Abstract
The effect of human-associated habitat degradation on tsetse populations is well established. However, more insights are needed into how gradual human encroachment into tsetse fly belts affect tsetse populations. This study investigated how wing vein length, wing fray categories, and hunger stages, taken as indicators of body size, age, and levels of access to hosts, respectively, in Glossina morsitans morsitans Westwood (Diptera: Glossinidae) and Glossina pallidipes Austen (Diptera: Glossinidae), varied along a transect from the edge into inner parts of the tsetse belt, in sites that had human settlement either concentrated at the edge of belt or evenly distributed along transect line, in north-eastern Zambia. Black-screen fly round and Epsilon traps were used in a cross-sectional survey on tsetse flies at three sites, following a transect line marked by a road running from the edge into the inner parts of the tsetse belt, per site. Two sites had human settlement concentrated at or close to the edge of the tsetse belt, whereas the third had human settlement evenly distributed along the transect line. Where settlements were concentrated at the edge of tsetse belt, increase in distance from the settlements was associated with increase in wing vein length and a reduction in the proportion of older, and hungry, tsetse flies. Increase in distance from human settlements was associated with improved tsetse well-being, likely due to increase in habitat quality due to decrease in effects of human activities.
Keywords: tsetse flies, Glossina morsitans morsitans, tsetse fly belt, settlements, human activities
Tsetse flies infest an estimated 8.7 million km2 and affect 38 countries in sub-Saharan Africa where they transmit human African trypanosomosis (HAT) (sleeping sickness) and animal African trypanosomiasis (AAT) (Kabayo 2002, Holmes 2013, Alsan 2015). The Eastern tsetse fly belt of Zambia, which is largely associated with the Luangwa Valley, is among the most affected areas in the country with regard to occurrence of both AAT and HAT (Anderson et al. 2011, Munangandu et al. 2012).
For a tsetse population to survive and sustain itself, it is critical that the flies are able to adequately reproduce, and have adequate access to blood meals, and to shelter from adverse climatic conditions (Leak 1999, Hargrove 2004). All stages of the tsetse life cycle are exclusively reliant on blood meals as the source of nourishment, indirectly through the female parent in the case of the larva and pupa stages (Hargrove 2004, English et al. 2016). This indicates the critical importance of availability and access to sources of blood meals, i.e., hosts and capacity of tsetse flies to seek and find these hosts (Hargrove 1999, Van den Bossche and Hargrove 1999, Hargrove 2001). Because tsetse flies have no capacity to internally regulate body temperature, they need to find shelter whenever the ambient temperatures are too high or too low (Leak 1999, Hargrove 2004). With regard to reproduction, it is vital for females to adequately provide for the nourishment of the larvae and to find larviposition sites that provide appropriate micro-environmental conditions for development of pupae to ensure successful pupation (Hargrove and Brady 1992).
Reduction in tsetse habitat quality diminishes the level of availability of the key environmental conditions necessary for the survival of tsetse flies, and entails increased prospects for occurrence of stress and consequently mortality in affected tsetse population (Reid et al. 2000, Schowalter 2012, Anderson et al. 2015). Occurrence of stress in tsetse flies could with time give rise to particular changes in the characteristics of tsetse population including, change in the body size of tsetse flies, change in the proportions of young and/or old flies, and to longer feeding intervals (i.e., a high proportion of tsetse flies staying hungry for periods much longer than normal; Hargrove and Brady 1992, Leak, 1999).
In large and continuous tsetse belts, particularly those associated with protected wildlife conservation areas, such as the Eastern tsetse belt in Zambia, reduction in tsetse habitat quality tends to manifest starting from the edges of the tsetse belt that constitutes the interface with human settlements and hence human activities (Anderson et al. 2015). As such, in the absence of a physical tsetse fly barrier, the edge (or limit) of a tsetse belt usually marks a zone of transition from favorable to unfavorable environmental conditions for tsetse flies (Robinson et al. 1997, Reid et al. 2000, Schowalter 2012).
Human settlements are associated with human activity, and human activity is a major factor in degradation of natural ecosystems (Reid et al. 2000). In this regard, the higher the human population density, the higher the level of human-associated ecosystem degradation tends to be (Reid et al. 2000, Sala et al. 2000, Anderson et al. 2015). In terrestrial ecosystems, such degradation largely takes the form of reduction in vegetation cover, particularly as a consequence of agriculture and other activities associated with human livelihoods (Mwanakasale and Songolo 2011, Timberlake and Chidumayo 2011). Humans also negatively affect tsetse habitat quality through their direct impact on availability of animals, by scaring away and/or killing wild animals that serve as sources of blood meals (Leak 1999). Thus, where tsetse flies exist, an increase in human population density and activity usually leads to a decline in the tsetse fly population density (Leak 1999, Reid et al. 2000, Ducheyne et al. 2009, Mwanakasale and Songolo 2011). Such observations have led to the argument that human-associated negative change in habitat quality could eventually result in disappearance of tsetse flies all together (Reid et al. 2000). However, human activity may also affect tsetse habitat quality favourably through keeping of livestock species such as cattle that serve as good hosts for tsetse flies (Van Den Bossche and Staak 1997). This indicates that tsetse habitat quality can be assessed based on the characteristics of the tsetse flies including wing body size, age, availability, and access to hosts.
