Abstract
Background
Breast cancer is the second most common cancer associated with brain metastases. The purpose of this study was to identify factors that impact the time to brain metastases in breast cancer patients at a single institution.
Methods
Single institution retrospective study that captured all consecutive stage 2 and stage 3 breast cancer patients from 2003 to 2010. Patient characteristics analyzed included age, hormone status, HER2 receptor status, grade, stage, and time from breast cancer diagnosis to brain metastasis.
Results
A total of 1218 patients were eligible for the final analysis. 849 (69.7%) patients were ER+/HER2−, 90 (7.4%) were HER2+, and 279 (22.9%) were triple-negative (TN). Overall, 74 patients (6.1%) developed brain metastases over a median follow up time of 92 months. Median times to brain metastases for HER2+, TN, and ER+/HER2− patients were 20, 26, and 57 months, respectively. Multivariate analysis demonstrated that TN disease (HR = 2.043, P = .015), grade (HR = 1.667, P = .024) and stage (HR = 3.851, P < .001) were independent risk factors for earlier brain metastases. Median times to brain metastases were 34 and 52 months for stage 3 and 2 patients, and 30, 49, and 71 months for grade 3, 2, and 1 tumors, respectively.
Conclusions
This single-institutional case series demonstrates that TN breast cancer, higher stage, and higher histologic grade are associated with earlier brain metastases in multivariate analysis. Additional prospective studies are warranted to investigate the impact of brain metastases screening on survival outcome in this high-risk defined group.
Keywords: brain metastases, breast cancer, HER2+ breast cancer, MRI screening, triple negative
Key Points.
Triple-negative breast cancer patients have a higher risk of earlier brain metastases.
Breast cancer stage and grade impact time to brain metastasis.
Importance of the Study.
Though breast cancer is the second most common cause of brain metastases, current guidelines do not recommend routine MRI screening of the brain in breast cancer patients. We identify risk factors in breast cancer patients that impact the time to brain metastases. We demonstrate that patients with triple-negative breast cancer, higher-grade tumors, and higher stage disease are at greater risk of earlier metastases. Early brain metastases screening may impact the prognosis, treatment options, and overall survival for this select group of high-risk patients, and should be studied further in additional prospective studies.
Breast cancer is the second leading cause of brain metastases after lung cancer. Central nervous system (CNS) involvement, including both brain and leptomeningeal disease, has been shown to occur in 10–30% of breast cancer patients.1,2 Previous studies have identified multiple independent risk factors for the development of CNS recurrence including: young age, African-American ethnicity, nodal status, high tumor grade, as well as having a triple-negative (TN) or HER2+ subtype.3–6 The incidence of brain metastases in breast cancer patients has increased due to in part improved survival time from novel treatment options, as well as higher detection rates via improved imaging techniques.3 The lifetime incidence rate of CNS metastases has been reported to be between 25–46% in TN patients and 30–55% in HER2+ patients.4–7
Brain metastasis occurs after median latency period of 32 months from the initial breast cancer diagnosis in patients of all tumor subtypes.8 Median survival time after brain metastases ranges from 4–10 months even after treatment, although there are several factors that impact survival time. The most validated prognostic factors that impact a median overall survival time in breast cancer patients with brain metastases are age, Karnofsky performance status (KPS), and tumor subtype, all of which are included in the Radiation Therapy Oncology Group (RTOG) Breast Cancer Graded Prognostic Analysis (BrGPA). Patients with TN breast tumors have been shown to have the worst median overall survival of 4.9–7.3 months, compared to 17.9–25.3 months in HER2+ patients and 15–17 months in hormone receptor positive-HER2 negative patients after the development of brain metastases.4,7–10
Although there is a clearly established group of breast cancer patients at increased risk of both brain metastases and shorter median survival time, there is currently no standard of care for routine brain metastases screening. Screening of asymptomatic patients with brain MRI is not currently recommended by the American Society of Clinical Oncology due to lack of evidence from prospective studies. However, due to the high burden of CNS involvement, there should be a low threshold for performing diagnostic brain imaging if patients report any new neurologic signs or symptoms.11 Identifying risk factors that are associated with both earlier and more aggressive CNS relapse could help us further define ideal candidates for brain metastases screening prior to symptom onset. This subgroup of patients would potentially benefit from more aggressive screening in the pre-symptomatic period that may ultimately impact the treatment regimen and overall survival after brain metastases. There are only a few studies that have explored risk factors associated with a shorter time to brain metastases in breast cancer patients, with the majority focusing on tumor subtype.7,12,13 The aim of this study was to identify various risk factors in breast cancer patients that affect the time to brain metastases with the hopes of defining a high-risk population that may benefit from earlier screening.
