMZ increased sensitivity to ATR inhibitors in in vitro and in vivo models of GBM cell lines.46 The role of MGMT promoter methylation status in determining response to DDR inhibitors needs to be investigated further in clinical trials.
In addition, the ATR-CHK1 pathway is activated in GSCs following RT, while the inhibition of ATR and CHK1 increases mitotic catastrophe and radiation sensitivity.47 Together, these data suggest that the addition of an ATR inhibitor could enhance GBM sensitivity to RT and increase the antitumor effects of TMZ, although overlapping normal tissue toxicity may limit the combination doses of either or both ATR inhibitor and TMZ.
To the best of our knowledge, there are no ongoing ATR inhibitor clinical trials for GBM (www.clinicaltrials.org). However, clinical trials of the combination of the ATR inhibitor AZD6738 and RT (NCT02223923) and the combination of the ATR inhibitor M6220 and chemo-RT (NCT02567422) are underway in patients with advanced solid cancers. M6220 is also being studied in combination with whole-brain RT in non-small cell lung cancer patients with brain metastases (NCT02589522). A novel, potent, selective ATR inhibitor, BAY1895344, appears to have an acceptable safety profile as monotherapy in patients with advanced solid cancers.48 CY-237 (TNT Medical), another selective ATR inhibitor with BBB penetration, is currently being tested in preclinical models of GBM at MD Anderson Cancer Center.
Other DDR Inhibitors
CHK1 and CHK2 translate signals from upstream ATR and ATM enzymes to downstream cell cycle effectors.49 Inhibitors of CHK1 and CHK2 have been tested in preclinical models of GBM, but have not, to date, been translated into clinical trials in GBM patients. The CHK1 inhibitor AZD7762 sensitized diverse primary human GBM isolates to RT, but was not developed further because of drug-induced cardiotoxicity in a phase I clinical trial.50 The CHK2 inhibitor PV1019 showed antiproliferative activity in combination with RT in U251 GBM cells.51
WEE1 is a downstream kinase of the ATR-CHK1 pathway and a key regulator of G2/M transition. WEE1 prolongs the G2 phase by regulating the activity of CDK1, allowing DDR mechanisms additional time for DNA repair. WEE1 is overexpressed in many cancers, including GBM.52 Small-molecule inhibitors of WEE1 sensitize GBM to TMZ and RT in vitro and also sensitize orthotopic mouse models of U251-FM GBM to such treatments.52 In addition, WEE1 is activated after PI3K inhibition in glioma cells, while WEE1 inhibition potentiates the effectiveness of the targeted inhibition of PI3K in vivo.53
One preclinical study demonstrated that the WEE1 inhibitor adavosertib (AZD1775) has poor BBB penetration in a GBM xenograft model.54 However, a phase 0 study of adavosertib in patients with recurrent GBM found that unbound adavosertib, which represents the pharmacologically active fraction of the drug, reaches therapeutic concentrations within the contrast-enhancing region of the tumor.55 A phase 1 trial of adavosertib in combination with standard RT and TMZ in newly diagnosed and recurrent GBM was conducted (NCT01849146). Grade 3 and 4 DLTs included neutropenia, thrombocytopenia and elevations in liver function tests. Simultaneously, the trial determined intratumoral drug distribution in patients with recurrent GBM. This trial measured drug concentrations of 8× and 2.6× greater than plasma in contrast enhancing and nonenhancing brain tumor, respectively.56
Another class of targetable enzymes that regulate DNA repair machinery are histone deacetylases (HDACs). DNA lesions need to be accessible to the DNA repair enzymes for proper DNA repair, a process that is modulated by post-translational modifications regulating the nucleosome structure. Histone acetylation and deacetylation via the HDAC family of proteins are among the most important processes involved in chromatin remodeling. Small molecule HDAC inhibitors have been developed and several have been approved by the FDA for the treatment of a variety of cancer. Preclinical studies of HDAC inhibitors in GBM were promising, but the result of clinical trials have largely been disappointing,57,58 possibly due to poor pharmacokinetic properties of these agents, intratumor heterogeneity in GBM and lack of biomarkers of response.
As summarized above, preclinical activity of modulators of the DNA damage pathways in GBM cell lines, xenograft and orthotopic mice models have resulted in early phase clinical trials. However, major limitations of these models need to be considered. Most commonly used GBM cell lines such as U87 lack tumor heterogeneity and have undergone decades of cell culture and genetic drift.59 The orthotopic mice models do not capture the immunocompetent tumor microenvironment and adequate cell–cell interactions which have evolved as essential elements of glioma biology.60 Incorporation of preclinical models that best capture the GBM tumor heterogeneity, maintain stemness of glioma cells and allow for study of novels agents in the intact tumor–brain immune microenvironment such as the QPP model (QkiL/L; PtenL/L; Trp53L/L)61 may result in more reliable estimates of efficacy in clinical trials of DDR inhibitors.
