Abstract
Background
The clinical value of whole brain radiotherapy (WBRT) for brain metastases (BM) is a matter of debate due to the significant side effects involved. Stereotactic radiosurgery (SRS) is an attractive alternative treatment option that may avoid these side effects and improve local tumor control. We initiated a randomized trial (NCT02353000) to investigate whether quality of life is better preserved after SRS compared with WBRT in patients with multiple brain metastases.
Methods
Patients with 4–10 BM were randomized between the standard arm WBRT (total dose 20 Gy in 5 fractions) or SRS (single fraction or 3 fractions). The primary endpoint was the difference in quality of life (QOL) at 3 months post-treatment.
Results
The study was prematurely closed due to poor accrual. A total of 29 patients (13%) were randomized, of which 15 patients have been treated with SRS and 14 patients with WBRT. The median number of lesions were 6 (range: 4–9) and the median total treatment volume was 13.0 cc3 (range: 1.8–25.9 cc3). QOL at 3 months decreased in the SRS group by 0.1 (SD = 0.2), compared to 0.2 (SD = 0.2) in the WBRT group (P = .23). The actuarial 1-year survival rates were 57% (SRS) and 31% (WBRT) (P = .52). The actuarial 1-year brain salvage-free survival rates were 50% (SRS) and 78% (WBRT) (P = .22).
Conclusion
In patients with 4–10 BM, SRS alone resulted in 1-year survival for 57% of patients while maintaining quality of life. Due to the premature closure of the trial, no statistically significant differences could be determined.
Keywords: brain metastases, quality of life, stereotactic radiotherapy, whole brain radiotherapy
Key Points.
SRS is a promising treatment option for patients with multiple brain metastases.
In patients with brain metastases, SRS resulted in >50% OS while maintaining QOL.
The main reason for poor inclusion was patient and referrer preference for SRS.
Importance of the Study.
Brain metastases (BM) are an important cause of morbidity and mortality. Therefore, optimal tumor control with preservation of quality of life is essential. Treatment has mainly consisted of whole brain radiotherapy (WBRT), but in the last decades, stereotactic radiotherapy (SRS) became available. Until recently, the Dutch guideline was to treat patients with multiple BM with WBRT. Several studies have shown that survival, quality of life, and neurological deterioration after SRS is equal between patients with multiple BM and those with 2–4 BM.
To our knowledge, this study is the first phase III randomized controlled trial investigating WBRT versus SRS for patients with multiple BM. This trial assesses quality of life, which is an important endpoint in this patient population, and shows that patients can be treated with SRS while maintaining quality of life. In addition, since the study was closed prematurely, we evaluated the reasons for poor accrual.
A significant proportion (20–40%) of patients with cancer develop brain metastases (BM).1 BM are an important cause of morbidity and mortality in patients with metastasized cancer. Therefore, optimal tumor control with preservation of quality of life (QOL) during patients’ remaining lifespan is essential. Traditionally, the treatment mainly consisted of radiotherapy, primarily whole brain radiotherapy (WBRT). The QUARTZ study showed that WBRT did not provide any benefit in QOL or survival over the best supportive care in selected vulnerable patients with non-small cell lung cancer (NSCLC).2 WBRT has significant side effects, such as hair loss, fatigue, and cognitive dysfunction, which may result in decreased QOL.3 In the last decades, stereotactic radiosurgery (SRS) became available, and has since become a widely used technique in the treatment for patients with BM. There are some important advantages of SRS over WBRT, that is, limiting radiation doses to the uninvolved brain and a high probability of local tumor control. SRS is available in all Dutch radiotherapy centers and due to recent technical advances, radiation can be delivered within half an hour. Until recently, the Dutch guideline was to treat patients with 1–3 BM with SRS and patients with 4 or more BM with WBRT. In 2017, a working group was established to review the current guidelines and SRS for a maximum of 10 BM with low volume is now supported by the renewed Dutch and UK guidelines. In the current era, in which targeted agents and immunotherapy are often also treatment options for BM, avoidance of elective brain irradiation is attractive.4 Multidisciplinary management is essential to determine the optimal treatment at the right moment.5
A nonrandomized study in a large cohort of favorable prognostic patients with low volume BM showed that survival after SRS in patients with 5–10 BM and 2–4 BM was comparable.6 Cumulative volume of BM was shown to be an important prognostic factor in patients with BM, just as much as performance status.6,7
In this randomized controlled study, WBRT was compared to SRS to determine if SRS is a better palliative treatment than WBRT in favorable prognostic patients with 4 to 10 low volume BM in terms of QOL at 3 months post-radiotherapy.
