Abstract
An effective control of malaria vectors requires an extensive knowledge of mechanisms underlying the resistance-phenotypes developed by these vectors against insecticides. We investigated Anopheles gambiae mosquitoes from Benin and Togo for their intensity of insecticide resistance and we discussed the involvement of genotyped mechanisms in the resistance-phenotypes observed. Three- to five-day-old adult mosquitoes emerged from field and laboratory An. gambiae larvae were assayed using WHO tube intensity tests against various doses of deltamethrin: 1× (0.05%); 2× (0.1%); 5× (0.25%); 7.5× (0.375%) and those of pirimiphos-methyl: 0.5× (0.125%); 1× (0.25%). Members of An. gambiae complex were screened in field populations using polymerase chain reaction (PCR) assays. The presence of kdrR(1014F/1014S) and ace-1R(119S) mutations was also investigated using TaqMan and PCR-RFLP techniques, respectively. Anopheles gambiae from field were very resistant to deltamethrin, whereas KisKdr and AcerKdrKis strains displayed 100% mortality rates at 2× the diagnostic dose. In contrast, the field mosquitoes displayed a low resistance-intensity against 1× the diagnostic dose of pirimiphos-methyl, whereas AcerKis and AcerKdrKis strains showed susceptibility at 0.5× the diagnostic dose. Anopheles gambiae s.s., Anopheles coluzzii, and Anopheles arabiensis were identified. Allelic frequencies of kdrR (1014F) and ace-1R (119S) mutations in the field populations varied from 0.65 to 1 and 0 to 0.84, respectively. The field An. gambiae displayed high-resistance levels against deltamethrin and pirimiphos-methyl when compared with those of the laboratory An. gambiae-resistant strains. These results exhibit the complexity of underlying insecticide resistance mechanisms in these field malaria vectors.
Keywords: Anopheles gambiae, phenotype, resistance mechanism, deltamethrin, pirimiphos-methyl
Graphical Abstract
Graphical Abstract.
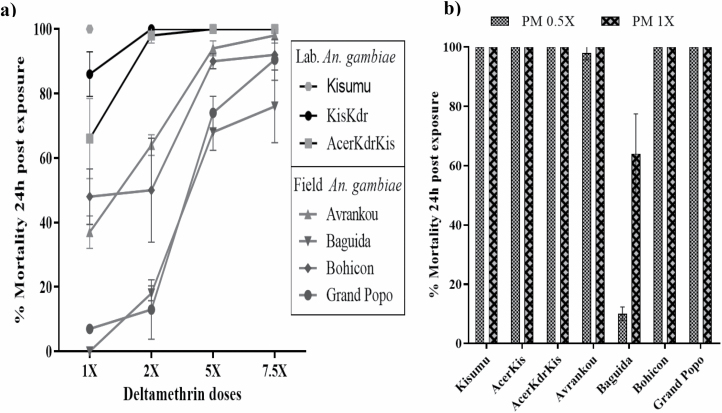
The field An. gambiae displayed high resistance levels against deltamethrin and pirimiphos-methyl when compared to those of the laboratory An. gambiae resistant strains. These results exhibit the complexity of underlying insecticide resistance mechanisms in these field malaria vectors. Figure. (a) Deltamethrin and (b) pirimiphos-methyl exposures.
Currently, the most effective way to prevent malaria transmission episodes remains the use of malaria vectors control trials alongside with chemical insecticides contained in long-lasting insecticide-treated nets (LLINs) and indoor residual spraying (IRS) formulations (Katureebe et al. 2016). Due to their properties, pyrethroid chemistries remain the only class of insecticides authorized for the treatment of LLINs (World Health Organization 2004, 2006). Pirimiphos-methyl insecticide (Actellic capsule suspension) has been recently used as an alternative molecule to control the pyrethroid-resistant Anopheles gambiae in the field (Fuseini et al. 2011, Rowland et al. 2013, Tchicaya et al. 2014).
Most often, the resistance-phenotypes reported in natural populations of mosquitoes relies on four relevant resistance mechanisms such as target-site insensitivity (Chandre et al. 1999), metabolic (Li et al. 2007), behavioral (Reddy et al. 2011, Russell et al. 2011, Moiroux et al. 2012), and cuticular (Vannini et al. 2014, Huang et al. 2018). Target-site resistance is induced by punctual mutation in specific gene encoding for specific protein that interact with target insecticide through its mechanisms of action. The known common target-site resistance mechanisms that occur in malaria vectors are the voltage-gated sodium channel (Vgsc) mutations encoded by L1014F or L1014S, N1575Y and the insensitivity acetylcholinesterase ace-1(G119S) mutations that cause resistance to pyrethroid/DDT and carbamate/organophosphate insecticides, respectively (Martinez-Torres et al. 1998, Ranson et al. 2000, Djogbénou et al. 2007, Jones et al. 2012). However, genetic technologies developed in the last 20 yr cannot yet allow researchers to specifically link the characterized mechanisms to the resistance-phenotypes observed in field-collected malaria vectors after susceptibility assays.
The resistance of malaria vectors to insecticides used in public health relies on a genetic phenomenon which once selected is transmitted from generation to generation (Corbel and N’Guessan 2013). Its evolution over time is mainly favored by the selection pressure exerted by the pesticides and other xenobiotics residues present in malaria vector environments (Diabate et al. 2002, Djouaka et al. 2008, Djogbénou et al. 2011, Nkya et al. 2014). Unfortunately, the widespread of insecticide resistance in natural populations of An. gambiae mosquitoes represent a threat for implementation of malaria prevention programs based on the use of insecticide compounds (Ranson et al. 2011, Aïkpon et al. 2013, Corbel and N’Guessan 2013, Mnzava et al. 2015). To slow the threats of emergence and spread of resistance on vector control measures, the Global plan for insecticide resistance management in malaria vectors (GPIRM) was launched in May 2012 and one of its objectives was to fill gaps in knowledge on mechanisms of insecticide resistance and the impact of current insecticide resistance management approaches (World Health Organization 2012).