In view of this background, our study investigated how the distribution of wing vein length (as indicator of body size), wing fray categories (as indicator of age), hunger stages (as indicator of level of relative availability of hosts or relative level of access to hosts), and ovarian age categories, in Glossina morsitans morsitans Westwood (Diptera: Glossinidae) and G. pallidipes, varied with increase in distance from the edge of the tsetse belt, and along a transect line, into the inner parts of the tsetse belt. This was undertaken in three sites that were devoid of cattle, two with the human population concentrated at or close to the edge of the tsetse belt, and the third with human population relatively evenly distributed along the transect line, in the eastern tsetse belt in north-eastern Zambia.
Materials and Methods
Study Areas and Design
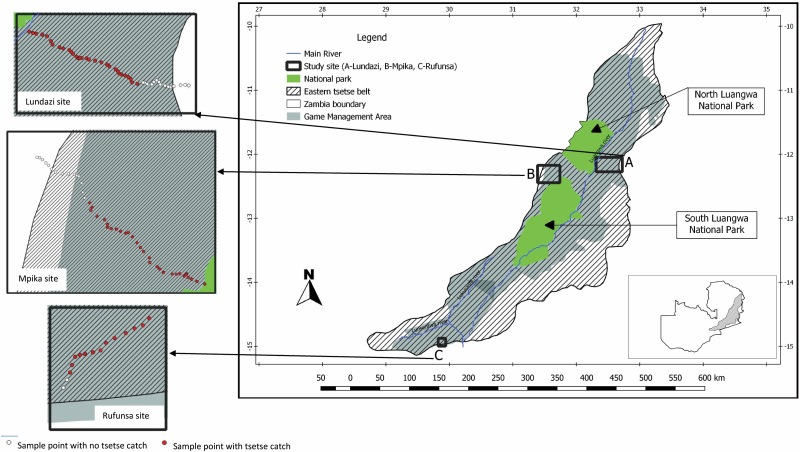
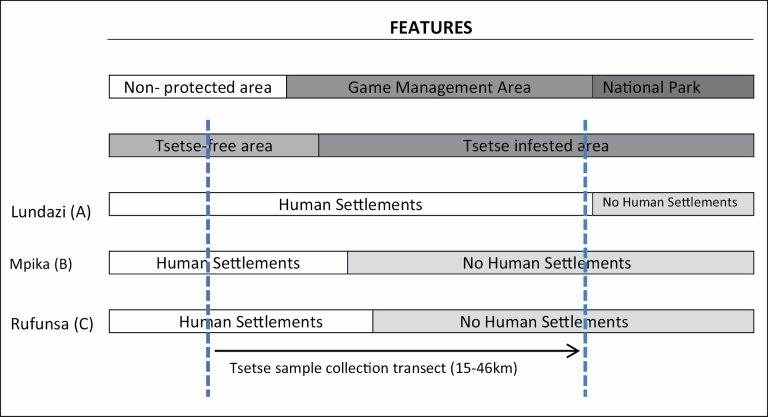
The study was undertaken in three sites located in Lundazi, Mpika, and Rufunsa districts, respectively (Fig. 1). The sites were selected based on absence of cattle, location and concentration of human settlements in relation to the edge of the tsetse belt, and presence of a road that ran from the edge into the inner parts of the tsetse belt, marking a transect line from the edge to a point located 15, 45, and 46 km inside the tsetse belt. With human settlements at and beyond the edge (outside) of the tsetse belt, the transect line (road) was taken to represent a potential gradient of increasing habitat quality, i.e., from the edge into the innermost part of the tsetse belt. In the Lundazi and Mpika sites, the last point on the transect line was located at the edge of the North Luangwa National Park (NLNP) and at the edge of the South Luangwa National Park (SLNP), respectively. In the Rufunsa site, the point that marked the end of the road, i.e., at the camp for wild life protection personnel (Wild life Scouts) in the area, well inside the Luano game management area (GMA), marked the last sample point in the site. In each site, the approximate edge of the tsetse belt was determined based on the maps by Evison and Kathuria (1982). From the approximate edge of the tsetse belt, the length of the transect line measured 15, 45, and 46 km in the Rufunsa, Mpika, and Lundazi sites, respectively (Figs. 2 and 3). The location of human settlements in relation to the edge of the tsetse belt is indicated in Fig. 2.
Fig. 1.
Location of the study sites and sample points in relation to the tsetse belt and wildlife conservation areas in north-eastern in Zambia.
Fig. 2.
Location of transect lines (for sample collection) in relation to location of the tsetse belt, human settlements and wildlife conservation areas, in the Lundazi, Mpika, and Rufunsa sites in north-eastern Zambia.