Methods
Study Design and Participants
In this prospective randomized phase III multicenter trial, patients were enrolled from different radiotherapy centers in the Netherlands. Patients referred for radiotherapy with 4–10 BM from solid tumors diagnosed on a high-resolution contrast-enhanced MRI scan were included. Patients were randomized between WBRT (standard arm) or SRS to the BM (study arm). Inclusion criteria were age ≥18 years, minimal 4 up to a maximum of 10 BM and a maximum cumulative gross tumor volume (GTV) of 30 cm3 based on the diagnostic cerebral MRI scan, Karnofsky performance status ≥70, any solid primary tumor (small cell lung carcinoma, germinoma, and lymphoma were excluded), and patients ability to provide written informed consent. Exclusion criteria were contra-indication for MRI, prior treatment for BM (ie, surgery, SRS, or WBRT), concurrent use of systemic therapy, and any brainstem metastasis with a planning target volume (PTV) of more than 20 cm3.
All patients willing to participate gave written informed consent. The study protocol was approved by the medical ethical committee of the Maastricht University Medical Center, the Netherlands (NCT02353000).
Randomization and Masking
After discussion within the multidisciplinary tumor board which included a neurosurgeon, medical oncologist, neuro-radiologist, pathologist, and radiation oncologist, all specialized in the treatment of BM, patients were selected for participation in the study. Patients were allocated to one of the two treatment arms at random using a permutated block randomization approach with a block size of 8. After randomization, baseline data, treatment characteristics, and follow-up data were entered by the treating physician or investigator at the participating center. Neither patients, clinicians, nor study statisticians were masked to treatment assignment.
Procedures
On a gadolinium contrast-enhanced (single – triple dose gadolinium was allowed) MRI (1.0T-3T) with a maximal slice thickness of 1.5 mm, the definitive number of BM and the definitive maximum lesion diameter in any direction of the largest BM was determined. The use of a planning CT with slice thickness of ≤3 mm was mandatory.
Whole Brain Radiotherapy
Patients randomized for WBRT were treated with 5 fractions of 4 Gy up to a total dose of 20 Gy delivered over 5 consecutive working days. The brain was contoured as the clinical target volume (CTV) up to the foramen magnum, where the CTV is equal to the PTV. To determine the size of the GTV of the metastases, all BM were contoured using MRI. Patients were positioned with a thermoplastic mask. The daily prescription dose was 4 Gy prescribed at the ICRU reference point and the 95% isodose was required to encompass 99% of the PTV. The maximum dose to the PTV did not exceed 107% of the prescribed dose. All radiation techniques that could meet the dose requirements were allowed. The following parameters were noted: the GTVs of all lesions separately and the PTV that received 95% of the prescription dose (V95%).
Stereotactic Radiotherapy
The dose (15 Gy up to 24 Gy in one fraction or 24 Gy in 3 fractions) was determined by the PTV of the largest BM or by brainstem location (24 Gy in 3 fractions). A volume-based dosing strategy was employed conforming to the National Neuro-Oncology consensus. If the V12Gy of healthy brain tissue surrounding an individual BM was more than 10 cm3, 24 Gy in 3 fractions of 8 Gy was allowed to minimize the risk of radionecrosis.8,9 The GTV was defined by contouring the outer contrast-enhancing border of the BM on T1 gadolinium-weighted MRI images. The PTV was defined by a 0–2 mm isotropic expansion of the GTV determined by the treating physician’s preference and the center’s set-up uncertainties. If a BM was within or adjacent to the brainstem, the PTV margin was defined at 0 mm in all centers to minimize the risk of brainstem necrosis.
Patients were immobilized in a supine position within a thermoplastic mask or stereotactic noninvasive frame, with or without bite block and other fixation methods. The planning CT scan with ≤3 mm thick contiguous slices was registered with a contrast-enhanced MRI scan. The maximum interval between the planning-MRI and the SRS treatment was 3 weeks. Single or multiple isocenters were allowed for delivering SRS according to the treating center preference. The following parameters were noted: maximum diameter of largest GTV and PTV, total GTV and PTV, maximum dose (D2%), mean dose (Dmean), Paddick’s gradient index, maximum dose OAR (D0.03cc), total V12Gy (brain minus GTVs), and V12Gy largest BM (surrounding brain of the largest BM minus GTV). All (serious) adverse events ((S)AE) reported spontaneously by the patient or observed by the investigator or his staff were recorded.
Outcomes
The primary objective was to determine if SRS is a better palliative treatment than WBRT for patients with 4–10 BM in terms of QOL at 3 months post-radiotherapy using the EQ5D EUROQOL score. The secondary endpoints were difference in QOL (EQ5D EUROQOL questionnaire) at 6, 9, and 12 months after radiation with respect to baseline. In addition, survival, Karnofsky score, WHO performance status, toxicity according to the CTCAE V4.0 criteria, salvage treatment and time to salvage after randomization, and Barthel index were evaluated at 3, 6, 9, and 12 months after treatment. Facultative secondary endpoints were neurocognition with the Hopkins Verbal Learning Test, quality of life EORTC BN20 brain module, quality of life EORTC QLQ-C30, and fatigue scale EORTC QLQ-FA13.