Strategies for Successful Clinical Development of DDR Inhibitors for GBM Patients
Clinical trials of DDR inhibitors in GBM are underway (Table 1) and the number of clinically available DDR and PARP inhibitors, some with BBB penetration, are on the rise (Table 2). Experience gained from clinical trials of PARP inhibitors and other targeted therapies in GBM suggests that the clinical development of DDR inhibitors for GBM treatment will be challenging owing to several factors, including the intracranial location of the tumor, limited BBB penetration, difficulties with repeated sampling, and overlapping toxicities of DDR inhibitors with RT and TMZ including myelotoxicity and neurotoxicity. One strategy to successfully develop these drugs is to first confirm their adequate BBB penetration and intratumoral pharmacodynamic endpoints. Strategies to minimize cumulative hematological toxicities of DDR inhibitors and conventional chemotherapy in ongoing clinical trials are needed. In addition, the clinical and radiographic manifestations of neurotoxicity of DDR inhibitors are largely unknown and need to be defined in order to limit central nervous toxicity. Parallel correlative studies should be carefully designed to lay the foundation for future rational combinatorial trials of DDR inhibitors with novel therapies, such as checkpoint inhibitors (CPIs).
Table 1.
Ongoing Clinical Trials of DDR Inhibitors in GBM
| Investigational Agent | Target | Combination Treatments | Disease Setting | Phase | MGMT Status for Inclusion | Treatment Arms | NCT ID |
|---|---|---|---|---|---|---|---|
| M3814 Nedisertib | DNA-PK | RT | ND GBM | I | MGMT-unmethylated | Stage I: RT/M3814 -> TMZ Stage II: RT/M3814 -> resection -> TMZ | NCT04555577 |
| CC-115 | DNA-PK and mTOR | RT | ND GBM | II | MGMT-unmethylated | RT/CC-115 -> CC-115 vs. RT/TMZ -> TMZ | NCT02977780 |
| AZD1390 | ATM | RT | Brain metastasis, ND and Rec GBM | I | MGMT-unmethylated | Arm A: AZD1390/RT (35 Gy in 10 Fx) (Rec GBM) Arm B: AZD1390/WBRT or PBRT (BM) Arm C: AZD1390/RT - > TMZ | NCT03423628 |
| AZD1775 Adavosertib | WEE1 | RT and TMZ | ND and Rec GBM | I | none | Arm I: AZD1775/RT/TMZ -> TMZ Arm II: RT/TMZ -> TMZ/AZD1775 | NCT01849146 |
ATM, ataxia telangiectasia-mutated; DDR, DNA damage response; DNA-PK, DNA-dependent protein kinase; Fx, fraction; GBM, glioblastoma; MGMT, O6-methylguanine-DNA methyltransferase; mTOR, mammalian target of rapamycin; ND, newly diagnosed; PBRT, partial brain radiation therapy; Rec, recurrent; RT, radiation therapy; TMZ, temozolomide; WBRT, whole brain radiation therapy.
Table 2.
Clinically Available DDR and PARP Inhibitors
| Target | Putative Biomarkers of Response in Gliomas | Investigational Agent | BBB Penetration | References |
|---|---|---|---|---|
| ATM | P53 mutation | AZD1390 | Yes | 39,43,62,63 |
| AZD0156 | No | |||
| ATR | MGMT promoter methylation | AZD6738 | Yes | 46 |
| BAY 1895344 | Unknown | |||
| VX+970 | Unknown | |||
| DNA-PK | P53 mutation | AZD7648 | Unknown | 29,30 |
| CC-115 | Yes | |||
| M3814 | Unknown | |||
| VX-984 | Yes | |||
| Chk1/Chk2 | N/A | AZD7762 | Unknown | |
| LY2606368 | Unknown | |||
| Chk1 | N/A | CCT245737 | Unknown | |
| GDC-0575 | Unknown | |||
| LY2606368 | Unknown | |||
| MK8776 | Unknown | |||
| SRA737 | Unknown | |||
| WEE1 | N/A | MK-1775 | Yes | 55 |
| RAD51 | N/A | CYT-0851 | Unknown | |
| PARP | IDH1/2 mutation | Niraparib | Yes | 64–72 |
| Olaparib | Yes | |||
| Pamiparib | Yes | |||
| Rucaparib | No | |||
| Talazoparib | No | |||
| Veliparib | Yes |
ATM, ataxia telangiectasia-mutated; ATR, ataxia telangiectasia and Rad3 related; BBB, blood–brain barrier; DDR, DNA damage response; DNA-PK, DNA-dependent protein kinase; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA methyltransferase.