Recently, several studies have attempted to reveal the association between genotype at the kdrR locus and occurrence of the pyrethroids/DDT resistance-phenotypes in wild populations of An. gambiae s.s. (Dabiré et al. 2009, Ibrahim et al. 2014, Djegbe et al. 2017). Meanwhile, other research works demonstrated a lack of such a correlation which means that kdrR genotyping is not the only predictor of the pyrethroids/DDT resistance-phenotypes (Matambo et al. 2007, N’Guessan et al. 2007, Abdalla et al. 2008) often observed in field-collected Anopheles mosquitoes. Overall, the role of kdrR mutation in the expression of pyrethroids/DDT resistance-phenotypes remains a matter of debate (Donnelly et al. 2009).
In order to enlighten the ongoing debate on the involvement of resistance mechanisms in phenotypic insecticide resistance occurring in An. gambiae, we performed herein the resistance-intensity assays against four colonies of well-known genotypes and four field-collected mosquitoes using two insecticides currently applied in malaria vector control interventions. The more likely reasons explaining why the specific characterization of resistance mechanisms which confer the resistance-phenotypes still matters are discussed.
Materials and Methods
Mosquito Strains
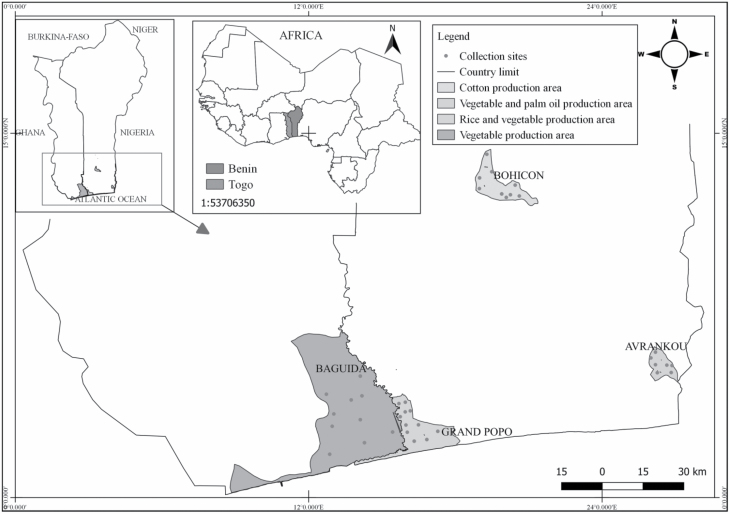
Larvae and pupae were collected from four selected localities in Benin and Togo (based on the levels of insecticide resistance recorded by these populations in previous studies) in 2018 using the techniques previously described (Service 1977). Prospected areas were Avrankou (6°33′42″ N–2°38′55″ E), Bohicon (7°11N–2°49E), Grand Popo (6°14′28″ N–1°37′60″ E) in Republic of Benin and Baguida (06°09′47″ N–01°19′05″ E) in Republic of Togo (Fig. 1). Collected larvae and pupae were transported in labeled plastic bottles to the insectary of Laboratory of Infectious Vector-Borne Diseases based at Regional Institute of Public Health/University of Abomey-Calavi (Benin) and reared to adults as the An. gambiae s.s. of well-known genotypes used. All mosquito strains were maintained under standard insectary conditions of 70 ± 8% relative humidity and 27 ± 2°C ambient temperature. The field samples used for resistance-intensity assays were females F0 adults that emerged from the collected larvae and pupae. The An. gambiae s.s. colonies of well-known genotypes and the field strains used for resistance-intensity assessments, susceptibility status, insecticide resistance mechanisms, and references are presented in Table 1.
Fig. 1.
Map of Benin and Togo showing the prospected breeding sites in Avrankou (vegetable and palm oil production area), Baguida (vegetable production area), Bohicon (cotton production area) and Grand Popo (rice and vegetable production area).
Table 1.
Resistance status of the different mosquito strains (field and laboratory Anopheles gambiae strains) used in this study
| Names of the strains | Type of the strains | Resistance profiles | Known resistance mechanisms | References |
|---|---|---|---|---|
| Kisumu | Laboratory strains sharing the same genetic background | Susceptible for all insecticides | None | Shute (1956) |
| KisKdr | Pyrethroids and DDT resistant | Homozygous for kdrR(1014F) allele | Alout et al. (2013) | |
| AcerKis | Carbamates and organophosphates resistant | Homozygous for ace-1R(119S) allele | Djogbénou et al. (2007) | |
| AcerKdrKis | Pyrethroids and DDT, carbamates and organophosphates resistant | Homozygous for both kdrR(1014F) and ace-1R(119S) mutations | Assogba et al. (2014) | |
| Avrankou | Field strain | Permethrin resistant | Unknown | Assogba et al. (2020) |
| Baguida | Field strain | Pyrethroids and DDT, carbamates and organophosphates resistant | Unknown | Amoudji et al. (2019) |
| Bohicon | Field strain | Pyrethroids and DDT resistant | Unknown | Djègbè et al. (2011) |
| Grand Popo | Field strain | Permethrin resistant | Unknown | Assogba et al. (2020) |
WHO Insecticide Resistance Tests for Determining Insecticide Resistance-Intensity
Intensity assays were performed on 3- to 5-d-old non-blood-fed females from both field and laboratory mosquitoes using the classical WHO susceptibility test kits (WHO 2016) with slight modifications. Filter papers impregnated with 0.5–7.5 times the diagnostic dose of the both deltamethrin (pyrethroid) and pirimiphos-methyl (organophosphate) were supplied by Liverpool School of Tropical Medicine (LSTM) and stored at 4°C before, during and after each test. These insecticides were chosen because they are currently used in West Africa for malaria vector control (deltamethrin in bed nets and pirimiphos-methyl in indoor residual spraying).