Materials and Methods
Participants
We identified consecutive patients diagnosed with breast cancer between January 1, 2003 and December 31, 2010 at the Robert H. Lurie Comprehensive Cancer Center of Northwestern. Patients with a primary breast cancer diagnosis, as well as those with brain metastases, were identified in the Electronic Data Warehouse by the correlating ICD-9 codes: 174.0, 174.1, 174.2, 174.3, 174.4, 174.5, 174.6, 174.8, 174.9, 198.3. Patients were excluded due to having a concurrent or previous separate primary cancer diagnosis or having incomplete documentation of immunohistological data in the electronic medical record. In addition, to focus our study on a high-risk group of patients who did not already present with metastases, we limited our study population to patients with stage 2–3 disease per the seventh edition of the American Joint Committee on Cancer (AJCC) cancer staging manual.14 Patient characteristics that were collected included age at primary diagnosis of breast cancer, hormone, and HER2 receptor status, clinical stage and histologic grade, as well as time from primary diagnosis to brain metastasis. Patients were followed from the time of primary diagnosis until June 1, 2016. The study was approved by the Institutional Review Board of Northwestern University.
Breast Cancer Subtype Definition and Surgical Pathology
The three clinical subtypes identified were defined as: ER+/HER2− (ER+, PR+/PR−, HER2−), HER2+ (ER−, PR−, HER2+), and TN (ER−, PR−, HER2−). Tumors were considered ER or PR positive if at least 1% of tumor cells stained positive on immunohistochemistry (IHC). Tumors were considered positive for human epidermal growth factor 2 (HER2) if they were scored 3+ by IHC, 2+ by IHC and HER2 amplified (ratio >2.0) by fluorescence in situ hybridization (FISH), or FISH ratio <2.0 but gene copy >6.15
Statistical Methods
Time to brain metastases was determined as the time in months from the date of primary breast cancer diagnosis to the date of brain metastasis diagnosis. Baseline characteristics were compared across tumor subtype groups by one-way ANOVA for age and Kruskal–Wallis test for histology, grade, and stage. Survival curves plotting the time to brain metastases according to breast cancer subtype, stage, and grade on a Kaplan–Meier curve were compared using the log-rank test. Patients were censored from the Kaplan–Meier analyses if they developed brain metastases, were lost to follow up, or if they experienced death. At-risk tables are provided for Kaplan–Meier curves. Both univariate and multivariate Cox proportional hazard models were performed to identify factors associated with earlier time to brain metastases, and the estimated hazard ratios were calculated. Variables in the Cox proportional hazard models are continuous. Univariate variables of P < .05 were included in the multivariate Cox proportional hazard model. All analyses were performed using R 3.3.2. The survival 3.3.2 and survminer 3.3.2 packages were used. Two-sided P values of < .05 were considered statistically significant.
Results
Patient Population and Baseline Characteristics
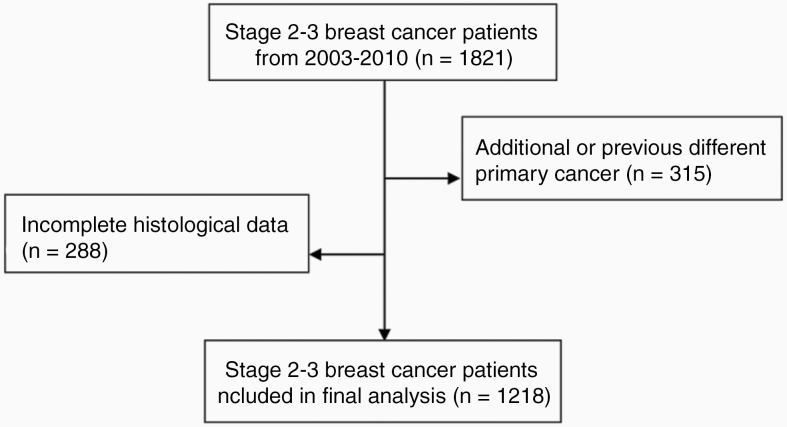
Patients were selected according to the process outlined in Figure 1. Out of 1821 breast cancer patients with stage 2 or 3 disease, 315 were excluded because they were diagnosed with a concurrent or previous different primary cancer and 288 patients were excluded due to incomplete histological data. Our final analysis included 1218 patients diagnosed with stage 2–3 primary breast cancer.