Statistical Analysis
Questionnaires measuring QOL with the EQ5D questionnaire were collected from patients at baseline and at 3 months post-treatment. A sample size calculation was done for a comparison of means with a 2-sided α of 0.05 and a power of 0.80, which led to a sample size of 230 patients (115 per group). Patient accrual started on July 1, 2016, but due to poor accrual, the trial was closed prematurely in July 2018. A comparison of the earlier described EQ5D scores between the SRS and WBRT groups was performed using an independent samples Student’s t-test with a 2-sided significance level α set at 0.05. Differences in secondary endpoints in time were analyzed using Kaplan–Meyer curves, including logrank test and ANOVA testing. Time-to-event data (eg, overall survival) were compared using Kaplan–Meier curves and logrank testing. Means were compared using independent samples Student’s t-tests. Statistical analysis was performed using SPSS® version 23 (IBM).
Results
Baseline Characteristics
Between July 2016 and May 2018, 29 patients were enrolled and randomly assigned to WBRT (14 patients) or SRS (15 patients). Median follow-up was 26 months. Baseline characteristics for both treatment arms are shown in Table 1. The most common primary cancer site was non-small cell lung cancer in both treatment arms (85% WBRT vs 80% SRS). In both arms, patients were in a favorable physical condition with a median Karnofsky score of 90 in the SRS group and 85 in the WBRT group. The median number of lesions per patient was 6 (range: 4–9) and the median total treatment volume was 13.0 cc (range: 1.8–25.9 cc). In the SRS group, there were 3 (20%) patients with 9 BM. Of the 15 patients treated with SRS, 13 (87%) patients were treated with single fraction SRS and 2 (13%) patients were treated with 3 fractions of 8 Gy. The median interval between the planning MRI and the actual SRS treatment was 7 days (range: 6–28). LINAC-based SRS was performed in 13 patients and CyberKnife SRS in 2 patients. No GammaKnife centers participated in this study. The mean maximum diameter of the GTV was 5.1 ± 6.6 mm. The complete plan quality for the SRS patients is described in Table 2.
Table 1.
Baseline Characteristics
| SRS Group (N = 15) | WBRT Group (N = 14) | |
|---|---|---|
| Sex | ||
| Female | 8 (53%) | 6 (42%) |
| Male | 7 (46%) | 8 (57%) |
| Age (years) | ||
| Median (range) | 59 (51–74) | 66 (51–85) |
| Mean (SD) | 60 (±7) | 65 (±10) |
| >65 | 5 (33%) | 7 (50%) |
| Primary cancer | ||
| NSCLC | 12 (80%) | 12 (85%) |
| Breast | - | 1 (7%) |
| Melanoma | 1 (7%) | - |
| Renal cell | - | 1 (7%) |
| Colorectal | 1 (7%) | - |
| Gastric | 1 (7%) | - |
| WHO | ||
| 0 | 2 (13%) | 5 (36%) |
| 1 | 9 (60%) | 6 (43%) |
| 2 | 4 (27%) | 3 (21%) |
| Karnofsky score | ||
| Median (range) | 90 (70–100) | 85 (60–100) |
| Number of metastases | ||
| 4 | 5 (33%) | 4 (29%) |
| 5 | 2 (14%) | 3 (21%) |
| 6 | 3 (20%) | 2 (14%) |
| 7 | 1 (7%) | 3 (21%) |
| 8 | 1 (7%) | 2 (14%) |
| 9 | 3 (20%) | |
| RPA Classification | ||
| I | 3 (20%) | 4 (29%) |
| II | 12 (80%) | 9 (64%) |
| III | - | 1 (7%) |
| DS-GPA | ||
| 0.5 | 2 (13%) | 3 (21%) |
| 1.0 | 4 (27%) | 2 (14%) |
| 1.5 | 2 (13%) | 5 (36%) |
| 2.0 | 5 (33%) | 2 (14%) |
| 2.5 | 1 (7%) | 2 (14%) |
| 3.0 | 1 (7%) | - |
| EQ5D Health state | ||
| Mean (SD) | 0.9 ± 0.1 | 0.8 ± 0.1 |
| EQ5D VAS score | ||
| Mean (SD) | 70 ± 20 | 77 ± 14 |
| Maximum diameter single GTV | ||
| 0.5–1.0 cm | - | 1 (7%) |
| 1.0–1.5 cm | 3 (21%) | 1 (7%) |
| 1.5–2.0 cm | 4 (29%) | 3 (21%) |
| 2.0–2.5 cm | 2 (14%) | 6 (43%) |
| 2.5–3.0 cm | 2 (14%) | 2 (14%) |
| >3.0 cm | 3 (21%) | 1 (7%) |
| Cumulative GTV | ||
| 0.1–5 cm3 | 5 (36%) | 5 (36%) |
| 5–10 cm3 | 3 (21%) | 3 (21%) |
| 10–15 cm3 | 3 (21%) | 4 (29%) |
| 15–20 cm3 | 3 (21%) | 1 (7%) |
| 20–25 cm3 | - | 1 (7%) |
DS-GPA, diagnosis-specific graded prognostic assessment; GTV, gross tumor volume; PTV, planning target volume; RPA, recursive partitioning analysis; SRS, stereotactic radiotherapy; WBRT, whole brain radiotherapy.