The doses of deltamethrin used were 0.05, 0.1, 0.25, and 0.375% (respectively termed 1×, 2×, 5×, and 7.5×) and those of pirimiphos-methyl were 0.125 and 0.25% (respectively termed 0.5× and 1×). Note that the strains used in the resistance-intensity experiments were not all exposed to the same insecticide (Table 2). The tests were implemented using batches of 20–30 females for 1 h at 70 ± 8% relative humidity and 27 ± 2°C ambient temperature. For a single insecticide dose, four batches were exposed against impregnated filter papers, whereas four other were exposed to nonimpregnated filter papers serving as controls. For deltamethrin insecticide, knocked-down mosquitoes were recorded at 10-min intervals along the hour of exposure. After insecticide exposures, final mortality rates were recorded 24-h holding period later during which a 10% honey solution was made available to survivors mosquitoes (WHO 2016).
Table 2.
Anopheles gambiae mosquitoes used in each intensity bioassays for deltamethrin and pirimiphos-methyl exposures
| Insecticides | Deltamethrin | Pirimiphos-methyl | ||||
|---|---|---|---|---|---|---|
| Intensity doses | 1× | 2× | 5× | 7.5× | 0.5× | 1× |
| Kisumu | Kisumu | |||||
| KisKdra | AcerKisb | |||||
| AcerKdrKisa | AcerKdrKisb | |||||
| Mosquito strains | Avrankou | Avrankou | ||||
| Baguida | Baguida | |||||
| Bohicon | Bohicon | |||||
| Grand Popo | Grand Popo | |||||
aHomozygous for kdrR(1014F) mutation.
bHomozygous for ace-1R(119S) mutation.
Identification of An. gambiae Species and Detection of kdrR(1014F/1014S) and ace-1R(119S) Mutations in Field-Collected Mosquitoes
In order to assess the real resistant allele frequencies as occurred in natural populations, Anopheles specimens were randomly selected from the batches of each field unexposed populations (control tubes) and genomic DNA from these mosquito samples was individually extracted using the DNA extraction protocol previously developed (Collins et al. 1987). The members of An. gambiae species present were identified using the SINE-PCR method (Santolamazza et al. 2008). The presence of both West and East African kdrR mutations was characterized in the same specimens applying a TaqMan real-time PCR assays (Bass et al. 2010). Specimens were also tested for ace-1R(119S) mutation using Weill et al.’s protocol (Weill et al. 2004). Genotyping results’ analysis was performed using a Mx3005Pro Software (Agilent Technologies, Stratagene, San Diego, CA).
Data Analysis
For different doses of deltamethrin bioassays, times for 50% knock-down of mosquitoes (KdT50) and their CIs were generated by probit analysis through a log-time probit model with Polo-plus 1.0 software (Russell et al. 1977).
The percentage mortality (24-h postinsecticide exposure) of the mosquitoes exposed against each dose of each insecticide was determined as the proportion of mosquitoes that died at the times the diagnostic doses of each insecticide.
To assign resistance-intensity levels, the mortalities obtained from the WHO intensity bioassays as described above were interpreted using the following criteria as a guide:
For deltamethrin
• Mortality between 98 and 100% at 2× the diagnostic dose indicates low resistance-intensity.
• Mortality between 98 and 100% at 5× the diagnostic dose indicates moderate resistance-intensity.
• Mortality between 98 and 100% at 7.5× the diagnostic dose confirms moderate resistance-intensity.
• Mortality <98% at 7.5× the diagnostic dose indicates high resistance-intensity.
For pirimiphos-methyl
• Mortality <98% at 1× the diagnostic dose indicates low resistance-intensity.
The main objective of the data analysis was to compare the levels of insecticide resistance-phenotype for the field populations to those of the laboratory strains of well-known genotypes used in present study.
Results
Knock-Down Time Effects of Deltamethrin
The knock-down time (KdT50) values induced by deltamethrin exposure at 1× the diagnostic dose were relatively higher for the field mosquitoes when compared with those of laboratory strains (Table 3). From 2×, the diagnostic dose and above, knock-down time (KdT50) values of the field populations showed two to four times increase in the mean KdT50 compared with the well-known resistant laboratory strains of An. gambiae (Table 3).
Table 3.
Knock-down times (KdT50 and KdT95) with deltamethrin of the field-collected and laboratory Anopheles gambiae mosquitoes
| Deltamethrin doses | Mosquito strains | N | Knock-down times with CI | |||
|---|---|---|---|---|---|---|
| KdT50, min | 95% CI | KdT95, min | 95% CI | |||
| 1× | KisKdr | 100 | 53.537 | (47.923–62.290) | 140.164 | (107.007–218.236) |
| Kisumu | 100 | 14.659 | (12.751–16.556) | 36.798 | (30.963–47.047) | |
| AcerKdrKis | 100 | 49.259 | (45.615–54.097) | 97.401 | (81.739–129.235) | |
| Avrankou | 100 | 67.390 | (57.222–84.425) | 398.278 | (258.241–758.440) | |
| Baguida | 100 | No kd | No kd | No kd | No kd | |
| Bohicon | 100 | 74.607 | (61.543–98.200) | 536.946 | (321.811–1173.022) | |
| Grand Popo | 108 | 186.085 | (111.776–986.760) | 715.898 | (270.373–18436.883) | |
| 2× | KisKdr | 100 | 26.015 | (23.847–28.283) | 59.661 | (52.189–71.187) |
| Kisumu | 100 | 11.297 | (8.175–14.377) | 33.583 | (24.498–61.482) | |
| AcerKdrKis | 100 | 30.128 | (26.993–33.429) | 58.461 | (50.206–73.483) | |
| Avrankou | 100 | 40.886 | (34.630–50.729) | 182.232 | (120.517–369.668) | |
| Baguida | 100 | No kd | No kd | No kd | No kd | |
| Bohicon | 100 | 47.722 | (40.691–59.010) | 265.192 | (172.598–521.039) | |
| Grand Popo | 92 | 168.252 | (108.897–472.839) | 802.533 | (331.040–6935.958) | |
| 5× | KisKdr | 100 | 17.906 | (15.465–20.411) | 40.980 | (33.878–55.066) |
| Kisumu | 100 | 8.936 | (6.054–11.565) | 27.654 | (20.045–52.464) | |
| AcerKdrKis | 100 | 17.315 | (15.213–19.432) | 40.673 | (34.542–51.236) | |
| Avrankou | 100 | 23.726 | (18.786–29.644) | 98.042 | (66.309–203.916) | |
| Baguida | 100 | 94.128 | (72.470–164.946) | 318.972 | (177.122–1224.283) | |
| Bohicon | 100 | 24.867 | (21.756–28.393) | 104.032 | (79.746 –152.937) | |
| Grand Popo | 100 | 27.571 | (25.467–29.866) | 98.798 | (83.633–122.038) | |
| 7.5× | KisKdr | 100 | 17.906 | (15.465–20.411) | 40.980 | (33.878–55.066) |
| Kisumu | 100 | 8.936 | (6.054–11.565) | 27.654 | (20.045–52.464) | |
| AcerKdrKis | 100 | 17.138 | (15.610–18.678) | 37.243 | (32.838–43.991) | |
| Avrankou | 100 | 21.208 | (16.829–26.207) | 94.858 | (64.944–186.126) | |
| Baguida | 100 | 80.850 | (66.039–120.226) | 236.477 | (147.908–641.252) | |
| Bohicon | 100 | 21.946 | (18.418–25.928) | 117.689 | (83.490–201.220) | |
| Grand Popo | 100 | 80.850 | (65.969–120.722) | 236.477 | (147.562–648.107) | |
KdT50, knock-down time for 50% of mosquitoes; KdT95, knock-down time for 95% of mosquitoes; N, sample sizes; No kd, complete loss of knock-down effect (<10% knock-down after 60 min exposure).