It is also important to determine the role of methylation status of MGMT and DDR genes in DDR inhibitor clinical trials. For example, TMZ preferentially sensitizes MGMT-deficient tumor cells to ATR inhibition in preclinical models,46 but similar observations have not been described with DNA-PK or ATM inhibition. We recommend inclusion of both MGMT promoter methylated and unmethylated GBM tumors in early phase clinical trials and to stratify based on MGMT status in order to detect early signals regarding the role of MGMT status in determining response to DDR inhibitors. In addition, hypomethylation of DDR genes have been shown to predict response benefit to TMZ in low-grade gliomas73 and poor prognosis in IDH wildtype GBM, in particular in MGMT promoter unmethylated tumors.74 It is yet to be determined if this observation is due to poor response to treatment due to hyperactivation of DDR genes or due to association between intrinsically aggressive GBM subtypes with DDR gene hypomethylation. Determining tumor DNA methylome of DDR genes could help answer this question in clinical trials of DDR inhibitors in GBM.
Conducting Window-of-Opportunity Trials
Multiple phase I and II clinical trials have investigated the safety and early efficacy of small-molecule inhibitors of various signaling pathways in GBM, but none has led to successful phase III registration trials. The challenge of performing repeated biopsies of intracranial tumors certainly contributes to the lack of successful drug development for GBM. The inclusion of planned, clinically indicated resections is likely clinically beneficial, and would add significant value to early-phase clinical trials.
We advocate conducting window-of-opportunity trials of investigational drugs at their maximum tolerated doses to determine the tumor and plasma concentrations of the drugs, which are difficult to ascertain from animal studies, and to determine pharmacodynamic endpoints within resected tumor tissue. In addition, the resection of brain tissue after treatment with DDR inhibitors allows researchers to determine the ability of the drugs to alter the tumor immune microenvironment. This design has been implemented in a study of the DNA-PK inhibitor M3814 in combination with RT in patients with newly diagnosed, MGMT-unmethylated GBM (NCT04555577).
Addressing BBB Penetration
Several PARP and DDR inhibitors are clinically available and cross the BBB to varying degrees (Table 2). Potential therapeutic agents for GBM should not be eliminated based solely on their poor BBB penetration in animal studies. As demonstrated in the case of the WEE1 inhibitor adavosertib,55 drug did not cross an intact BBB in mice but reached therapeutic concentrations in enhancing regions of human GBM. Carefully designed window-of-opportunity trials in which agents are administered prior to surgery can best determine the respective BBB penetration in human. This is particularly important when drugs are given in combination with RT, as studies in both humans and animals have demonstrated that RT increases focal BBB disruption.75–77 Therefore, appropriately sequencing systemic drug administration in relation to RT or BBB-modifying agents like bevacizumab is crucial in the development of therapeutic drugs for GBM. Moreover, given the potential toxicity of DDR agents in normal brain tissue, it may be beneficial to spare the normal, nonirradiated brain from exposure to DDR inhibitors.
Combining DDR Inhibitors With CPIs
Despite the relative success of CPIs in various other solid cancers, their clinical development in GBM has been challenging 78–81. The lack of clinical efficacy of CPIs in GBM is suspected to be due to the known low mutation burden of GBM, a feature that may potentially be overcome with the use of PARP and other DDR inhibitors.78,82 By inducing S-Phase DNA damage, it is hypothesized that DDR and PARP inhibitors can enhance the tumor mutational burden and increase the neo-antigen load,83 which has been shown to be a marker of response to immunotherapy in various solid tumors.84 DNA damage is also known to enhance signaling pathways that activate the innate immune response, such as the cGAS-STING pathway, and elevation of PD-L1 expression.85,86 Tumors with microsatellite instability are known to have significantly higher tumor mutational burden and neoantigen load, resulting in increased T-cell infiltration, augmented recognition, and cytotoxicity by the adaptive immune system.87 The FDA has granted the first tumor-agnostic approval for pembrolizumab based on the presence of high microsatellite instability or MMR deficiency and high tumor mutation burden.88 The responsiveness of MMR-deficient tumors to immunotherapy exemplifies the interplay between DDR deficiency and immunotherapy.
The effective use of CPIs against GBM has been reported in patients with tumors with a high mutational burden resulting from germline impairment in DNA repair genes.89,90 Therapeutic interventions that increase tumor mutational burden may overcome CPI resistance in GBM. Preclinical data in breast cancer and small cell lung cancer demonstrate in vivo efficacy of dual anti-PD-L1 and PARP inhibitors in immunocompetent xenograft models.86,91 Several trials of PARP and DDR inhibitors and CPIs in patients with different solid tumors are ongoing.92 Chemotherapy-related hypermutation did not promote a response to anti-PD-1 blockade in a large retrospective study.93 It remains to be determined whether PARP and DDR inhibitors-related hypermutation augments a response to CPI in GBM clinical trials.