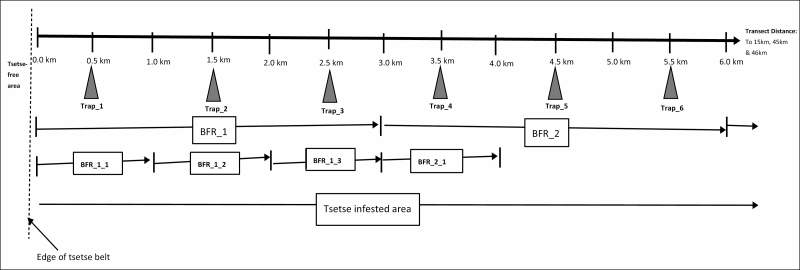
Fig. 3.
Relative location of Black-screen fly rounds (BFRs) and Epsilon traps in the transect line in the Lundazi, Mpika, and Rufunsa sites in north-eastern Zambia.
In all the sites, the transect line was such that the start point (at the edge of the tsetse belt) fell in location that was at the highest altitude (above sea level), and the last point was at the lowest altitude, i.e., in the transect line. Each of the transect lines ran from a point (at edge of tsetse belt) located either in the plateau area or in the transition zone from the plateau to the valley areas, to a location that fell in or close to the valley floor. The difference in altitude between the start point and end point was 1485 and 675 m in the Mpika site, 805 and 561 m in the Lundazi site, and 928 and 786 m in the Rufunsa site.
Among the criteria used to select the study sites was absence of a cattle population, and this was intended to eliminate presence of cattle as a factor in habitat quality in the respective sites, considering the importance of cattle (where they exist in a tsetse infested area) as hosts for tsetse flies (Van Den Bossche and Staak 1997). For each of the sites, information was collected from the district veterinary office on the distribution of cattle owning households in the veterinary camps that covered parts of the eastern tsetse belt in the district—i.e., through the veterinary assistants that manned the respective camps. It was this information, largely based on registers of cattle owners and their location (per veterinary camp), and in combination with the other criteria indicated above, that guided the selection of the particular sites. The map in Fig. 1 is created with application of QGIS (version 3.4 [Madeira]).
Collection of Tsetse Samples
Collection of samples of tsetse flies in each transect line was undertaken as indicated in Figs. 2 and 3. Two methods of tsetse sampling techniques, the Black-screen man fly round (BFR) and the Epsilon trap, were used and deployed as indicated in Fig. 3. The BFR was applied based on the method described by Potts (1930) except that it involved a pair of operators that carried a 1.5 × 1.0 m whole-black fabric screen, with the pole carried horizontally on their shoulders and the screen hanging from a pole between them and kept vertical by weighting with a second horizontal pole at the bottom. In addition, methyl ethyl ketone (Butanone) and 1-octen-3-ol (Octenol) were used as odour attractants, with the butanone dispensed through 500-ml brown bottles at about 200 mg/h, and the octanol dispensed through 250-µm thick polythene sachets with evaporation surface measuring 50 mm (length) × 50 mm (width) × 2 (sides) (Shereni 1984, Vale and Hall 1985, Willemse 1991). The length of each BFR and the relative location of the traps in the transect line were as indicated in Fig. 3. The pair of BFR operators stopped after every 100 m for about 2 min to capture any tsetse flies that alighted on the screen, on the ground, and on surrounding vegetation. Each BFR was walked two times during the day, in the morning (between 06:00 and 09:00) and in the afternoon (between 16:00 and 18:30), i.e., over a period of 2 to 3 h per BFR, considering that tsetse flies are most active (e.g., hunting for hosts) in the hours just before and/or just after sunrise, and just before and/or just after sunset, when the temperatures are usually ideal for them to do so, i.e., neither too high nor too low (Leak 1999). The start and end points of each BFR were marked in advance through brazing of marks on trees located on road-side, and so were points located every 500 m from the starting point, with use of a vehicle odometer. An Epsilon trap was deployed (set up) at a point falling that fell as indicated in Fig. 3, but was deployed (set up) at a point that was located 5 to 10 m away from the BFR sample point that was located in the middle of the road—i.e., the location that served as the sample point for both the BFR and the epsilon trap—the points for which GPS coordinates were recorded as such. In each trap site, the trap was deployed at least 2 days before the BFR was conducted in the particular sample point in the transect line—and a trap remained in each site for a period of about 20 h—i.e., from about 14:00 h on day 1 to about 10:00 h the following day (on day 2).
Samples were aggregated to ‘Sample points’ in the transect line each having covered a 1 km stretch of each BFR, and also to a ‘Section’ of the transect line that covered a stretch equivalent to one BFR (3 km) in the Rufunsa site (due to the shorter length of the transect line in the site), and two BFRs (6 km) in the Lundazi and Mpika sites—with the first ‘sample point’ and first ‘section’ located closest to the edge of the tsetse belt—and the last located furthest away from the edge of the tsetse belt.
Three BFRs were undertaken during any one sample collection session, i.e., with three pairs of operators (i.e., six operators). The same team of operators undertook deployment of Epsilon traps and collection of samples from the traps. Each pair of operators was led by a Tsetse Control Technician (TCT) or Tsetse Control Assistant (TCA) that had many years of experience with field application of the two tsetse survey methods. Two well trained technicians identified tsetse catches to species and sex, and undertook dissections to establish ovarian categories, on site. Examination of the samples for wing fray categories and for measurement of wing vein length was undertaken much later after the field sample collection assignments.