Insecticide Resistance-Phenotypes
The levels of resistance-phenotype for deltamethrin and pirimiphos-methyl in the field populations (Avrankou, Baguida, Bohicon, and Grand Popo) were assessed and compared to those of the laboratory strains of well-known genotypes (see supplementary data: Phenotypic Insecticide Resistance).
For Deltamethrin
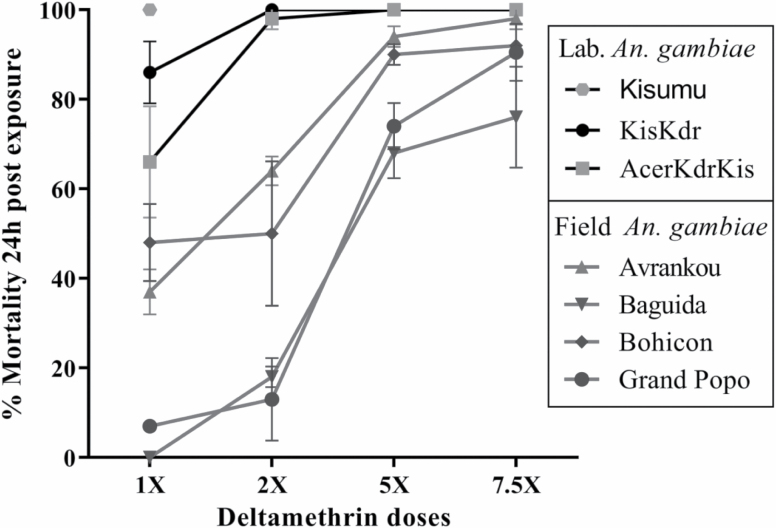
Using the criteria explained above in present study, Kisumu was as expected, susceptible at 1× the diagnostic dose, whereas KisKdr, AcerKdrKis, Avrankou, Baguida, Bohicon, and Grand Popo mosquitoes displayed mortalities of less than 98%. At 2×, the diagnostic dose, both KisKdr and AcerKdrKis strains showed susceptibility (100% mortality), whereas Grand Popo, Bohicon, Baguida, and Avrankou samples were still resistant and confirmed moderate-resistant, respectively, against 7.5× the diagnostic dose (Fig. 2). In addition, even if they were purely homozygote for kdrR(1014F) allele, KisKdr and AcerKdrKis displayed low intensity of resistance and the field mosquitoes showed relatively high resistance-intensity against deltamethrin insecticide.
Fig. 2.
Levels and evolutions of resistance-phenotype for the field-collected Anopheles gambiae in deltamethrin exposure compared to those of laboratory strains of well-known genotypes. Error bars indicate 95% CIs.
For Pirimiphos-Methyl
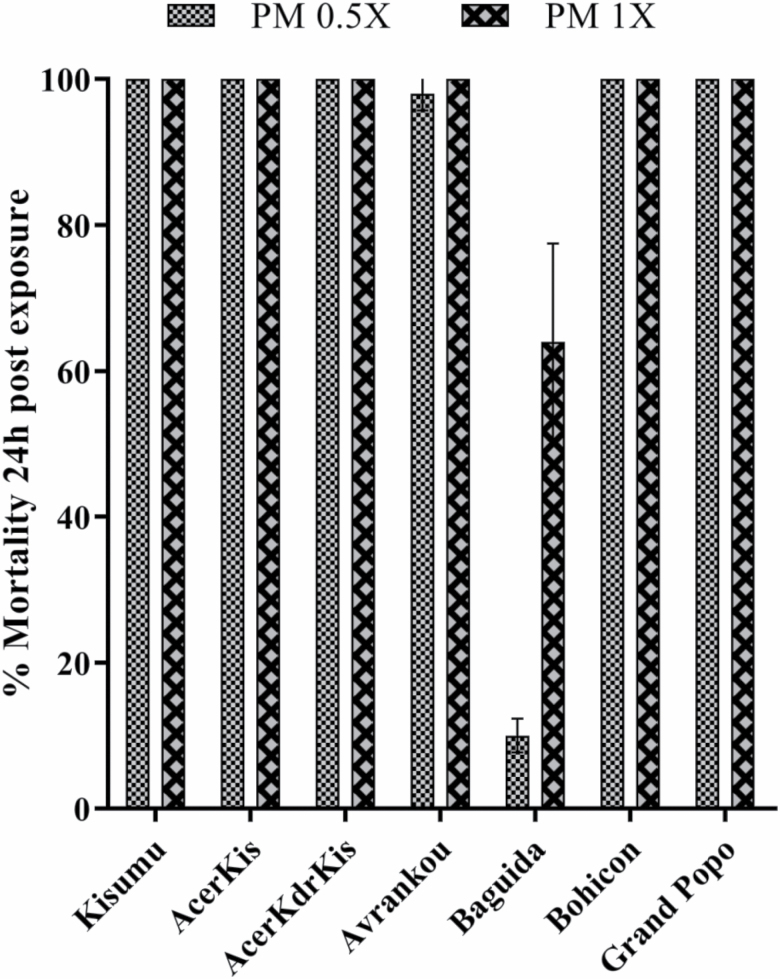
Based on the criteria explained above, all laboratory strains (Kisumu, AcerKis, and AcerKdrKis) exposed to 0.5× the diagnostic dose showed susceptibility while Baguida mosquitoes were still resistant against 1× the diagnostic dose with mortality of less than 98% (Fig. 3). Even if they were purely homozygote for ace-1R(119S) allele, AcerKis and AcerKdrKis displayed susceptibility phenotypes and Baguida An. gambiae recorded low resistance-intensity (64% mortality) against 1× the diagnostic dose of pirimiphos-methyl insecticide.