The glioma tumor microenvironment should be evaluated after exposure to PARP and DDR inhibitors through window-of-opportunity studies to determine if the inhibition of DNA repairs pathways elevate the tumor neoantigen load and increase alterations in its immune cell composition to lay the foundation for future rationale combinatorial studies.94
Considering Modified Dosing Regimens
Combinations of PARP and DDR inhibitors with cytotoxic chemotherapy and RT have the potential to exacerbate hematological and gastrointestinal adverse events and acute RT toxicities. The frequency of G3-4 myelosuppression with the use of PARP inhibitors and TMZ was seen 54%–63% of patients with melanoma.95,96 Similarly, administration of velaparib plus standard RT and concurrent TMZ was not tolerable in GBM patients as a result of hematological toxicities.97 Combination of TMZ and DDR inhibitors have not been conducted but are expected to be complicated with similar hematological toxicities, based on combination of ATR inhibitors with other cytotoxic chemotherapy resulting in 50% G3-4 toxicity. Myelotoxicity as a result of TMZ is dose dependent, and lower effective doses of TMZ may be warranted in trials of PARP and DDR inhibitors.98,99 In addition, an intermittent dosing schedule may facilitate dose escalation with minimal risk of hematological toxicity.100
Combinations of PARP and DDR inhibitors with RT and/or TMZ should also account for intracranial dose-limiting toxicities. Given the untested nature of intracranial radiation and DDR inhibitors, both intracranial and extracranial DLTs should be defined. Intracranial DLTs should account for early and late cerebral edema, focal necrosis, neurocognitive deficits, and cerebrovascular disease. Low starting doses of DDR inhibitors and prolonged monitoring should be considered in phase I trials to ensure safety and tolerability.
Carefully designed dose combinations in terms of dose intervals and sequencing, as well as close attention to the timing of RT in relation to the administration of PARP and DDR inhibitors in early-phase trials will aid in the successful clinical development of these inhibitors.
Overcoming Mechanisms of Resistance
Lessons learnt from years of failed clinical trials of molecularly targeted therapy in GBM should be considered and applied in preclinical and clinical studies of DDR inhibitors.101 Intratumoral heterogeneity and redundant signaling pathways are among the most important factors contributing to the resistance to novel therapeutic agents in GBM. To overcome these hurdles, researchers should use preclinical models that encompass these key hallmarks of GBM to assess the efficacy of novel agents. Cell lines, GSCs, and genetically engineered mouse models lack tumor heterogeneity; therefore, novel clinical models that capture such heterogeneity, such as tumor organoids, mixing of bar-coded cell lines, and patient-derived tumor explants, are likely to be better suited for the investigation of novel agents.102 Because several pathways are involved in DDR, the inhibition of one such pathway might lead to the upregulation of, and compensation by, an alternative pathway. Therefore, in parallel with clinical trials, preclinical studies focused on identifying mechanisms of resistance are needed to guide the design of future combinatorial approaches.
Conclusion
High-throughput genomic analyses have uncovered frequent alterations in DNA damage pathways in GBM. DNA damage pathways are upregulated in GSCs, the cells responsible for RT and chemotherapy resistance in GBM. Inhibitors of DDR enzymes have great potential to improve the outcomes of GBM patients. Novel study designs with close attention to BBB penetration of the drugs, target engagement in resected brain tissue, biomarkers of response, modified dosing regimens, and mechanisms of resistance are needed for the successful development of DDR inhibitors in GBM.
Acknowledgments
Editorial support was provided by Joe Munch in Scientific Publications Services, Research Medical Library at the University of Texas MD Anderson Cancer Center. We would like to thank Ahmed Shehalbeldin, MD for graphic design of Figure 1.
Conflict of interest statement. JdG: Grant or research support: Sanofi-Aventis, AstraZeneca, EMD-Serono; Eli Lilly, Novartis, Deciphera Pharmaceuticals, Mundipharma. Paid consultancies: Celldex; Deciphera Pharmaceuticals, AbbVie, FivePrime Therapeutics, Inc., GW Pharma, Carthera, Eli Lilly, Kadmon, Boston Biomedical Inc., Taiho Pharmaceuticals, Kairos Venture Investments, Syneos Health, Monteris, Agios, Mundipharma Research, GenomiCare, Blue Earth Diagnostics, Del Mar Pharmaceuticals, Insightec, Voyager Therapeutics, Inc., Merck & Co., Tocagen, Bioasis Technologies, Inc., ResTORbio, Inc. Advisory board membership: Genentech, Celldex, Foundation Medicine, Inc., Novogen, Deciphera, AstraZeneca, Insys Therapeutics, Merck, Eli Lilly, Novella Clinical, Karyopharm Therapeutics, Blue Earth Diagnostics, Kiyatec, Vanquish Oncology, Orsenix, Insightec, Prelude Therapeutics, Debiopharm Therapeutics, Inc., Janssen Global Services, LLC. Data safety monitoring board membership: VBL Therapeutics (VB111; glioblastoma); Novella (ICT-107; glioblastoma); VBI Vaccines, Inc (VBI-1901; glioblastoma). Stock ownership: Ziopharm Oncology, Gilead. Company employment (spouse): Ziopharm Oncology. TAY: Research support (to institution): Artios, AstraZeneca, Bayer, Clovis, Constellation, Cyteir, Eli Lilly, EMD Serono, Forbius, F-Star, GlaxoSmithKline, Genentech, ImmuneSensor, Ipsen, Jounce, Karyopharm, Kyowa, Merck, Novartis, Pfizer, Ribon Therapeutics, Regeneron, Repare, Sanofi, Scholar Rock, Seattle Genetics, Tesaro, and Vertex Pharmaceuticals. Consultancies: Almac, Aduro, AstraZeneca, Atrin, Axiom, Bayer, Bristol Myers Squibb, Calithera, Clovis, Cybrexa, EMD Serono, F-Star, Guidepoint, Ignyta, I-Mab, Jansen, Merck, Pfizer, Repare, Roche, Rubius, Schrodinger, Seattle Genetics, Varian, and Zai Labs. WKAY: Advisory Board: DNAtrix, Denovo, Quadriga, ILCT. Consultancy: Roche. NKM, DK, VB, XL, SK, and KSG dont have conflicts of interest.