Examination of Tsetse Fly Samples
Measurement of wing vein length, examination/assessment for wing fray categories, hunger stages, and ovarian age categories were undertaken with application of the procedures described by the FAO (1982). In the procedures, 1) levels of wing fray (level of damage to the wings) falls in six (06) categories (i.e., categories 1 to 6)—with category 1 having the least damage and category 6 having the most damage to the wings; 2) hunger stages, based on the appearance of the abdomen, ranging from stage 1 (newly and fully fed tsetse flies) to stage 4 (hungry tsetse flies); and 3) ovarian categories, based on the state of each of the fours ovarioles in relation to release of ova, ranging from ovarian category 0 (newly emerged adult) to category 7 or higher for tsetse flies that are 70 to 80 d old or older—noting that this is the highest category that can be determined in a straight forward manner through the method (Leak 1999). Wing vein length, wing fray categories, and ovarian categories have been applied by several authors, e.g., Allsopp (1985) and Hargrove et al. (2019).
Data Analyses
The linear regression model was used to determine and measure association between wing vein length and increase in distance from the edge of the tsetse belt. The proportional ordinal logistic regression (POLR) model was applied to determine and measure association between wing fray categories, hunger stages, and ovarian age categories, and increase in distance from the edge of the tsetse belt. This was done in R version 3.6.2, and specifically with use of the MASS package in the case of the POLR (R Core Team 2016).
For each of the species of tsetse flies, the analyses were carried out on data for the categories ‘males & females’, ‘males only’, and ‘females only’. 95% confidence intervals were considered in all the estimates reported, and a P-value of less than or equal to 0.05 was considered statistically significant.
Results
Tsetse Fly Samples Collected
A total of 1,731 tsetse samples were collected, 1,279 G. m. morsitans (846 males and 433 females), and 452 Glossina pallidipes Austen (Diptera: Glossinidae) (206 males and 246 female). In each of the transect lines, the first tsetse fly was caught at a distance of 2.5, 14.5, and 18.5 km from start point of the transect line (i.e., from the estimated edge of the tsetse belt) in the Rufunsa, Lundazi, and Mpika sites, respectively. The distribution of the two species and sexes of the tsetse flies in relation to the two sample collection methods, study sites, and sections of transect lines is shown in Table 1. As shown in the table, the first two sections of the transect lines, in all the sites, recorded none or very few G. pallidipes. Most of the tsetse flies, particularly male G.m. morsitans, were captured in the BFRs. In the last two sections, Epsilon traps captured relatively more G. pallidipes than did BFRs. Tsetse sample collection was undertaken over a period of 2 wk in the Rufunsa site and 3 wk per site in the Lundazi and Mpika sites, during the period September to December 2012. Samples collected were made up of two species, G. m. morsitans and G. pallidipes.
Table 1.
No. of samples of tsetse flies collected per section of transect distance in the Lundazi, Mpika, and Rufunsa sites in north-eastern Zambia
| Site/Section | Number of tsetse fly samples—Black-screen Fly rounds | Total | Number of tsetse fly samples—Epsilon Traps | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gmm_M | Gmm_F | Gmm_F_Ova | Gp_M | Gp_F | Gp_F_Ova | Gmm_M | Gmm_F | Gmm_F_Ova | Gp_M | Gp_F | Gp_F_Ova | |||
| Lundazi | ||||||||||||||
| Section 1 | 19 | 11 | 7 | 0 | 0 | 0 | 30 | 6 | 9 | 5 | 0 | 0 | 0 | 15 |
| Section 2 | 45 | 16 | 9 | 2 | 1 | 1 | 64 | 15 | 12 | 6 | 4 | 6 | 3 | 37 |
| Section 3 | 26 | 9 | 4 | 5 | 3 | 2 | 43 | 11 | 10 | 8 | 3 | 8 | 3 | 32 |
| Section 4 | 21 | 14 | 6 | 9 | 5 | 4 | 49 | 5 | 18 | 8 | 9 | 16 | 10 | 48 |
| Section 5 | 46 | 20 | 12 | 8 | 7 | 4 | 81 | 21 | 10 | 4 | 7 | 18 | 13 | 56 |
| Total | 157 | 70 | 38 | 24 | 16 | 11 | 267 | 58 | 59 | 31 | 23 | 48 | 29 | 188 |
| Mpika | ||||||||||||||
| Section 1 | 28 | 10 | 8 | 0 | 0 | 0 | 38 | 9 | 7 | 4 | 0 | 0 | 0 | 16 |
| Section 2 | 65 | 22 | 15 | 0 | 0 | 0 | 87 | 14 | 17 | 12 | 0 | 0 | 0 | 31 |
| Section 3 | 49 | 18 | 11 | 8 | 7 | 5 | 82 | 17 | 15 | 11 | 11 | 18 | 10 | 61 |
| Section 4 | 104 | 42 | 28 | 35 | 23 | 14 | 204 | 39 | 31 | 8 | 30 | 41 | 24 | 141 |
| Total | 246 | 92 | 62 | 43 | 30 | 20 | 411 | 79 | 70 | 35 | 41 | 59 | 34 | 249 |
| Rufunsa* | ||||||||||||||
| Section 1 | 59 | 18 | 0 | 1 | 1 | 0 | 79 | 15 | 11 | 0 | 2 | 1 | 0 | 29 |
| Section 2 | 25 | 10 | 0 | 1 | 3 | 0 | 39 | 9 | 13 | 0 | 2 | 6 | 0 | 30 |
| Section 3 | 51 | 22 | 0 | 6 | 9 | 0 | 88 | 21 | 16 | 0 | 14 | 21 | 0 | 72 |
| Section 4 | 91 | 25 | 0 | 28 | 18 | 0 | 162 | 35 | 27 | 0 | 21 | 34 | 0 | 117 |
| Total | 226 | 75 | 0 | 36 | 31 | 0 | 368 | 80 | 67 | 0 | 39 | 62 | 0 | 248 |
Gmm—Glossina morsitans morsitans; Gp—Glossina pallidipes; M—male; F—female; Ova—Ovarian dissection.