Fig. 3.
Levels of resistance-phenotype for the field-collected Anopheles gambiae in pirimiphos-methyl (PM) exposure compared to those of laboratory strains of well-known genotypes. Error bars indicate 95% CIs.
Detection of kdrR(1014F/1014S) and ace-1R(119S) Mutations in Natural Populations
Species identification was performed on a total of 320 individuals of Anopheles mosquitoes (80 from each locality). Anopheles gambiae s.s., An. coluzzii, and An. arabiensis species were identified. Overall, 13 An. gambiae s.l. were An. gambiae s.s. (16.25%) and 67 (83.75%) belonged to An. coluzzii among Avrankou specimens. All of Baguida individuals investigated were An. gambiae s.s. From Bohicon Anopheles mosquitoes, 39 were An. gambiae s.s. (48.75%), 36 were An. coluzzii (45%) and 5 (6.25%) were An. arabiensis. All of Grand Popo samples were An. coluzzii. These specimens were then genotyped for Vgsc-kdrR(1014F/1014S) and ace-1R(119S) mutations to evaluate their frequencies in natural populations of An. gambiae s.l. tested. The Vgsc-kdrR(1014F) West resistance allele frequencies were relatively high (ranging 0.65–1) among all the field An. gambiae s.l. populations (Table 4). From Avrankou, Baguida, Bohicon, and Grand Popo kdr resistant specimens, 67.74, 100, 96.1, and 76% of individuals were detected homozygotes [RR], respectively, and the remaining are heterozygotes [RS]. No individual was detected bearing kdrR(1014S) East resistance allele.
Table 4.
Frequency of the kdrR(1014F) and ace-1R(119S) mutations in the field-collected Anopheles gambiae s.l.
| Locality | Anopheles coluzzii | Anopheles gambiae s.s. | Anopheles arabiensis | Frequency mutation in field An. gambiae s.l. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kdr R (1014F) | ace-1 R (119S) | kdr R (1014F) | ace-1 R (119S) | kdr R (1014F) | ace-1 R (119S) | ||||||||||
| n s | F | n s | F | n s | F | n s | F | n s | F | n s | F | n l | F (kdrR) | F (ace-1R) | |
| Avrankou | 67 | 0.73 | 67 | 0 | 13 | 0.23 | 13 | 0 | – | – | – | – | 80 | 0.65 | 0 |
| Baguida | – | – | – | – | 80 | 1 | 80 | 0.84 | – | – | – | – | 80 | 1 | 0.84 |
| Bohicon | 36 | 0.90 | 36 | 0 | 39 | 1 | 39 | 0.01 | 5 | 0.8 | 5 | 0 | 80 | 0.94 | 0.006 |
| Grand Popo | 80 | 0.83 | 80 | 0.05 | – | – | – | – | – | – | – | – | 80 | 0.83 | 0.05 |
n s, number of mosquitoes tested by species; nl, total number of mosquitoes tested; F, allele frequency; –, no data.
In Baguida mosquito specimens, the ace-1R(119S) point mutation was detected at high frequency (0.84). From these ace-1 resistant mosquitoes, 83.56% of individuals were homozygotes [RR] and the remaining were heterozygotes [RS]. However, relatively low ace-1R(119S) frequencies were detected among the other field mosquitoes (Table 4) (see supplementary data: Phenotypic Insecticide Resistance).
Discussion
Specific mechanisms involved in the insecticide resistance-phenotypes occurring in natural populations of malaria vectors remain unclear. With regard to the kdrR(1014F or 1014S) mutations, the current question is whether the levels of resistance-phenotype observed toward pyrethroids in the field Anopheles vectors is associated or not with the frequency of these resistance alleles (Donnelly et al. 2009). The present study was conducted in order to enhance the debate about the question above. Here, we have compared knock-down times and mortality values of An. gambiae strains bearing the well-known target-site insensitivity mechanisms at homozygous state (KisKdr, AcerKis, and AcerKdrKis) with those of field-collected populations (Avrankou, Baguida, Bohicon, and Grand Popo).