Authorship Statement.
N.K.M., T.A.Y., A.Y., and Jd.G.: Concept development and manuscript writing. Provided critical feedback, contributed to the concept and organization, edited the manuscript: D.K., V.B., X.L., S.K., and K.S.G.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro Oncol. 2016;18(10):1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Research N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma J, Benitez JA, Li J, et al. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;36(6):690–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu S, Gao F, Zheng S, et al. EGFR amplification induces increased DNA damage response and renders selective sensitivity to talazoparib (PARP Inhibitor) in Glioblastoma. Clin Cancer Res. 2020;26(6):1395–1407. [DOI] [PubMed] [Google Scholar]
- 7. Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100(9):5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caldecott KW. Protein ADP-ribosylation and the cellular response to DNA strand breaks. DNA Repair (Amst). 2014;19:108–113. [DOI] [PubMed] [Google Scholar]
- 9. Finzel A, Grybowski A, Strasen J, Cristiano E, Loewer A. Hyperactivation of ATM upon DNA-PKcs inhibition modulates p53 dynamics and cell fate in response to DNA damage. Mol Biol Cell. 2016;27(15):2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 11. Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22(4):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caporali S, Falcinelli S, Starace G, et al. DNA damage induced by temozolomide signals to both ATM and ATR: role of the mismatch repair system. Mol Pharmacol. 2004;66(3):478–491. [DOI] [PubMed] [Google Scholar]
- 13. Quinn JA, Jiang SX, Reardon DA, et al. Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma. J Clin Oncol. 2009;27(8):1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tawbi HA, Villaruz L, Tarhini A, et al. Inhibition of DNA repair with MGMT pseudosubstrates: phase I study of lomeguatrib in combination with dacarbazine in patients with advanced melanoma and other solid tumours. Br J Cancer. 2011;105(6):773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jette N, Lees-Miller SP. The DNA-dependent protein kinase: a multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog Biophys Mol Biol. 2015;117(2-3):194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drouet J, Frit P, Delteil C, de Villartay JP, Salles B, Calsou P. Interplay between Ku, Artemis, and the DNA-dependent protein kinase catalytic subunit at DNA ends. J Biol Chem. 2006;281(38):27784–27793. [DOI] [PubMed] [Google Scholar]
- 17. Luo Q, Guo H, Kuang P, et al. Sodium fluoride arrests renal G2/M phase cell-cycle progression by activating ATM-Chk2-P53/Cdc25C signaling pathway in mice. Cell Physiol Biochem. 2018;51(5):2421–2433. [DOI] [PubMed] [Google Scholar]
- 18. You Z, Chahwan C, Bailis J, Hunter T, Russell P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol. 2005;25(13):5363–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. [DOI] [PubMed] [Google Scholar]
- 20. Stracker TH, Usui T, Petrini JH. Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (amst). 2009;8(9):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Majd N, Yap TA, Yung WKA, de Groot J. The promise of poly(ADP-Ribose) polymerase (PARP) inhibitors in Gliomas. Journal of Immunotherapy and Precision Oncology. 2020;3(4):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stover EH, Konstantinopoulos PA, Matulonis UA, Swisher EM. Biomarkers of response and resistance to DNA repair targeted therapies. Clin Cancer Res. 2016;22(23):5651–5660. [DOI] [PubMed] [Google Scholar]
- 24. Goodwin JF, Knudsen KE. Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 2014;4(10):1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dietlein F, Thelen L, Reinhardt HC. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014;30(8):326–339. [DOI] [PubMed] [Google Scholar]
- 26. Riabinska A, Daheim M, Herter-Sprie GS, et al. Therapeutic targeting of a robust non-oncogene addiction to PRKDC in ATM-defective tumors. Sci Transl Med. 2013;5(189):189ra178. [DOI] [PubMed] [Google Scholar]
- 27. Lan T, Zhao Z, Qu Y, et al. Targeting hyperactivated DNA-PKcs by KU0060648 inhibits glioma progression and enhances temozolomide therapy via suppression of AKT signaling. Oncotarget. 2016;7(34):55555–55571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Xu H, Liu T, et al. Temporal DNA-PK activation drives genomic instability and therapy resistance in glioma stem cells. JCI Insight. 2018;3(3):e98096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timme CR, Rath BH, O’Neill JW, Camphausen K, Tofilon PJ. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic Xenografts. Mol Cancer Ther. 2018;17(6):1207–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Q, Guo Y, Liu X, et al. Therapeutic implications of p53 status on cancer cell fate following exposure to ionizing radiation and the DNA-PK inhibitor M3814. Mol Cancer Res. 2019;17(12):2457–2468. [DOI] [PubMed] [Google Scholar]