*Ovarian dissections not done in the site.
Wing Vein Length
The results are shown in Supp Fig. 1A and D (online only) and in Tables 2 and 3. In the case of G. m. morsitans in the Lundazi and Rufunsa sites, an increase in distance by 1 km was associated with an increase in wing vein length by 0.003 (0.002–0.005; P < 0.0001) and by 0.005 (0.002−0.007; P < 0.0001) in ‘males + females’; by 0.004 (0.002–0.005; P < 0.0001) and by 0.005 (0.003–0.007; P < 0.0001) in ‘males only’; and by 0.004 (0.002–0.005; P < 0.0001) and by 0.005 (0.003–0.007; P < 0.0001) in ‘females only’, in the two sites, respectively. No association between wing vein length in G. m. morsitans and increase in distance was observed in the Lundazi site. In the Rufunsa sites, we observed an association between increase in distance and wing vein length, with 1km increase in distance associated with a 0.007 (0.001–0.014) increase in wing vein length in ‘males + females’ (P = 0.013) for G. pallidipes.
Table 2.
Variation of wing vein length with increase in distance away from the edge of the tsetse belt in samples of Glossina morsitans morsitans and Glossina pallidipes in the Lundazi, Mpika, and Rufunsa sites in north-eastern Zambia
| Lundazi | Mpika | Rufunsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G. m. morsitans | G. pallidipes | G. m. morsitans | G. pallidipes | G. m. Morsitans | G. pallidipes | |||||||
| Males+ Females |
Males only |
Females only |
Males+ Females |
Males+ Females |
Males only |
Females only |
Males+ Females |
Males+ Females |
Males Only |
Females only | Males+ Females |
|
| Coefficient | 0.0005 | 0.001 | 0.0003 | 0.002 | 0.003 | 0.004 | 0.002 | 0.003 | 0.005 | 0.005 | 0.006 | 0.007 |
| Confidence interval | – | – | – | – | 0.002–0.004 | 0.002–0.005 | 0.0005–0.004 | – | 0.002–0.007 | 0.003–0.007 | 0.002–0.01 | 0.001–0.014 |
| P-value | 0.283 | 0.086 | 0.669 | 0.133 | < 0.0001 | < 0.0001 | 0.010 | 0.167 | < 0.0001 | < 0.0001 | 0.004 | 0.013 |
Table 3.
Results (PORL regression test): Distribution of wing fray categories, hunger stages and ovarian categories in tsetse flies in relation to increase in distance away from the edge of the tsetse belt in the Lundazi, Mpika, and Rufunsa sites in north-eastern Zambia
| Site | Wing fray categories | Hunger stages | Ovarian categories | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G. m. morsitans | G. pallidipes | G. m. morsitans | G. pallidipes | G. m. morsitans | G. pallidipes | |||||
| Males+ Females | Males only |
Females only | Males+ Females | Males+ Females |
Males only |
Females only | Males+ Females | Females | Females | |
| Lundazi site | ||||||||||
| Coefficient | −0.005 | −0.005 | 0.003 | −0.031 | −0.015 | −0.008 | −0.009 | −0.033 | −0.004 | −0.014 |
| P-value | 0.648 | 0.738 | 0.876 | 0.249 | 0.166 | 0.526 | 0.526 | 0.231 | 0.880 | 0.767 |
| Mpika site | ||||||||||
| Coefficient | −0.042 | −0.048 | −0.054 | −0.038 | −0.056 | −0.056 | −0.042 | −0.034 | −0.039 | −0.074 |
| Odds Ratio | 0.959 | 0.954 | 0.947 | – | 0.946 | 0.952 | 0.959 | – | – | – |
| Confidence Interval | 0.939–0.980 | 0.928–0.979 | 0.913–0.982 | – | 0.925–0.966 | 0.928−0.978 | 0.924–0.996 | – | – | * |
| P-value | < 0.001* | < 0.001* | 0.003* | 0.260 | < 0.0001* | < 0.0001* | 0.028* | 0.303 | 0.117 | 0.162 |
| Rufunsa site† | ||||||||||
| Coefficient | −0.088 | −0.084 | −0.073 | −0.094 | −0.097 | −0.082 | −0.112 | −0.096 | – | |
| Odds Ratio | 0.915 | 0.919 | 0.930 | – | 0.907 | 0.922 | 0.894 | – | – | |
| Confidence Interval | 0.873–0.959 | 0.870–0.972 | 0.815−0.980 | – | 0.866–0.951 | 0.872–0.973 | 0.815–0.980 | – | – | |
| P-value | < 0.001* | 0.003* | 0.011* | 0.118 | < 0.0001* | 0.004* | 0.016* | 0.114 | ||
*Significant result.