Table 2.
Plan Quality
| SRS Group | |
|---|---|
| Prescribed dose | |
| 1 × 16 Gy (BM in brainstem) | 3 (21%) |
| 1 × 18 Gy | 2 (14%) |
| 1 × 21 Gy | 4 (29%) |
| 1 × 24 Gy | 3 (22%) |
| 3 × 8 Gy | 2 (14%) |
| GTV max diameter largest GTV (cm) | 5.1 ± 6.6 |
| Total GTV (cm3) | 8.9 ± 6.7 |
| Total PTV (cm3) | 13.0 ± 8.8 |
| Maximum dose PTV (D2%) (Gy) | 26.4 ± 3.8 |
| Mean brain dose (brain-GTV) (Gy) | 3.0 ± 1.2 |
| Paddick conformity index (Volume (50% of prescribed dose)/Volume (prescribed dose) |
5.1 ± 1.7 |
| Total V12Gy (brain exclusive GTVs) | 31.0 ± 24.0 |
| V12Gy largest BM | 16.0 ± 15.6 |
BM, brain metastases; GTV, gross tumor volume; PTV, planning target volume; SRS, stereotactic radiotherapy.
Quality of Life and Toxicity
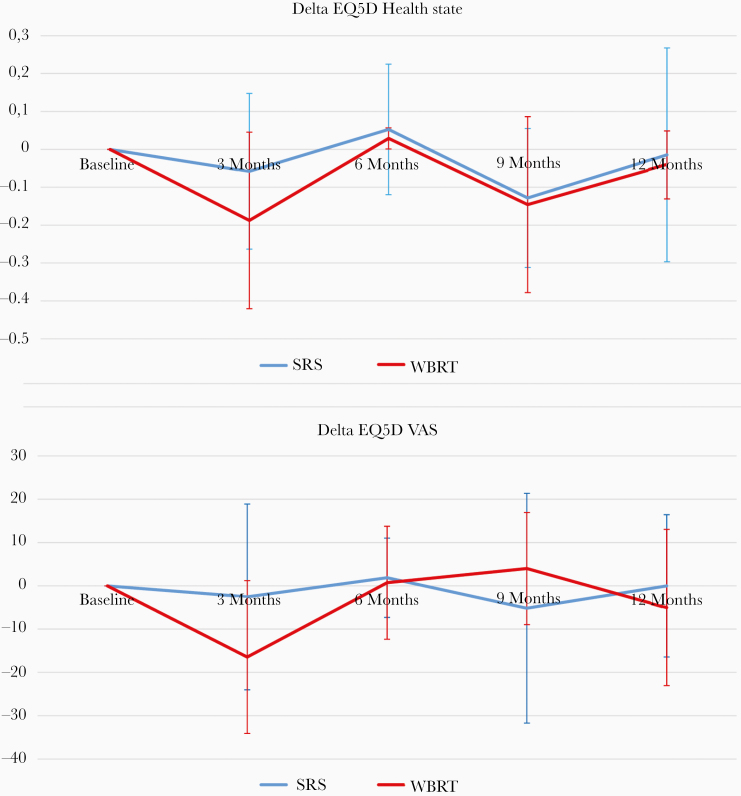
The EQ-5D health state and EQ-5D VAS score at baseline were 0.9 and 70 (SRS) and 0.8 and 77 (WBRT), respectively. The difference, which was not significant, on EQ5D health state and EQ5D VAS score between WBRT and SRS (3, 6, 9, and 12 months), is illustrated in Figure 1. Overall, the EQ5D health state and EQ5D VAS score decreased by 0.1 and 10 points (Table 3). QOL EQ5D health state at 3 months post-radiotherapy decreased in the WBRT group by 0.2 ± 0.2, compared with 0.1 ± 0.2 in the SRS group (P = .23). The EQ5D VAS score (patient’s perspective on quality of life) decreased by 16 points in the WBRT group versus 3 points in the SRS group (P = .15). No significant difference was observed in QOL after 3 months between WBRT and SRS.
Figure 1.
Difference between stereotactic radiotherapy and whole brain radiotherapy in EQ5D health state and EQ5D VAS score. SRS, stereotactic radiotherapy; WBRT, whole brain radiotherapy.
Table 3.