Figure 1.
Patient selection process. 1821 breast cancer patients with stage 2 or 3 disease identified of which 315 excluded due to a concurrent or previous different primary cancer diagnosis, 288 excluded due to incomplete histological data. 1218 patients with stage 2–3 primary breast cancer included in the final analysis.
Baseline characteristics are displayed in Table 1. The median follow-up time was 92 months. The median age at primary diagnosis of breast cancer was at 52 years. 969 (79.6%) patients were diagnosed with infiltrating ductal carcinoma (IDC), 120 (9.9%) patients with infiltrating lobular carcinoma (ILC), and 129 (10.6%) patients with mixed infiltrating ductal and lobular carcinoma. Of the clinical subtypes, 849 (69.7%) patients were ER+/HER2−, 90 (7.4%) patients were HER2+, and 279 (22.9%) patients were TN. The majority of all tumors had a histologic grade of 2 (45.5%) or 3 (37.5%). Overall, 878 (72.1%) patients were diagnosed with stage 2 disease compared to 340 patients (27.9%) with stage 3. At baseline, both the HER2+ and TN groups had significantly greater percentage of IDC tumors (P < .001), higher grade tumors (P < .001), and higher clinical stage (P = .048) compared to the ER+/HER2− group. This representative sample is consistent with previous studies.7,9,13,16
Table 1.
Baseline Characteristics
| Number of Patients (%) | |||||
|---|---|---|---|---|---|
| Characteristics | HER2+ Subtype (n = 90) | TN Subtype (n = 279) | ER/HER2– Subtype (n = 849) | All Subtypes (n = 1218) | P Value |
| Median age (years) | 52 | 51 | 52 | 52 | .094 |
| Histology | |||||
| IDC | 85 (94.4%) | 266 (95.3%) | 618 (72.8%) | 969 (79.6%) | <.001 |
| ILC | 0 (0.0%) | 3 (1.1%) | 117 (13.8%) | 120 (9.9%) | |
| Mixed | 5 (5.6%) | 10 (3.6%) | 114 (13.4%) | 129 (10.6%) | |
| Grade | |||||
| 1 | 0 (0.0%) | 5 (1.8%) | 202 (23.8%) | 207 (17.0%) | <.001 |
| 2 | 29 (32.2%) | 36 (13.0%) | 489 (57.6%) | 554 (45.5%) | |
| 3 | 61 (67.8%) | 238 (85.3%) | 158 (18.6%) | 457 (37.5%) | |
| Stage | |||||
| 2 | 57 (63.3%) | 193 (69.2%) | 628 (74.0%) | 878 (72.1%) | .048 |
| 3 | 33 (36.7%) | 86 (30.8%) | 221 (26.0%) | 340 (27.9%) | |
| Tumor subtype | |||||
| HER2+ | 90 (7.4%) | ||||
| TN | 279 (22.9%) | ||||
| ER+/HER2− |
849 (69.7%) | ||||
IDC, infiltrating ductal carcinoma; ILC, infiltrating lobular carcinoma; TN, triple negative.
Incidence of Brain Metastases
Table 2 outlines the incidence of brain metastases according to tumor subtype, histology, grade, and stage. Over the course of the study from the time of primary diagnosis to June 1, 2016, 74 patients (6.1%) developed brain metastases. Of these 74 patients with CNS relapse, there were 9 HER2+ patients, and 30 TN patients, and 35 ER+/HER2− patients. The incidence of brain metastases by clinical subtype was 10.0% for HER2+ patients, 10.8% for TN patients, and 4.1% for ER+/HER2− patients (P < .001). The incidence of brain metastases was 3.5% and 12.6% for all stage 2 and 3 patients, respectively (P < .001). Tumors of IDC histology and higher grade were associated with a higher incidence of brain metastases (P = .015 and P < .001, respectively).