- 31. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munster P, Mita M, Mahipal A, et al. First-in-human phase I study of a dual mTOR kinase and DNA-PK inhibitor (CC-115) in advanced malignancy. Cancer Manag Res. 2019;11:10463–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma DJ, Galanis E, Anderson SK, et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17(9):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS. Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res. 2013;33(4):1657–1660. [PubMed] [Google Scholar]
- 35. Wick W, Gorlia T, Bady P, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 36. Eich M, Roos WP, Nikolova T, Kaina B. Contribution of ATM and ATR to the resistance of glioblastoma and malignant melanoma cells to the methylating anticancer drug temozolomide. Mol Cancer Ther. 2013;12(11):2529–2540. [DOI] [PubMed] [Google Scholar]
- 37. Barlow C, Hirotsune S, Paylor R, et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86(1):159–171. [DOI] [PubMed] [Google Scholar]
- 38. Zhang T, Cronshaw J, Kanu N, Snijders AP, Behrens A. UBR5-mediated ubiquitination of ATMIN is required for ionizing radiation-induced ATM signaling and function. Proc Natl Acad Sci USA. 2014;111(33):12091–12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biddlestone-Thorpe L, Sajjad M, Rosenberg E, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res. 2013;19(12):3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golding SE, Rosenberg E, Adams BR, et al. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle. 2012;11(6):1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vecchio D, Daga A, Carra E, et al. Pharmacokinetics, pharmacodynamics and efficacy on pediatric tumors of the glioma radiosensitizer KU60019. Int J Cancer. 2015;136(6):1445–1457. [DOI] [PubMed] [Google Scholar]
- 42. Karlin J, Allen J, Ahmad SF, et al. Orally bioavailable and blood-brain barrier-penetrating ATM inhibitor (AZ32) radiosensitizes intracranial gliomas in mice. Mol Cancer Ther. 2018;17(8):1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Durant ST, Zheng L, Wang Y, et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci Adv. 2018;4(6):eaat1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knizhnik AV, Roos WP, Nikolova T, et al. Survival and death strategies in glioma cells: autophagy, senescence and apoptosis triggered by a single type of temozolomide-induced DNA damage. PLoS One. 2013;8(1):e55665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aasland D, Götzinger L, Hauck L, et al. Temozolomide induces senescence and repression of DNA repair pathways in glioblastoma cells via activation of ATR-CHK1, p21, and NF-κB. Cancer Res. 2019;79(1):99–113. [DOI] [PubMed] [Google Scholar]
- 46. Jackson CB, Noorbakhsh SI, Sundaram RK, et al. Temozolomide sensitizes MGMT-deficient tumor cells to ATR inhibitors. Cancer Res. 2019;79(17):4331–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahmed SU, Carruthers R, Gilmour L, Yildirim S, Watts C, Chalmers AJ. Selective inhibition of parallel DNA damage response pathways optimizes radiosensitization of glioblastoma stem-like cells. Cancer Res. 2015;75(20):4416–4428. [DOI] [PubMed] [Google Scholar]
- 48. Bono JSD, Tan DSP, Caldwell R, et al. First-in-human trial of the oral ataxia telangiectasia and Rad3-related (ATR) inhibitor BAY 1895344 in patients (pts) with advanced solid tumors. J Clin Oncol. 2019;37(15_suppl):3007-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3(5):421–429. [DOI] [PubMed] [Google Scholar]
- 50. Tang Y, Dai Y, Grant S, Dent P. Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol Ther. 2012;13(6):379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jobson AG, Lountos GT, Lorenzi PL, et al. Cellular inhibition of checkpoint kinase 2 (Chk2) and potentiation of camptothecins and radiation by the novel Chk2 inhibitor PV1019 [7-nitro-1H-indole-2-carboxylic acid {4-[1-(guanidinohydrazone)-ethyl]-phenyl}-amide]. J Pharmacol Exp Ther. 2009;331(3):816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mir SE, De Witt Hamer PC, Krawczyk PM, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18(3):244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu S, Wang S, Gao F, et al. Activation of WEE1 confers resistance to PI3K inhibition in glioblastoma. Neuro Oncol. 2018;20(1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pokorny JL, Calligaris D, Gupta SK, et al. The efficacy of the Wee1 inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood–brain barrier in glioblastoma. Clin Cancer Res. 2015;21(8):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanai N, Li J, Boerner J, et al. Phase 0 Trial of AZD1775 in first-recurrence glioblastoma patients. Clin Cancer Res. 2018;24(16):3820–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alexander BM, Ahluwalia MS, Desai AS, et al. Phase I study of AZD1775 with radiation therapy (RT) and temozolomide (TMZ) in patients with newly diagnosed glioblastoma (GBM) and evaluation of intratumoral drug distribution (IDD) in patients with recurrent GBM. J Clin Oncol. 2017;35(15_suppl):2005-2005. [Google Scholar]