Difference in Quality of Life After 3 Months in Relative to Baseline
| SRS | WBRT | Total | P-value | |
|---|---|---|---|---|
| EQ5D Health state | −0.06 ± 0.21 | −0.19 ± 0.24 | −0.12 ± 0.22 | .23 |
| EQ5D VAS score | −2.6 ± 21.5 | −16.4 ± 17.6 | −9.5 ± 20.4 | .15 |
| Karnofsky score | 3 ± 17 | −4 ± 16 | −0.5 ± 17 | .34 |
Mean + standard deviation (SD).
SRS, stereotactic radiotherapy; WBRT, whole brain radiotherapy.
There were few adverse events in the study population. Two patients in the SRS group had an epileptic seizure after SRS, but both patients already had epileptic seizures prior to SRS. The seizures were controlled with medication. There were no epileptic seizures in the WBRT group. Toxicity mainly consisted of hair loss in the WBRT group, with 6 patients experiencing grade 2 hair loss. None of the patients in the SRS group suffered from grade 2 hair loss. Fatigue (CTCAE 4.0 grade 2) was registered in 3 patients within the WBRT group and 2 patients treated with SRS.
Oncologic Outcome
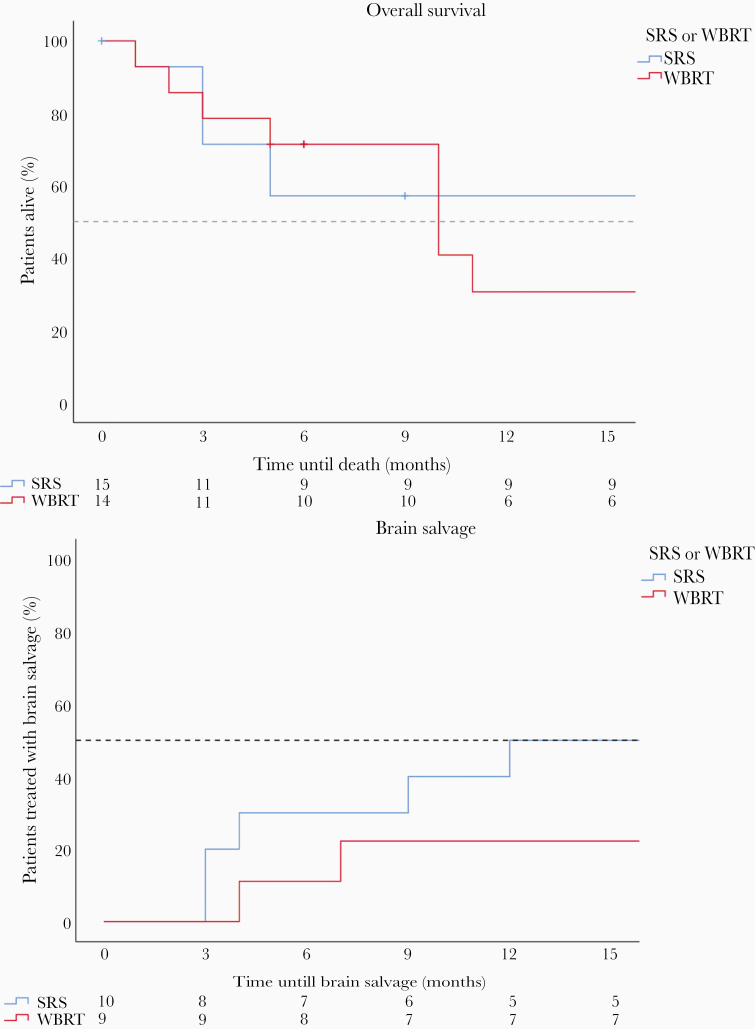
Using Kaplan–Meyer analysis, the median overall survival (OS) was 10 months in the WBRT group and the median OS was not reached in the SRS group after 1-year follow-up (duration of the study). The actuarial 1-year survival rate was 31% versus 57% in the WBRT and SRS groups, respectively (P = .52). The crude 1-year survival rate was 6/14 (43%) in the WBRT group and 9/15 (60%) in the SRS group. The actuarial 1-year brain salvage-free survival (BSFS) was 78% (WBRT) and 50% (SRS) (P = .22). Figure 2 illustrates the Kaplan–Meyer curve for OS and BSFS. There was no significant difference between the groups.
Figure 2.
(A) Kaplan–Meier survival curve. (B) Kaplan–Meier curve brain salvage. SRS, stereotactic radiotherapy; WBRT, Whole brain radiotherapy.
Reasons for Poor Accrual
The trial closed prematurely due to poor accrual. Multiple reasons were provided by the local investigators at the institutes. The main reason for poor accrual was the patient’s or referrer’s preference for treatment with SRS over WBRT. These and other reasons are also summarized in Table 4. Several patients could not participate in the trial due to the strict volume criteria, or had more than 10 BM.
Table 4.