Table 2.
Incidence of Brain Metastases by Factor
| Number of Patients With Brain Metastases (n = 74) | P Value | |
|---|---|---|
| Tumor subtype | ||
| HER2+ (n = 90) | 9 (10.0%) | <.001 |
| TN (n = 279) | 30 (10.8%) | |
| ER+/HER2– (n = 849) | 35 (4.1%) | |
| Histology | ||
| IDC (n = 969) | 67 (6.9%) | .015 |
| ILC (n = 120) | 4 (3.3%) | |
| Mixed (n = 129) | 3 (2.3%) | |
| Grade | ||
| 1 (n = 207) | 5 (2.4%) | <.001 |
| 2 (n = 554) | 24 (4.3%) | |
| 3 (n = 457) | 45 (9.8%) | |
| Stage | ||
| 2 (n = 878) | 31 (3.5%) | <.001 |
| 3 (n = 340) | 43 (12.6%) | |
| Total (n = 1218) | 74 (6.1%) |
Time to Brain Metastases
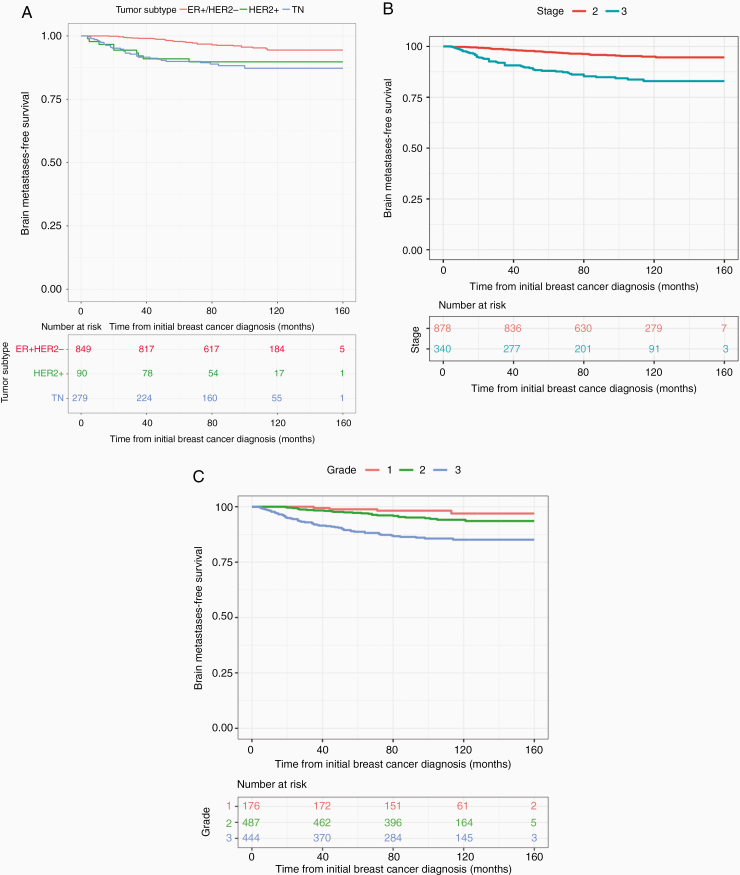
The time from primary diagnosis of breast cancer to brain metastases according to tumor subtype, stage, and grade is shown in Figure 2. The median follow-up time was 92 months. As shown in Figure 2A, both HER2+ and TN patients tended to have a shorter time to brain metastases compared to ER+/HER2− patients in Figure 2A (P < .0001). For the 9 HER2+ and 30 TN patients who developed brain metastases, the median time to CNS involvement was 20 months (95% CI 12.9–32.9 months) and 26 months (95% CI 18.9–33.1 months), respectively, compared to 57 months (95% CI 47.6–64.3 months) for the 35 ER+/HER2− patients with CNS involvement.
Figure 2.