†No dissection for ovarian categories undertaken in the site.
Wing Fray Categories and Hunger Stages
The outcomes were as shown in Supp Fig. 1B and 1C (online only), and Tables 2 and 3.
In the case of G. m. morsitans in the Rufunsa and Mpika sites, an increase in the distance was associated with a reduction in the likelihood of having an increase, and not a reduction, in wing fray categories and hunger stages, as follows.
Wing Fray Categories
In ‘males + females’, an 1 km increase in distance was associated with a 4.1% (2–6%) and 8.5% (4.1–12.7%) reduction in the likelihood of an increase, against a reduction, in wing fray categories in tsetse flies caught, in the Mpika and Rufunsa sites, respectively. Similarly for ‘males only’, the increase in distance was associated with a 4.6% (2.1–7.2%) and 8.1% (2.8–13.0%) reduction in ‘males only’, and a 5.3% (1.8–8.7%) and 7.0% (2.0–18.5%) reduction in the likelihood of an increase, as opposed to reduction, in wing fray category in ‘females only’ in tsetse flies caught, in the two sites, respectively.
Hunger Stages
An increase in distance by 1 km was associated with a reduction in the likelihood of finding tsetse flies with high levels of hunger stage, and not low levels of hunger stage, by 5.4% (3.4–7.5%) and 9.3% (4.9–13.4%) in ‘male + females’; by 4.8% (2.2–7.2%) and 7.8% (2.7–12.8%) in ‘males only’; and by 4.1% (0.4–7.6%) and 10.6% (2.0–18.5%) in ‘females only’, in the two sites, respectively.
For G. pallidipes, in all the three sites, there was no association between increase in distance from the edge of the tsetse belt, and wing fray categories and hunger stages. Similarly, with regard to ovarian categories in both species, the model indicated no relationship with increase in distance from from the edge of the tsetse belt.
Results suggested that in the Mpika and Rufunsa sites, and for G. m. morsitans tsetse flies, increase in distance from the edge into the inner parts of the tsetse belt was associated with an increase in the proportion of flies with larger body size, and with reduction in the proportion of older flies (and as such an increase in the proportion of younger flies). The results further suggested that increase in this distance was associated with reduction in the proportion of hungry flies, in turn suggesting an increase in the proportion of nonhungry flies of the species. In the Lundazi site, the results suggested that there was no relationship between increase in the distance indicated, and the distribution of wing vein length, wing fray categories, and hunger stages. Similarly, in the case on in the three sites, the outcomes suggested nonexistence of a relation between increase in the particular distance and the distribution of wing vein length, wing fray categories, and hunger stages in the species.
Discussion
This study indicated that in the Mpika and Rufunsa sites, increase in distance away from the human settlements was associated with an increase in the proportion of larger tsetse flies, reduction in the proportion of older flies, and reduction in the proportion of hungry tsetse flies. This was in agreement with observations that have been made in the past on effects of habitat degradation on the short- and long-term well-being and age structure of tsetse populations (FAO 1982, Hargrove 1994, Leak 1999). No association was observed between increase in this distance and the distribution of wing vein length, wing fray categories, and hunger stages in G. m. morsitans in the Lundazi site, where human settlement was relatively evenly distributed from the edge to the innermost parts of the tsetse belt. This further suggested that the nature of the distribution of human settlements had influence on the distribution of wing vein length, wing fray category, and hunger state in G. m. morsitans, in the three sites, and also that of wing vein length in G. pallidipes in the Rufunsa site.
In sampling tsetse populations, as applied in this study, the number of tsetse flies caught per unit effort, e.g., per fly round and/or per trap per day, and the characteristics of the samples collected are affected to varying degrees by the type of sampling method (Leak, 1999). This is largely due to variation in the nature of response of different categories of tsetse flies (e.g., species, sex, and age) to particular sampling devices or methods (Vale and Phelps 1978). In this regard, literally all available tsetse sampling tools or methods have some level of biasness (Vale 1974, Vale and Phelps 1978, Vale and Hall 1985, Leak 1999). With use of the two methods having been implemented by the same field teams in each transect line, it may be assumed that the biasness or systematic error of each of the methods and/or devices was consistent (constant) throughout the length of each transect lines such that any variation in the numbers caught per sample point and the characteristics of the samples collected, with increase in distance along each of the transect lines, may be attributed to other factors and not to the biasness and/or weaknesses of the sampling methods or devices.