Results from intensity bioassays showed that all of the well-known homozygous resistant individuals for kdrR(1014F) mutation (KisKdr and AcerKdrKis strains) died at 2× the diagnostic dose of deltamethrin, whereas significant survival percentages: 2, 24, 8, and 9% of adult female mosquitoes from Avrankou, Baguida, Bohicon, and Grand Popo localities (field strains) were recorded at 7.5× the diagnostic dose, respectively. Moreover, the molecular biology analysis among all the field strains revealed globally, the presence of An. gambiae species (An. coluzzii, An. gambiae s.s., and An. arabiensis) with very high frequency of kdrR(1014F) mutation (Table 4). Furthermore, by using the average knock-down time (KdT50) data obtained with the range of deltamethrin doses and the WHO bioassay method, it was shown that the knock-down time expressed in the field An. gambiae is approximately two to four times higher than the one displayed by the insecticide-resistant laboratory strains (resistance ratio calculated for the field strains using KisKdr KdT50 value as denominator; Table 3). The present results show clearly that the observed phenotypes (in terms of resistance level) in the field populations is not associated only with the presence of the resistance allele kdrR(1014F). Comments above on the kdrR mutation can also be applied to the ace-1R(119S) mutation. The findings have shown that, at 1× the diagnostic dose of pirimiphos-methyl, all resistant homozygous specimens for the ace-1R(119S) mutation of laboratory strains used were killed, whereas, except the 100% mortalities displayed by Avrankou, Bohicon, and Grand Popo mosquitoes, only 64% of death was recorded especially in Baguida An. gambiae. Therefore, it can also be deducted here that the levels of resistance-phenotype observed against pirimiphos-methyl in this field An. gambiae population was not only associated with the presence of the resistance allele ace-1R(119S) at the ace-1 locus.
In most insecticide resistance studies using wild populations, the target-site insensitivity-mediated resistance such as kdrR(L1014F, L1014S & N1575Y), Rdl and ace-1R(G119S), is one of the most common resistance characterized in malaria vectors (Djogbénou et al. 2011, Alemayehu et al. 2017, Nardini et al. 2017, Camara et al. 2018). However, the role of metabolic resistance mechanisms through activities of the P450 and GST genes is increasingly being detected in An. gambiae vectors across different sites (Ochomo et al. 2013, Mitchell et al. 2014, Awolola et al. 2018, Stica et al. 2019). Thus, in this study, the wider discrepancies in the levels of resistance-phenotype observed between the field and laboratory resistant An. gambiae exposed against various doses of deltamethrin (Fig. 2) indicate that metabolic insecticide resistance mechanisms like P450-monooxygenase could contribute to the pyrethroid resistance-phenotypes observed.
Malaria control highly depends on an effective programmatic-scale vector control with wide distribution of insecticide-treated nets and the large-scale indoor residual spraying campaigns which have contributed to the recent decline in morbidity and mortality in endemic countries (Katureebe et al. 2016). Unfortunately, insecticide resistance is a cause of a great concern for vector control and it threatens to reverse these gains. To monitor the insecticide resistance mechanisms, relatively affordable diagnostic methods for the detection of target-site insecticide resistance mutations have been carried out and can be used in the endemic countries and also used to monitor their evolution in natural populations of malaria vectors (Martinez-Torres et al. 1998, Weill et al. 2004, Bass et al. 2010, Badolo et al. 2012). As for metabolic resistance mechanisms, there are still no tools to monitor their evolution in time and space due to their complex molecular basis despite their probable greater operational impact on malaria control (Corbel and N’Guessan 2013, David Jean-Philippe et al. 2013, Liu 2015). This is then posing a serious additional threat for malaria vector control measures. Furthermore, it is also possible that unknown insecticide resistance mechanisms may be occurring in wild populations of the dominant Afro-tropical malaria vectors An. gambiae. In this case, we cannot argue that the metabolic resistance plus the target-site insensitivity mechanisms may be the cause of the higher levels of insecticide resistance-phenotype observed. Recent studies have revealed the presence of previously undetectable insecticide resistance mechanisms in African malaria vectors An. gambiae (Balabanidou et al. 2018, 2019; Ingham et al. 2018). That illustrates thereby the complexity of mechanisms involved in the resistance-phenotypes observed in malaria vectors An. gambiae.
Our findings provide evidence that target-site insensitivity mutations alone cannot induce the resistance-phenotypes that occur in natural populations of An. gambiae mosquitoes. Therefore, the main concern of entomologists and other actors of the related disciplines would be to work for a better characterization of the specific resistance mechanisms that contribute to this insecticide resistance phenomenon in order to help appropriate decision-making process for an effective management of resistant Anopheles vectors.
Supplementary Material
Acknowledgments
We express our sincere thanks to David Weetman and Martin Donnelly (LSTM) for providing WHO insecticide-impregnated papers. We would like to acknowledge Emilienne Fiogbe for her helpful English writing assistance. This study and AAM received financial support by grant to LSD from Wellcome Trust (grant 109917/Z/15/Z).
References Cited
- Abdalla, H, Matambo T S, Koekemoer L L, Mnzava A P, Hunt R H, and Coetzee M. 2008. Insecticide susceptibility and vector status of natural populations of Anopheles arabiensis from Sudan. Trans. R. Soc. Trop. Med. Hyg. 102: 263–271. [DOI] [PubMed] [Google Scholar]