Overall time to brain metastases according to the following factors: (A) Tumor subtype. HER2 + 20 months (12.9–32.9); TN 26 months (18.9–33.1); ER+/HER2− 57 months (47.6–64.3) (P < .0001); (B) Stage. 34 months (26.0–42.0) for stage 3 patients; 52 months (42.8–61.2) for stage 2 patients (P < .0001); (C) Grade. 30 months (22.9–37.0) for grade 3 tumors; 49 (37.5–60.5) for grade 2 tumors; 71 (44.5–97.5) months for grade 1 tumors (P < .0001).
Figure 2B demonstrates that stage 3 patients of all subtypes have a shorter interval to CNS involvement compared to stage 2 patients (P < .0001). The median time to brain metastases was 34 (95% CI 26.0–42.0) vs. 52 (95% CI 42.8–61.2) months for stage 3 vs. 2 patients with brain metastases of all tumor subtypes.
Figure 2C demonstrates that grade 3 patients had shorter onset to brain metastases compared to grade 2 and 1 (P < .0001). The median time to brain metastases was 30 (95% CI 22.9–37.0), 49 (95% CI 37.5–60.5), and 71 (95% CI 44.5–97.5) months for grade 3, 2, and 1 patients.
Univariate and Multivariate Cox Proportional Hazard Model
Results for both univariate and multivariate analysis are shown in Table 3. In univariate analysis, compared to ER+/HER2− breast cancer patients, both HER2+ (HR = 2.639, P = .009) and TN (HR = 3.062, P ≤ .001) tumor subtypes were independently associated with a shorter time to CNS recurrence. Both grade (HR = 2.334, P < .001) and stage (HR = 4.062, P < .001) were also predictive of earlier brain metastases. Neither age nor histology was associated with brain metastases.
Table 3.
Univariate and Multivariate Cox Proportional Hazard Models
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk Factor | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age | 0.982 (0.964–1) | .055 | – | – |
| Tumor subtype | ||||
| HER2+ vs. ER+/HER2- | 2.639 (1.269–5.491) | .009 | 1.592 (0.728–3.484) | .244 |
| TN vs. ER+/HER2- | 3.062 (1.880–4.989) | <.001 | 2.043 (1.149–3.632) | .015 |
| Histology | ||||
| ILC vs. IDC | 0.462 (0.168–1.266) | .133 | – | – |
| Mixed vs. IDC | 0.326 (0.103–1.037) | .058 | – | – |
| Grade | 2.334 (1.600–3.405) | <.001 | 1.667 (1.069–2.598) | .024 |
| Stage | 4.062 (2.559–6.448) | <.001 | 3.851 (2.422–6.123) | <.001 |
In multivariate analysis, the TN (HR = 2.043, P = .015) tumor subtype, grade (HR = 1.667, P = .024), and stage (HR = 3.851, P < .001) remained independent prognostic factors for a shorter time to brain metastasis. The HER2+ tumor subtype was not statistically associated with a shorter time to brain metastasis in this multivariate analysis.
Adjusting for Adjuvant Trastuzumab Treatment in Multivariate Analysis
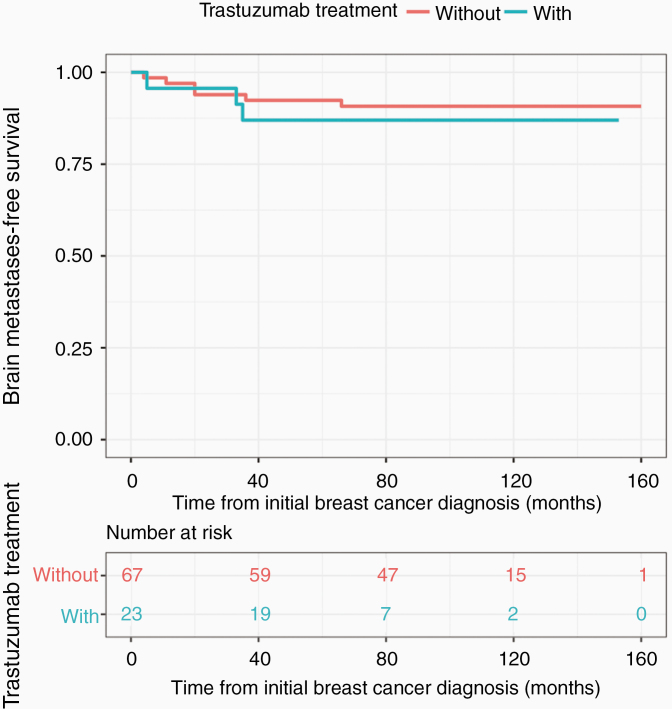
A separate Kaplan–Meier curve was performed for HER2+ patients (n = 90) specifically to investigate the effect of adjuvant trastuzumab treatment on the interval to CNS involvement. Of the HER2+ patients, 23 patients (25.6%) received adjuvant trastuzumab treatment. As shown in Figure 3, there was no significant difference between HER2+ patients with vs. without trastuzumab treatment (P = .6). 95% confidence intervals are also shown in Figure 3.