In the same vein, temporal variation in weather conditions over a 3-wk period within the month of September in the Mpika site, within the month of October in the Lundazi site, and over a period of 2 wk within the month of November in the Rufunsa site was unlikely to have affected the outcome of the study—considering that comparison of wing vein, wing fray categories, and hunger stages in the tsetse fly samples was made among sample points in the transect line per site and not among the sites. Considering that section 1 in the Lundazi site, and sections 1 and 2 in the Mpika, did not record any catch of G. pallidipes, and the number of flies of the species recorded was relatively low per section and per transect, it follows that this was likely to have negatively affected the analyses. This could in turn explain the inability of the model to detect any relationship between increase in distance (from age of tsetse belt) and variation in wing vein length, wing fray categories and hunger stages (except in the case of the Rufunsa site) among samples of G. pallidipes in the study. In the Rufunsa site, the number of G. pallidipes samples collected was relatively higher than in the Lundazi and Mpika sites, thus giving test results that were similar to that obtained in the case of G. m. morsitans samples, where the number of samples of the species was relatively higher than in the other two sites—i.e., where the results were similar to those obtained for G. m. morsitans
Tsetse-infested areas that are least exposed to human activity are known to be subjected to the least levels of tsetse fly habitat deterioration, and the rate of decline in habitat quality tends to be dependent on the nature and pattern of manifestation of the human-associated factors involved (Reid et al. 2000, Schowalter 2012, Anderson et al. 2015, Matawa et al. 2019). Considering the difference in the relative proximity to the human settlements, among the sections of the transect in the Mpika and Rufunsa sites, this link between of human activities and degradation of tsetse habitat quality could explain the observed relationship between increase in distance away from the human settlements, and distribution of body size, age, and hunger state in G. m. morsitans, in the two sites. In a study undertaken in parts of the eastern tsetse fly belt in Zambia by Ducheyne et al. (2009), tsetse apparent density was highest in areas that had low levels of human-associated habitat fragmentation, and was lowest where the level of such habitat fragmentation was high. This indicates that increase in human activities in tsetse-infested areas, usually associated with proximity to human settlements, give rise to a reduction in tsetse fly population density, and this is usually a consequence of reduction in tsetse habitat quality (Reid et al. 2000, Ducheyne et al. 2009). This association between human activity, habitat quality, and tsetse population density could explain the relatively lower numbers of tsetse flies captured in the first section of the transect lines (located closest to human settlements) and also the observed distribution of body size, age, and hunger state in G. m. morsitans in the study.
As indicated earlier, the well-being and survival of tsetse flies is dependent on habitat quality with regard to adequate availability of key needs, mainly, suitable climatic conditions, vegetation cover for shelter during adverse weather conditions, and adequate access to sources of blood meals (Hargrove 1988, Hargrove 2004). Thus, suboptimal levels of these requirements, or their elimination, in a tsetse-infested area, will diminish the well-being of tsetse flies, thereby giving rise to increased levels of occurrence of stress and density independent mortality in the affected tsetse population (Hargrove 2004, Van den Bossche et al. 2010). Considering that higher levels of effects of human activity are associated with higher levels of degradation of tsetse habitat (Reid et al. 2000, Ducheyne et al. 2009), it may be taken that in the Mpika and Rufunsa sites, the level of habitat degradation was highest in locations that were closest to the human settlements, and lowest in locations that were furthest away from the human settlements—thus indicating a higher prospect of exposure to stress for tsetse flies that inhabited locations closest to the human settlements, than in tsetse flies that inhabited locations that were further away from the human settlements.
Occurrence of stress in tsetse flies is associated with exposure to conditions that are below the optimum requirements for the tsetse flies, particular with regard to detrimental exposure to unfavorable weather conditions such as significant reduction or disappearance of vegetation cover and lack of access to blood meals as a consequence of human activities (Leak 1999, Hargrove 2004). Nutrition in adult tsetse flies is directly dependent on access to sources of blood meals (i.e., hosts) such that a significant reduction in the supply of hosts in an area entails diminished prospects for tsetse flies to find hosts, resulting in starvation associated stress in affected tsetse flies (Van den Bossche and Hargrove 1999, Hargrove 2001). Occurrence of stress has the potential to result in serious negative implications for the survival, distribution, reproduction, and vectorial capacity of the individual tsetse fly (Reid et al. 2000, Hargrove 2004, Akoda et al. 2009).