Main Reasons for Poor Inclusion
| Reason | Number of Institutes* |
|---|---|
| Radiation Oncologist preference | 2 |
| Patient’s preference | 4 |
| Referrer’s preference (preference for SRS) | 3 |
| SRS standard of care | - |
| Other | 2 |
SRS, stereotactic radiotherapy.
*Participating institutes could have multiple reasons for poor accrual.
Discussion
In the last decade, the management of BM has seen dramatic changes. For several primary tumors, targeted agents and immunotherapy have become available as treatment options for BM. This questions the need for elective irradiation of the brain in the setting of BM. Previously, SRS was only suitable for patients with a limited number of BM due to limitations in technology. Improvements in SRS technology now allow wide use of SRS treatments for multiple metastases within an acceptable treatment time.10 Recently, the first matched-pair analysis to compare SRS versus WBRT for patients with multiple BM was published. Patients (N = 128) with multiple BM were matched pairwise for potential prognostic factors, such as age, Karnofsky performance score, initial number of BM, and RPA class. The authors found a median OS of 16 months in the SRS group versus 8 months in the WBRT group. Extracerebral tumor control and an excellent clinical performance were favorable prognostic factors. The results of this study suggest that SRS is a feasible and effective treatment option for patients with multiple BM.11 We observed relatively high compliance of the QOL questionnaires since the EQ5D is a short questionnaire and a research nurse contacted the patient by telephone. In studies in which patients need to fill in extensive questionnaires by themselves, compliance is usually much lower. This supports the use of the EUROQOL EQ5D questionnaire in patients with a relatively poor prognosis.
To our knowledge, this is the first phase III randomized controlled trial investigating WBRT versus SRS for patients with 4–10 BM that evaluated QOL, overall survival, and brain failure-free survival. A case-matched, retrospective cohort trial by Yamamoto et al. compared treatment results for patients with 10 or more BM versus 2–9 metastases.12 The primary endpoint was OS, whereas the secondary endpoints consisted, among others, of neurological deterioration and death. Considering the incidence of neurological deterioration (defined as any brain disease-caused neurological worsening), there was no difference between the groups, including radiation-related complications. They concluded that treatment with SRS was feasible for patients with multiple BM.
It is crucial that BM treatment contributes to the maintenance of a good neurological state and QOL. With effective BM treatment, such as SRS, the cause of death in many patients is nowadays extracranial disease progression.12,13 There has been an increasing interest in QOL as an indicator of outcomes in studies for patients with advanced cancer. Therefore, the chosen primary endpoint in this trial was QOL 3 months post-radiotherapy. We consider it to be highly important that maintenance of QOL for patients treated with SRS for multiple BM was not inferior for patients treated with WBRT since WBRT carries the risk of inducing neurocognitive deterioration. Furthermore, the risk of serious complications due to SRS is comparable in patients treated for multiple BM to patients with a limited number of BM, therefore a reduced decrease or even an increase in QOL is expected.13–15 We hypothesized that QOL in the SRS group was better preserved than in the WBRT group, even longer than 3 months post-treatment. Sheehan et al. showed that QOL was likely to improve in patients treated with SRS. Worsening of the overall EQ5D was associated with an increased number of BM.16 A very recent study confirmed that QOL is preserved in patients treated with SRS, in which upfront WBRT is an independent predictor of QOL deterioration.17 The results of this study showed a decline in the EQ5D of 0.1 in the SRS group versus 0.2 in the WBRT group, which meets our expectations and shows that QOL is preserved at least as good in the SRS group as in the WBRT group. More mature results of randomized trials are needed to definitely confirm our hypothesis.
Several other trials are currently recruiting patients to evaluate the role of SRS in patients with more than 4 BM. A randomized phase III trial (NCT01592968) at the MD Anderson Cancer Center is randomizing patients with 4–15 BM to SRS alone versus WBRT alone. The ENCEPHALON trial is a randomized controlled trial which is recruiting patients to evaluate WBRT alone versus SRS in patients with 1–10 BM from small cell lung cancer (NCT03297788).