Figure 3.
Time to brain metastases in HER2+ patients with vs. without trastuzumab. With trastuzumab 34 months (14.0–52.0); without trastuzumab 20 months (2.2–37.8) (P = .6).
Both univariate and multivariate analysis for this HER2+ population as shown in Table 4 revealed that time to brain metastases was not dependent on age at primary diagnosis, stage, grade, or adjuvant trastuzumab treatment.
Table 4.
Multivariate Cox Proportional Hazard Model of Risk Factors for Time to Brain Metastasis in HER2+ Patients
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Risk Factor | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age at primary breast cancer diagnosis | 0.983 (0.933–1.036) | .529 | 0.990 (0.937–1.047) | .734 |
| Stage | 2.227 (0.598–8.297) | .233 | 2.285 (0.595–8.784) | .229 |
| Grade | 0.379 (0.102–1.41) | .148 | 0.369 (0.097–1.397) | .142 |
| Adjuvant trastuzumab | 1.452 (0.363–5.806) | .598 | 1.244 (0.271–5.712) | .779 |
Discussion
This retrospective, the single-institution study examined different breast cancer characteristics, including stage, grade, and tumor subtype, that impact the time to development of brain metastases. Our study was based on a population of 1218 patients with a primary breast cancer diagnosis and to the authors’ knowledge is one of the largest analyses looking at the time to brain metastases formation.6,7,12,13,17–19 Our study is also the first large retrospective analysis to specifically examine the impact of grade on time to brain metastases development.
We confirm that stage 2/3 HER2+ and TN patients have a higher incidence of brain metastases compared to stage 2/3 ER+/HER2− patients (10.0% and 10.8% vs. 4.1%, respectively, P < .001), as reported previously.3–8 These percentages appear less than prior studies as our study excluded both stage 1 and stage 4 patients and therefore is likely excluding a significant portion of breast cancer patients with CNS involvement, including those with CNS involvement at initial cancer diagnosis. When stratifying the incidence of brain metastases according to stage for these patients, we report the incidence to be 7.0% and 15.2% for stage 2 and 3 HER2+ patients, and 9.3% and 14.0% for stage 2 and 3 TN patients, respectively. Additionally, we demonstrate that IDC histology, higher grade, and higher stage are associated with a greater risk of CNS involvement, as shown in previous studies.3–6,20,21
We demonstrate that tumor subtype impacts the interval from primary breast cancer diagnosis to brain metastases development. The median times to brain metastases were 20 (12.9–32.9), 26 (18.9–33.1), and 57 (37.5–74.6) months for the 9 HER2+, 30 TN, and 35 ER+/HER2− patients in our study, respectively (P < .0001). Previous studies have reported a median interval of 18–31 months and 14–35 months between primary diagnosis and CNS recurrence for HER2+ and TN patients, respectively, compared to 63.5 months in ER+/HER2− patients.7,12,13,16,22,23 Again these prior analyses included all stage breast cancer patients and were not limited to stage 2–3 disease as in our study.
Univariate analysis revealed that both HER2+ (HR = 2.639, P = .009) and TN (HR = 3.062, P < .001) breast cancer were associated with a shorter time to CNS metastases. Multivariate analysis confirmed that the TN tumor subtype remained a risk factor for earlier brain metastases (HR = 2.043, P = .015). However, the interval to brain metastases no longer depended on HER2 positivity in this multivariable model (HR = 1.592, P = .244). This is a finding consistent with a recent study by Darlix et al., and suggests that though HER2+ breast cancer is associated with a higher incidence of brain metastases, it seems to have less of an effect on the time interval based on our results.1,3–10,13,19,20,24 Due to the limited cohort size of our HER2+ population with stage 2–3 disease, our results may underestimate the effect of HER2 positivity on the interval to brain metastases.