- 57. Galanis E, Anderson SK, Miller CR, et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: results of Alliance N0874/ABTC 02. Neuro Oncol. 2018;20(4):546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghiaseddin A, Reardon D, Massey W, et al. Phase II study of bevacizumab and vorinostat for patients with recurrent world health organization grade 4 malignant glioma. Oncologist. 2018;23(2):157–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Allen M, Bjerke M, Edlund H, Nelander S, Westermark B. Origin of the U87MG glioma cell line: good news and bad news. Sci Transl Med. 2016;8(354):354re353. [DOI] [PubMed] [Google Scholar]
- 60. Lenting K, Verhaak R, Ter Laan M, Wesseling P, Leenders W. Glioma: experimental models and reality. Acta Neuropathol. 2017;133(2):263–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shingu T, Ho AL, Yuan L, et al. Qki deficiency maintains stemness of glioma stem cells in suboptimal environment by downregulating endolysosomal degradation. Nat Genet. 2017;49(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blake SM, Stricker SH, Halavach H, et al. Inactivation of the ATMIN/ATM pathway protects against glioblastoma formation. Elife. 2016;5. https://elifesciences.org/articles/08711#content [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pike KG, Barlaam B, Cadogan E, et al. The identification of potent, selective, and orally available inhibitors of ataxia telangiectasia mutated (ATM) kinase: the discovery of AZD0156 (8-{6-[3-(Dimethylamino)propoxy]pyridin-3-yl}-3-methyl-1-(tetrahydro-2 H-pyran-4-yl)-1,3-dihydro-2 H-imidazo[4,5- c]quinolin-2-one). J Med Chem. 2018;61(9):3823–3841. [DOI] [PubMed] [Google Scholar]
- 64. Sulkowski PL, Corso CD, Robinson ND, et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. 2017;9(375):eaal2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Wild AT, Turcan S, et al. Targeting therapeutic vulnerabilities with PARP inhibition and radiation in IDH-mutant gliomas and cholangiocarcinomas. Sci. Adv. 2020;6(17):eaaz3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lu Y, Kwintkiewicz J, Liu Y, et al. Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-Mediated DNA Repair. Cancer Res. 2017;77(7):1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hanna C, Kurian KM, Williams K, et al. Pharmacokinetics, safety, and tolerability of olaparib and temozolomide for recurrent glioblastoma: results of the phase I OPARATIC trial. Neuro Oncol. 2020;22(12):1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Parrish KE, Cen L, Murray J, et al. Efficacy of PARP inhibitor rucaparib in orthotopic glioblastoma xenografts is limited by ineffective drug penetration into the central nervous system. Mol Cancer Ther. 2015;14(12):2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sun K, Mikule K, Wang Z, et al. A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models. Oncotarget 2018;9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiong Y, Guo Y, Liu Y, et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia. 2020;22(9):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Donawho CK, Luo Y, Luo Y, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13(9):2728–2737. [DOI] [PubMed] [Google Scholar]
- 72. Kizilbash SH, Gupta SK, Chang K, et al. Restricted delivery of talazoparib across the blood-brain barrier limits the sensitizing effects of PARP inhibition on temozolomide therapy in Glioblastoma. Mol Cancer Ther. 2017;16(12):2735–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bady P, Kurscheid S, Delorenzi M, et al. The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol. 2018;135(4):601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kessler T, Berberich A, Sadik A, et al. Methylome analyses of three glioblastoma cohorts reveal chemotherapy sensitivity markers within DDR genes. Cancer Med. 2020;9(22):8373–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23(18):4127–4136. [DOI] [PubMed] [Google Scholar]
- 76. Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13(6):1675–1683. [DOI] [PubMed] [Google Scholar]