- Aïkpon, R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, and Akogbéto M. 2013. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit. Vectors. 6: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemayehu, E, Asale A, Eba K, Getahun K, Tushune K, Bryon A, Morou E, Vontas J, Van Leeuwen T, Duchateau L, . et al. 2017. Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasit. Vectors. 10: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alout, H, Ndam N T, Sandeu M M, Djégbe I, Chandre F, Dabiré R K, Djogbénou L S, Corbel V, and Cohuet A. 2013. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One 8: e63849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoudji, A D, Ahadji-Dabla K M, Hien A S, Apétogbo Y G, Yaméogo B, Soma D D, Bamogo R, Atcha-Oubou R T, Dabiré R K, and Ketoh G K. 2019. Insecticide resistance profiles of Anopheles gambiae s.l. in Togo and genetic mechanisms involved, during 3-year survey: is there any need for resistance management? Malar. J. 18: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assogba, B S, Djogbénou L S, Saizonou J, Milesi P, Djossou L, Djegbe I, Oumbouke W A, Chandre F, Baba-Moussa L, Weill M, . et al. 2014. Phenotypic effects of concomitant insensitive acetylcholinesterase (ace-1®) and knockdown resistance (kdr®) in Anopheles gambiae: a hindrance for insecticide resistance management for malaria vector control. Parasit. Vectors. 7: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assogba, B S, Pasteur N, Makoundou P, Unal S, Baba-Moussa L, Labbé P, and Weill M. 2020. Dynamic of resistance alleles of two major insecticide targets in Anopheles gambiae (s.l.) populations from Benin, West Africa. Parasit. Vectors. 13: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola, T S, Adeogun A, Olakiigbe A K, Oyeniyi T, Olukosi Y A, Okoh H, Arowolo T, Akila J, Oduola A, and Amajoh C N. 2018. Pyrethroids resistance intensity and resistance mechanisms in Anopheles gambiae from malaria vector surveillance sites in Nigeria. PLoS One 13: e0205230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolo, A, Okado K, Guelbeogo W M, Aonuma H, Bando H, Fukumoto S, Sagnon N, and Kanuka H. 2012. Development of an allele-specific, loop-mediated, isothermal amplification method (AS-LAMP) to detect the L1014F kdr-w mutation in Anopheles gambiae s. l. Malar. J. 11: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanidou, V, Grigoraki L, and Vontas J. 2018. Insect cuticle: a critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 27: 68–74. [DOI] [PubMed] [Google Scholar]
- Balabanidou, V, Kefi M, Aivaliotis M, Koidou V, Girotti J R, Mijailovsky S J, Juárez M P, Papadogiorgaki E, Chalepakis G, Kampouraki A, . et al. 2019. Mosquitoes cloak their legs to resist insecticides. Proc. Biol. Sci. 286: 20191091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, C, Nikou D, Vontas J, Donnelly M J, Williamson M S, and Field L M. 2010. The Vector Population Monitoring Tool (VPMT): high-throughput DNA-based diagnostics for the monitoring of mosquito vector populations. Malar. Res. Treat. 2010: 190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara, S, Koffi A A, Ahoua Alou L P, Koffi K, Kabran J K, Koné A, Koffi M F, N’Guessan R, and Pennetier C. 2018. Mapping insecticide resistance in Anopheles gambiae (s.l.) from Côte d’Ivoire. Parasit. Vectors. 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandre, F, Darrier F, Manga L, Akogbeto M, Faye O, Mouchet J, and Guillet P. 1999. Status of pyrethroid resistance in Anopheles gambiae sensu lato. Bull. World Health Organ. 77: 230–234. [PMC free article] [PubMed] [Google Scholar]
- Collins, F H, Mendez M A, Rasmussen M O, Mehaffey P C, Besansky N J, and Finnerty V. 1987. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 37: 37–41. [DOI] [PubMed] [Google Scholar]
- Corbel, V, and N’Guessan R. 2013. Distribution, mechanisms, impact and management of insecticide resistance in malaria vectors: a pragmatic review, pp. 579–633. In S. Manguin (ed.), Anopheles mosquitoes - new insights into malaria vectors. InTech. See: http://www.intechopen.com/books/. [Google Scholar]
- Dabiré, K R, Diabaté A, Namountougou M, Toé K H, Ouari A, Kengne P, Bass C, and Baldet T. 2009. Distribution of pyrethroid and DDT resistance and the L1014F kdr mutation in Anopheles gambiae s.l. from Burkina Faso (West Africa). Trans. R. Soc. Trop. Med. Hyg. 103: 1113–1120. [DOI] [PubMed] [Google Scholar]
- David, J-P, H. Mahmoud I, Alexia C-P, and Ingraham P M J. 2013. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philos. Trans. R. Soc. B Biol. Sci. 368: 20120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate, A, Baldet T, Chandre F, Akoobeto M, Guiguemde T R, Darriet F, Brengues C, Guillet P, Hemingway J, Small G J, . et al. 2002. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 67: 617–622. [DOI] [PubMed] [Google Scholar]
- Djègbè, I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, Akogbéto M, and Corbel V. 2011. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar. J. 10: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djegbe, I, Dramane G, Zeukeng F, Akogbeto M, and Djouaka R. 2017. Relationship between kdr L1014F genotypes and phenotypic-resistance to pyrethroids and DDT insecticides in Anopheles gambiae s.l. Int. J. Innov. Appl. Stud. 20: 983–993. [Google Scholar]
- Djogbénou, L, Weill M, Hougard J M, Raymond M, Akogbéto M, and Chandre F. 2007. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J. Med. Entomol. 44: 805–810. [DOI] [PubMed] [Google Scholar]
- Djogbénou, L, Pasteur N, Akogbéto M, Weill M, and Chandre F. 2011. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med. Vet. Entomol. 25: 256–267. [DOI] [PubMed] [Google Scholar]
- Djouaka, R F, Bakare A A, Coulibaly O N, Akogbeto M C, Ranson H, Hemingway J, and Strode C. 2008. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics 9: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly, M J, Corbel V, Weetman D, Wilding C S, Williamson M S, and W CBlack, 4th. 2009. Does kdr genotype predict insecticide-resistance phenotype in mosquitoes? Trends Parasitol. 25: 213–219. [DOI] [PubMed] [Google Scholar]
- Fuseini, G, Ebsworth P, Jones S, and Knight D. 2011. The efficacy of ACTELLIC 50 EC, pirimiphos methyl, for indoor residual spraying in Ahafo, Ghana: area of high vector resistance to pyrethroids and organochlorines. J. Med. Entomol. 48: 437–440. [DOI] [PubMed] [Google Scholar]
- Huang, Y, Guo Q, Sun X, Zhang C, Xu N, Xu Y, Zhou D, Sun Y, Ma L, Zhu C, . et al. 2018. Culex pipiens pallens cuticular protein CPLCG5 participates in pyrethroid resistance by forming a rigid matrix. Parasit. Vectors. 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, S S, Manu Y A, Tukur Z, Irving H, and Wondji C S. 2014. High frequency of kdr L1014F is associated with pyrethroid resistance in Anopheles coluzzii in Sudan savannah of northern Nigeria. BMC Infect. Dis. 14: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham, V A, Wagstaff S, and Ranson H. 2018. Transcriptomic meta-signatures identified in Anopheles gambiae populations reveal previously undetected insecticide resistance mechanisms. Nat. Commun. 9: 5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C M, Liyanapathirana M, Agossa F R, Weetman D, Ranson H, Donnelly M J, and Wilding C S. 2012. Footprints of positive selection associated with a mutation (N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 109: 6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katureebe, A, Zinszer K, Arinaitwe E, Rek J, Kakande E, Charland K, Kigozi R, Kilama M, Nankabirwa J, Yeka A, . et al. 2016. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: a prospective observational study. PLoS Med. 13: e1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X, Schuler M A, and Berenbaum M R. 2007. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 52: 231–253. [DOI] [PubMed] [Google Scholar]