We also performed a separate subanalysis for HER2+ patients and exposure to trastuzumab which showed that adjuvant trastuzumab did not affect the time to CNS metastases. Though this finding has also been reported previously,7 our results conflict with a more recent study that showed HER2+ patients treated with trastuzumab had a shorter interval to brain metastases compared to those without trastuzumab (3.1 vs. 4.9 years).13 The actual role of trastuzumab in the metastatic potential of breast cancer to the CNS is not well understood. The improved overall survival time due to systemic disease control from trastuzumab administration may at the same time place patients at higher risk for brain metastases, as more time is allowed for brain metastases to develop. However, the results regarding the impact of trastuzumab treatment on the incidence of brain metastases in HER2+ patients remain mixed.25–28 Additional meta-analyses of HER2+ patients are required to further elucidate the effect of trastuzumab on the interval to brain metastases.
In this study, we also demonstrate that breast cancer tumors of higher histologic grade have an increased risk of earlier metastases to the brain (HR = 1.667, P = .024), as shown in previous studies.13 We report, for the first time in the authors’ knowledge, the median times to brain metastases by grade as 30, 49, and 71 months for all grade 3, 2, and 1 tumors, respectively.
Because our primary goal was to investigate the time course of brain metastatic progression in a group of high-risk patients who had not already been diagnosed with brain metastatic disease, we only included patients diagnosed with stage 2–3 disease. Here we demonstrate that when limiting the patient population to stage 2–3 disease, a higher stage at primary breast cancer diagnosis still correlates with a shorter interval to CNS metastases (HR = 3.851, P < .001). This suggests there is a substantial difference in risk for earlier CNS involvement when just comparing stage 3 vs. 2 disease. In our study, the median time to brain metastases was 34 vs. 52 months for all stage 3 vs. 2 patients. This finding confirms the results from a smaller study by et Saraf et al which reported a median interval time of 29 vs. 54 months for stage 3 and 2 patients, respectively.16 Additionally, one recent study noted that both HER2+ and TN patients had a higher risk of presenting with brain metastases at primary diagnosis of breast cancer, further suggesting the increased need for early MRI screening for this cohort of patients who are also at risk of de novo metastatic breast cancer to the CNS.29 Future studies are warranted to further characterize the time course of brain metastases in breast cancer patients with visceral metastases, which we did not assess in our study.
There are several limitations to our study. The study population only included primary breast cancer patients diagnosed between 2003 and 2010 at a single institution, and as the timeframe of our study begins prior to the introduction of adjuvant trastuzumab for HER2+ disease in 2005, our results may not reflect the contemporary breast cancer patient population undergoing current standard of care treatments. This raises the possibility of selection bias. Additional areas of selection bias include the exclusion of ER/HER2+ subtype due to limitations in data acquisition, as well as the exclusion of 603 patients due to a concomitant or previous different primary cancer diagnosis or incomplete histological data. Kaplan–Meier analysis of the patients in this study did include competing events which may have underestimated the risk of and time to brain metastases in censored patients. Furthermore, our study was limited to stage 2–3 patients based off of the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual for breast cancer given the timeframe of data collection.14 This limitation in stage may explain why our reported brain metastases incidence is considerably lower than what is reported in other contemporary studies (6.1% vs. 10–16%),1–7 and may have introduced sampling bias. Future studies will need to revisit how the interval to brain metastases is affected by clinical stage as defined by current clinical guidelines.
Despite these limitations, this study demonstrates that breast cancer patients with triple negative disease, higher grade tumors, and higher stage disease have a propensity for earlier CNS recurrence compared to other breast cancer patients. There are currently no recommendations for routine brain metastases screening due to a lack of proven survival benefit in previous limited studies.4,11 Brain metastases screening is, however, better studied in patients with stage II–IV non-small cell lung cancer, who are routinely evaluated for asymptomatic CNS relapse per current National Comprehensive Cancer Network guidelines.30 Based on our results, additional prospective trials are needed to investigate the utility of earlier MRI screening in this particular subgroup of breast cancer patients at risk of earlier brain metastases. Surveillance brain imaging for asymptomatic high-risk breast cancer patients may not only provide more accurate prognostic information, but ultimately modify clinical decision-making, adjust treatment options, and improve overall survival and quality of life.