Our study has several important limitations. As already mentioned, the trial was closed prematurely due to poor accrual. Therefore, only a 29 out of 230 patients planned for analysis were included in the study. Due to this small study population, no statistically significant differences could be determined between the 2 treatment arms. The majority of patients enrolled had metastases from non-small cell lung cancer (83%). However, lung cancer patients form a majority in almost all brain metastases trials and there is no evidence that QOL and cognitive effect vary between different primary cancers.18–20 Another potential limitation is that clinicians and participants could not be blinded for treatment allocation, which is typical for trials evaluating various forms of radiotherapy. We observed a relatively favorable prognosis in both the SRS and WBRT arms, which is explained by the stringent patient selection using relatively low volume BM and favorable performance status. It has been proven that these factors have prognostic significance.7,11,21,22 SRS is an attractive palliative treatment option for multiple brain metastases due to the avoidance of alopecia and the fatigue of elective brain irradiation. Nowadays, very efficient LINAC-based solutions are available in which SRS for multiple BM can be delivered within half an hour with a high treatment plan quality.23
Clinical trial inclusion failure is common in radiation oncology, but factors contributing to this failure are not well understood. Nguyen et al. compared complete and incomplete trials to identify predictors of trial failure. They undertook a review of RCT’s involving radiotherapy (external beam RT in at least one arm of the study), in which 134 studies were included. A third of the studies failed, with almost 58% due to poor accrual. An increase in failure was seen over time, with failure rates from 12% before 2007 up to 40% in 2012.24 Since poor accrual was the main reason, the study was closed prematurely, we asked the participating radiotherapy centers about the reasons for poor inclusion. The main reason appeared to be patient’s and referrer’s preference in favor of SRS. One institute indicated that the radiation oncologist’s preference played a role, with the most important consideration that the side effects of WBRT were undesirable in patients with a very favorable performance status (Karnofsky performance status of 90 or 100). A considerable number of patients could not be included because they did not meet the volume criteria and/or had more than 10 BM. In hindsight, the inclusion criteria may have been too strict, in particular, the maximum diameter of the BM. It can also be concluded that such a phase III trial can no longer take place in the Netherlands because SRS has become the preferred treatment choice over WBRT in favorable prognostic patients with several systemic treatment options available.
SRS is an attractive treatment option for patients with multiple BM to avoid elective brain irradiation and optimize local tumor control in the current era of personalized medicine, especially for patients who have targeted agents and immunotherapy as treatment options. Future studies will determine if all BM need to be irradiated with SRS if systemic therapy is available and if hypofractionated SRS may avoid the incidence of radionecrosis compared to single fraction SRS with the aim of optimal maintenance of quality of life.25,26
In conclusion, in patients with 4–10 BM, SRS alone resulted in 1-year survival in 57% of patients while maintaining QOL. Due to premature closure of the trial, mainly as a result of patient’s and referrer’s preference for SRS, no statistically significant differences could be determined between SRS alone and WBRT. Mature results of ongoing trials in the United States and Canada are awaited to define the role of SRS in the setting of multiple BM.
Acknowledgment
The authors would like to thank the data safety commission (Dr. An Hoeben, Dr. Monique Anten, and Dr. Peter Koehler), in particular Rody Zuidema and Ruud Hoeben for their commitment and contribution to this trial.
Funding
The funders provided financial support but had no other role in the study design, data collection, analysis, interpretation, or writing of the report. The MAASTRO Clinic has a research agreement with Varian Medial Systems, Palo Alto in which funding was provided for this trial. Varian was not involved in writing the study protocol or manuscript, the storage of data, nor the analysis of data. The Data Center was responsible for the collection and maintenance of the data. The authors acknowledge financial support from the Dutch Technology Foundation STW (grant n° 10696 DuCAT), which is the applied science division of NWO, and the Technology Programme of the Ministry of Economic Affairs. The authors also acknowledge financial support from the EU 7th framework program (ARTFORCE - n° 257144, REQUITE - n° 601826), SME Phase 2 (RAIL – n°673780), the European Program H2020-PHC-2015 (BD2decide - n°210274050), and Kankeronderzoekfonds Limburg from the Limburg Health Foundation. The corresponding author and principal investigator of the study had full access to all the data and final responsibility.
Conflict of interest statement. D.R.: Bristol-Myers Squibb, investigator initiated study, advisory board, all paid to the institution. AstraZeneca, investigator initiated study, advisory board, all paid to the institution. Boehringer Ingelheim, investigator initiated study, advisory board, all paid to the institution. Philips, advisory board, all paid to the institution. Olinks, investigator initiated study, all paid to the institution. A.B.: ViewRay.Inc, speaker fee and travel reimbursement. J.Z., D.H., C.H., P.T., J.V., D.E., F.L., A.S., E.D., P.L., B.O., A.S., L.V., and R.W. have nothing to disclose.
Authorship Statement.
Writing of study protocol: J.Z., P.L., A.B., F.L., D.E., A.S., and C.H. Local investigators: J.Z., A.B., P.-P.T., L.V., A.S.-K., R.W., E.D., B.O., and J.V. Analysis of data: D.H., J.Z. Writing of manuscript: D.H., J.Z., A.B., F.L., D.E., A.S., C.H., E.D., R.W., B.O., J.V., P.-P.T., D.R., P.L., A.S.-K., and L.V.