- Liu, N. 2015. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol. 60: 537–559. [DOI] [PubMed] [Google Scholar]
- Martinez-Torres, D, Chandre F, Williamson M S, Darriet F, Bergé J B, Devonshire A L, Guillet P, Pasteur N, and Pauron D. 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect. Mol. Biol. 7: 179–184. [DOI] [PubMed] [Google Scholar]
- Matambo, T S, Abdalla H, Brooke B D, Koekemoer L L, Mnzava A, Hunt R H, and Coetzee M. 2007. Insecticide resistance in the malarial mosquito Anopheles arabiensis and association with the kdr mutation. Med. Vet. Entomol. 21: 97–102. [DOI] [PubMed] [Google Scholar]
- Mitchell, S N, Rigden D J, Dowd A J, Lu F, Wilding C S, Weetman D, Dadzie S, Jenkins A M, Regna K, Boko P, . et al. 2014. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS One 9: e92662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnzava, A P, Knox T B, Temu E A, Trett A, Fornadel C, Hemingway J, and Renshaw M. 2015. Implementation of the global plan for insecticide resistance management in malaria vectors: progress, challenges and the way forward. Malar. J. 14: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux, N, Gomez M B, Pennetier C, Elanga E, Djènontin A, Chandre F, Djègbé I, Guis H, and Corbel V. 2012. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 206: 1622–1629. [DOI] [PubMed] [Google Scholar]
- Nardini, L, Hunt R H, Dahan-Moss Y L, Christie N, Christian R N, Coetzee M, and Koekemoer L L. 2017. Malaria vectors in the Democratic Republic of the Congo: the mechanisms that confer insecticide resistance in Anopheles gambiae and Anopheles funestus. Malar. J. 16: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Guessan, R, Corbel V, Akogbéto M, and Rowland M. 2007. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg. Infect. Dis. 13: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkya, T E, Poupardin R, Laporte F, Akhouayri I, Mosha F, Magesa S, Kisinza W, and David J P. 2014. Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasit. Vectors. 7: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochomo, E, Bayoh M N, Brogdon W G, Gimnig J E, Ouma C, Vulule J M, and Walker E D. 2013. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Med. Vet. Entomol. 27: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson, H, Jensen B, Vulule J M, Wang X, Hemingway J, and Collins F H. 2000. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 9: 491–497. [DOI] [PubMed] [Google Scholar]
- Ranson, H, N’guessan R, Lines J, Moiroux N, Nkuni Z, and Corbel V. 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27: 91–98. [DOI] [PubMed] [Google Scholar]
- Reddy, M R, Overgaard H J, Abaga S, Reddy V P, Caccone A, Kiszewski A E, and Slotman M A. 2011. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar. J. 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, M, Boko P, Odjo A, Asidi A, Akogbeto M, and N’Guessan R. 2013. A new long-lasting indoor residual formulation of the organophosphate insecticide pirimiphos methyl for prolonged control of pyrethroid-resistant mosquitoes: an experimental hut trial in Benin. PLoS One 8: e69516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, R M, Robertson J L, and Savin N E. 1977. POLO: a new computer program for probit analysis. Bull. Entomol. Soc. Am. 23: 209–213. [Google Scholar]
- Russell, T L, Govella N J, Azizi S, Drakeley C J, Kachur S P, and Killeen G F. 2011. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolamazza, F, Mancini E, Simard F, Qi Y, Tu Z, and della Torre A. 2008. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 7: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service, M W. 1977. A critical review of procedures for sampling populations of adult mosquitoes. Bull. Entomol. Res. 67: 343–382. [Google Scholar]
- Shute, G T. 1956. A method of maintaining colonies of East African strains of Anopheles gambiae. Ann. Trop. Med. Parasitol. 50: 92–94. [DOI] [PubMed] [Google Scholar]
- Stica, C, Jeffries C L, Irish S R, Barry Y, Camara D, Yansane I, Kristan M, Walker T, and Messenger L A. 2019. Characterizing the molecular and metabolic mechanisms of insecticide resistance in Anopheles gambiae in Faranah, Guinea. Malar. J. 18: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchicaya, E S, Nsanzabana C, Smith T A, Donzé J, de Hipsl M L, Tano Y, Müller P, Briët O J, Utzinger J, and Koudou B G. 2014. Micro-encapsulated pirimiphos-methyl shows high insecticidal efficacy and long residual activity against pyrethroid-resistant malaria vectors in central Côte d’Ivoire. Malar. J. 13: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini, L, Reed T W, and Willis J H. 2014. Temporal and spatial expression of cuticular proteins of Anopheles gambiae implicated in insecticide resistance or differentiation of M/S incipient species. Parasit. Vectors. 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill, M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, and Raymond M. 2004. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 13: 1–7. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2004. Global strategic framework for integrated vector management. World Health Organization, Geneva, Switzerland. https://www.who.int/wer/2011/wer8613.pdf. [Google Scholar]
- World Health Organization. 2006. Pesticides and their application : for the control of vectors and pests of public health importance.World Health Organization, Geneva, Switzerland. Available from WHO/CDS/NTD/WHOPES/GCDPP/2006.1. [Google Scholar]
- World Health Organization. 2012. Global plan for insecticide resistance management in malaria vectors. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/4484/9789241564472_eng.pdf. [Google Scholar]
- WHO. 2016. “Geneva: World malaria Report” 2016, World Health Organization, test procedures for insecticide resistance monitoring in malaria vector mosquitoes, Geneva, Switzerland, 2nd edn. World Health Organization, Geneva, Switzerland [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.