- 77. Lemasson B, Serduc R, Maisin C, et al. Monitoring blood-brain barrier status in a rat model of glioma receiving therapy: dual injection of low-molecular-weight and macromolecular MR contrast media. Radiology. 2010;257(2):342–352. [DOI] [PubMed] [Google Scholar]
- 78. Majd N, de Groot J. Challenges and strategies for successful clinical development of immune checkpoint inhibitors in glioblastoma. Expert Opin Pharmacother. 2019;20(13):1609–1624. [DOI] [PubMed] [Google Scholar]
- 79. Reardon DA, Nayak L, Peters KB, et al. Phase II study of pembrolizumab or pembrolizumab plus bevacizumab for recurrent glioblastoma (rGBM) patients. J Clin Oncol. 2018;36(15_suppl):2006-2006.29763342 [Google Scholar]
- 80. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: checkmate 143. Neuro Oncol. 2017;19(suppl_3):iii21-iii21. [Google Scholar]
- 81. Bristol-Myers Squibb. Bristol-Myers Squibb Announces Phase 3 CheckMate -498 Study Did Not Meet Primary Endpoint of Overall Survival with Opdivo (nivolumab) Plus Radiation in Patients with Newly Diagnosed MGMT-Unmethylated Glioblastoma Multiforme. May 9, 2019. https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-phase-3-checkmate-498-study-did
- 82. de Groot J, Penas-Prado M, Alfaro-Munoz K, et al. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol. 2020;22(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pilié PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-Mutant cancers. Clin Cancer Res. 2019;25(13):3759–3771. [DOI] [PubMed] [Google Scholar]
- 84. Viale G, Trapani D, Curigliano G. Mismatch repair deficiency as a predictive biomarker for immunotherapy efficacy. Biomed Res Int. 2017;2017:4719194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Parkes EE, Walker SM, Taggart LE, et al. Activation of STING-Dependent innate immune signaling by S-Phase-Specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109(1). https://academic.oup.com/jnci/article/109/1/djw199/2905926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov. 2019;9(5):646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22(11):1342–1350. [DOI] [PubMed] [Google Scholar]
- 88. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 90. Johanns TM, Miller CA, Dorward IG, et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 2016;6(11):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jiao S, Xia W, Yamaguchi H, et al. PARP Inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yap TA, Plummer R, Azad NS, Helleday T. The DNA damaging revolution: PARP inhibitors and beyond. Am Soc Clin Oncol Educ Book. 2019;39:185–195. [DOI] [PubMed] [Google Scholar]
- 93. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Majd N, Dasgupta P, de Groot J. Immunotherapy for Neuro-Oncology. In: Naing A, Hajjar J, eds. Immunotherapy. Cham: Springer International Publishing; 2020:183–203. [DOI] [PubMed] [Google Scholar]
- 95. Middleton MR, Friedlander P, Hamid O, et al. Randomized phase II study evaluating veliparib (ABT-888) with temozolomide in patients with metastatic melanoma. Ann Oncol. 2015;26(10):2173–2179. [DOI] [PubMed] [Google Scholar]
- 96. Plummer R, Lorigan P, Steven N, et al. A phase II study of the potent PARP inhibitor, Rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother Pharmacol. 2013;71(5):1191–1199. [DOI] [PubMed] [Google Scholar]
- 97. Kleinberg L, Supko JG, Mikkelsen T, et al. Phase I adult brain tumor consortium (ABTC) trial of ABT-888 (veliparib), temozolomide (TMZ), and radiotherapy (RT) for newly diagnosed glioblastoma multiforme (GBM) including pharmacokinetic (PK) data. J Clin Oncol. 2013;31(15_suppl):2065-2065.23650415 [Google Scholar]
- 98. Piotrowski A, Puduvalli V, Wen P, et al. ACTR-39. Pamiparib in combination with radiation therapy (RT) and/or temozolomide (TMZ) in patients with newly diagnosed or recurrent/refractory (R/R) glioblastoma (GBM); phase 1b/2 study update. Neuro Oncol. 2019;21(Supplement_6):vi21-vi22. [Google Scholar]
- 99. Shih K, Schiff D, Kim L, et al. ACTR-30. Phase 1B/2 study to assess the clinical effects of pamiparib (BGB-290) in combination with radiation therapy (RT) and/or temozolomide (TMZ) in patients with newly diagnosed or recurrent/refractory glioblastoma (GBM). Neuro Oncol. 2018;20(Suppl 6):vi17–vi18. [Google Scholar]
- 100. Fulton B, Short SC, James A, et al. PARADIGM-2: two parallel phase I studies of olaparib and radiotherapy or olaparib and radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma, with treatment stratified by MGMT status. Clin Transl Radiat Oncol. 2018;8:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Touat M, Idbaih A, Sanson M, Ligon KL. Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol. 2017;28(7):1457–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep. 2018;23(4):1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]