References
- 1. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 2. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinkham MB, Sanghera P, Wall GK, Dawson BD, Whitfield GA. Neurocognitive effects following cranial irradiation for brain metastases. Clin Oncol. 2015;27(11):630–639. [DOI] [PubMed] [Google Scholar]
- 4. Verduin M, Zindler JD, Martinussen HM, et al. Use of systemic therapy concurrent with cranial radiotherapy for cerebral metastases of solid tumors. Oncologist. 2017;22(2):222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartgerink D, van der Heijden B, De Ruysscher D, et al. Stereotactic radiosurgery in the management of patients with brain metastases of non-small cell lung cancer: indications, decision tools and future directions. Front Oncol. 2018;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0 [DOI] [PubMed] [Google Scholar]
- 7. Routman DM, Bian SX, Diao K, et al. The growing importance of lesion volume as a prognostic factor in patients with multiple brain metastases treated with stereotactic radiosurgery. Cancer Med. 2018;7:757–764. doi: 10.1002/cam4.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zindler JD, Thomas CR, Hahn SM, Hoffmann AL, Troost EGC, Lambin P. Increasing the therapeutic ratio of stereotactic ablative radiotherapy by individualized isotoxic dose prescription. J Natl Cancer Inst. 2016;108(2):djv305. doi: 10.1093/jnci/djv305 [DOI] [PubMed] [Google Scholar]
- 9. Zindler JD, Schiffelers J, Lambin P, Hoffmann AL. Improved effectiveness of stereotactic radiosurgery in large brain metastases by individualized isotoxic dose prescription: an in silico studyVerbesserte Effektivität der stereotaktischen Radiochirurgie bei großen Hirnmetastasen durch individualisierte is. Strahlentherapie und Onkol. 2018;194:560–569. doi: 10.1007/s00066-018-1262-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahgal A, Ma L, Chang E, et al. Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat. 2009;8(4):271–280. [DOI] [PubMed] [Google Scholar]
- 11. El Shafie RA, Celik A, Weber D, et al. A matched-pair analysis comparing stereotactic radiosurgery with whole-brain radiotherapy for patients with multiple brain metastases. J Neurooncol. 2020;147(3):607–618. [DOI] [PubMed] [Google Scholar]
- 12. Yamamoto M, Kawabe T, Sato Y, et al. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2-9 versus 10 or more tumors. J Neurosurg. 2014;121Suppl:16–25. [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto M, Kawabe T, Sato Y, et al. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1-4 vs ≥ 5 tumors: clinical article. J Neurosurg. 2013;118(6):1258–1268. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto M, Kawabe T, Higuchi Y, et al. Delayed complications in patients surviving at least 3 years after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85(1):53–60. doi: 10.1016/j.ijrobp.2012.04.018 [DOI] [PubMed] [Google Scholar]
- 15. Kocher M, Wittig A, Piroth MD, et al. Stereotactic radiosurgery for treatment of brain metastases: a report of the DEGRO working group on stereotactic radiotherapy. Strahlentherapie und Onkol. 2014;190:521–532. doi: 10.1007/s00066-014-0648-7 [DOI] [PubMed] [Google Scholar]
- 16. Sheehan JP, Grills I, Chiang VL, et al. Quality of life outcomes for brain metastasis patients treated with stereotactic radiosurgery: pre-procedural predictive factors from a prospective national registry. J Neurosurg. 2019;131:1848–1854. doi: 10.3171/2018.8.JNS181599 [DOI] [PubMed] [Google Scholar]
- 17. Bunevicius A, Lavezzo K, Shabo L, McClure J, Sheehan JP. Quality-of-life trajectories after stereotactic radiosurgery for brain metastases. J Neurosurg. 2020:1–9. doi: 10.3171/2020.4.jns20788 [DOI] [PubMed] [Google Scholar]
- 18. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallabanda M, García-Berrocal MI, Romero J, et al. Brain metastases treated with radiosurgery or hypofractionated stereotactic radiotherapy: outcomes and predictors of survival. Clin Transl Oncol. 2020;22(10):1809–1817. [DOI] [PubMed] [Google Scholar]
- 22. Mengue L, Bertaut A, Ngo Mbus L, et al. Brain metastases treated with hypofractionated stereotactic radiotherapy: 8 years experience after Cyberknife installation. Radiat Oncol. 2020;15(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas EM, Popple RA, Wu X, et al. Comparison of plan quality and delivery time between volumetric arc therapy (rapidarc) and gamma knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75(4):409–418. doi: 10.1227/NEU.0000000000000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen TK, Nguyen EK, Warner A, Louie AV, Palma DA. Failed randomized clinical trials in radiation oncology: what can we learn? Int J Radiat Oncol Biol Phys. 2018;101(5):1018–1024. [DOI] [PubMed] [Google Scholar]
- 25. Loo M, Pin Y, Thierry A, Clavier JB. Single-fraction radiosurgery versus fractionated stereotactic radiotherapy in patients with brain metastases: a comparative study. Clin Exp Metastasis. 2020;37(3):425–434. [DOI] [PubMed] [Google Scholar]
- 26. Koide Y, Tomita N, Adachi S, Tanaka H, Tachibana H, Kodaira T. Retrospective analysis of hypofractionated stereotactic radiotherapy for tumors larger than 2 cm. Nagoya J Med Sci. 2019;81(3):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]