Funding
This study was supported by Lavin Family Brain Metastasis Research Grant; Specialized Program of Research Excellence (SPORE) for Translational Approaches to Brain Cancer (P50CA221747).
Conflict of interest statement. The authors declare that they have no conflicts of interest.
Authorship Statement
Experimental design: T.C., P.K. Implementation: T.C. Analysis and interpretation of data: T.C., K.D., C.SM., P.K.
References
- 1. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. [DOI] [PubMed] [Google Scholar]
- 2. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. [DOI] [PubMed] [Google Scholar]
- 3. Witzel I, Oliveira-Ferrer L, Pantel K, Müller V, Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phillips C, Jeffree R, Khasraw M. Management of breast cancer brain metastases: a practical review. Breast. 2017;31:90–98. [DOI] [PubMed] [Google Scholar]
- 5. Pestalozzi BC, Zahrieh D, Price KN, et al. ; International Breast Cancer Study Group (IBCSG) . Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–944. [DOI] [PubMed] [Google Scholar]
- 6. Nam BH, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45(16):2792–2798. [DOI] [PubMed] [Google Scholar]
- 8. Leone JP, Leone BA. Breast cancer brain metastases: the last frontier. Exp Hematol Oncol. 2015;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niikura N, Hayashi N, Masuda N, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103–112. [DOI] [PubMed] [Google Scholar]
- 10. Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramakrishna N, Temin S, Chandarlapaty S, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014; 32(19): 2100–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112(3):467–472. [DOI] [PubMed] [Google Scholar]
- 13. Darlix A, Griguolo G, Thezenas S, et al. Hormone receptors status: a strong determinant of the kinetics of brain metastases occurrence compared with HER2 status in breast cancer. J Neurooncol. 2018;138(2):369–382. [DOI] [PubMed] [Google Scholar]
- 14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 15. Wolff AC, Hammond ME, Hicks DG, et al. ; American Society of Clinical Oncology; College of American Pathologists . Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saraf A, Grubb CS, Hwang ME, et al. Breast cancer subtype and stage are prognostic of time from breast cancer diagnosis to brain metastasis development. J Neurooncol. 2017;134(2):453–463. [DOI] [PubMed] [Google Scholar]
- 17. Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venkitaraman R, Joseph T, Dhadda A, Chaturvedi A, Upadhyay S. Prognosis of patients with triple-negative breast cancer and brain metastasis. Clin Oncol (R Coll Radiol). 2009;21(9):729–730. [DOI] [PubMed] [Google Scholar]
- 19. Evans AJ, James JJ, Cornford EJ, et al. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (R Coll Radiol). 2004;16(5):345–349. [DOI] [PubMed] [Google Scholar]
- 20. Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107(4):696–704. [DOI] [PubMed] [Google Scholar]
- 21. Jain S, Fisher C, Smith P, Millis RR, Rubens RD. Patterns of metastatic breast cancer in relation to histological type. Eur J Cancer. 1993;29A(15):2155–2157. [DOI] [PubMed] [Google Scholar]
- 22. Hines SL, Vallow LA, Tan WW, et al. Clinical outcomes after a diagnosis of brain metastases in patients with estrogen-and/or human epidermal growth factor receptor 2-positive versus triple-negative breast cancer. Annals Oncol. 2008; 19(9):1561–1565. [DOI] [PubMed] [Google Scholar]
- 23. Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer. 2012;106(3):440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. [DOI] [PubMed] [Google Scholar]
- 25. Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: the dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109(2):231–239. [DOI] [PubMed] [Google Scholar]
- 26. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 27. Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244–248. [DOI] [PubMed] [Google Scholar]
- 28. Nie F, Yang J, Wen S, et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer. 2012;118(21):5198–5209. [DOI] [PubMed] [Google Scholar]
- 29. Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H, Jeong SH, Jeong BH, et al. Incidence of brain metastasis at the initial diagnosis of lung squamous cell carcinoma on the basis of stage, excluding brain metastasis. J Thorac Oncol. 2016;11(3):426–431. [DOI] [PubMed] [Google Scholar]