Nutrition (supply of energy fat reserves) and general well-being of the nonadult stages in the life cycle (i.e., the larva and pupa stages), and as such that of the teneral stage of the adult, is wholly dependent on the nutrition and well-being of the female parent, and as such occurrence of nutritional or any other kind of stress in the female parent has serious negative implications for the survival and fitness of these particular stages in the life cycle of the tsetse fly (Leak 1999, Hargrove 2004, English et al. 2016). For this reason, diminished levels of nourishment in females entails diminished nourishment for the larva and pupa stages, resulting in newly emerged adults that are smaller in body size and with diminished levels of fat reserves—and thus with reduced prospects for survival. Consequently, an adult tsetse fly is most vulnerable to stress, and hence to mortality, during the period before accessing its first bloodmeal, due to low fat reserves and underdeveloped wing muscles, resulting in limited capacity for flight to find hosts and/or to escape predators. This indicates that adverse deterioration in tsetse habitat quality tends to have the most negative impact (particularly in terms of mortality) on newly emerged teneral adults (Van den Bossche and Hargrove 1999, Hargrove et al. 2018). The size of individual adult tsetse flies is determined at the time of emergence from the pupal stage, i.e., it does not change thereafter, and as such body size in tsetse flies provides an indirect measure of levels of exposure to factors such as nutritional stress and mortality in the parent and/or earlier generations of a tsetse population (Leak 1999, Van den Bossche and Hargrove 1999). Against this background, it may be taken that variation in body size, age structure, and levels of access to hosts, in a tsetse population, could serve as an indicator of variation in levels of exposure to unfavourable environmental conditions in the existing population and, in the parent and/or earlier generations of the tsetse population (Leak 1999, Van den Bossche and Hargrove 1999, Reid et al. 2000, English et al. 2016).
When habitat degradation diminishes availability of sources of blood meals, tsetse flies are deprived of their bloodmeal sources due to reduced tsetse-host contact and hence an increase in feeding intervals (Hargrove and Brady 1992, Loder 1997, Leak 1999). This could explain the higher likelihood in this study of finding hungry G. m. morsitans flies in locations that were close to the edge of the tsetse belt, and hence within or close to human settlements, than in locations that were far away inside the tsetse belt, in the Mpika and Rufunsa sites. This could similarly explain the relatively larger proportion of smaller sized and older G. m. morsitans flies in locations that were close to human settlements (at or close to the edge of tsetse belt) than in locations that were located further away inside the tsetse belt. Such outcomes, as observed in the locations that fell within and/or close to the edge of the tsetse belt, and also within or close to human settlements, may be associated with occurrence of stress in one or more of the parent generations of the tsetse flies (Hargrove 1999, Leak 1999, Van den Bossche and Hargrove 1999).
Areas with similar environmental characteristics in the context of environmental conditions, and hence habitat quality, tend to offer similar prospects for existence, and hence for survival of tsetse flies, and this forms the basis for models that have been applied to predict tsetse presence and distribution (Robinson et al. 1997, Rogers 2000, Cecchi et al. 2008). In the study area, increase in distance away from the edge (into the inner parts of the tsetse belt) also entailed transition from the high altitude locations (plateau areas) towards the low lying (low altitude) locations in the Luangwa valley (in the Lundazi and Mpika sites) and in the Luano valley (in the Rufunsa site). Altitude affects tsetse well-being and hence distribution indirectly, through changes on climatic factors (Leak 1999). The difference in altitude, in the study area, is associated with an increase in mean temperatures and associated change in climatic conditions and related agro-ecological conditions that in turn influence the distribution of human settlements and the nature of human activities (Reid et al. 2000; Anderson et al, 2015). Thus, similar kinds and levels of human activity tend to have similar impact on the environment (Reid et al. 2000, Tilman 2001, Schowalter 2012), and this could relate to the situation that was obtaining in the Lundazi site (in the study) where human settlements were distributed relatively evenly from the edge to the innermost section of the transect line. As such, it may be taken that in the particular site, the effects of the human settlement were likely to have also been evenly distributed along the transect line.
Although it had been envisaged (in the study) to have dissections for ovarian age categories (ovarian ageing) undertaken in each of the study sites, this could not be undertaken in the Rufunsa site due to budgetary constraints at the time of sample collection in the area. Furthermore, owing to the challenge of keeping tsetse samples adequately fresh from the time of collection (in the BFRs and in the traps) to the camp site for such dissections to be performed, only a relatively small proportion of females were dissected for ovarian categories (ovarian aging). This entailed that where tsetse dissection did get done (in the Rufunsa and Mpika sites), a relatively large proportion of the sample points had no female flies dissected. Similarly, samples of G. pallidipes were considerably few and with most collected far inside the tsetse belt, in comparison to samples of G. m. morsitans. These factors could explain the inability of the model to detect associations with regard to ovarian categories and G. pallidipes.
Conclusion
We conclude that in the Mpika and Rufunsa study sites in the eastern tsetse belt in north-eastern Zambia, an increase in distance from the edge into the inner parts of the tsetse belt was associated with an increase in the proportion of bigger sized flies and reduction in the proportion of old flies and that of hungry flies, in G. m. morsitans. The findings suggested the existence of a gradient of increasing tsetse habitat quality along the transect line from the edge into the inner parts of the tsetse belt. Considering that human settlement was concentrated at and close to the edge of the tsetse belt in the two sites, this in turn suggested that such a gradient could have been due to the existence of a gradient of reducing levels of human-associated tsetse habitat degradation, in each of the two sites. The findings could potentially have implications on prospects for shrinking of the tsetse belt and also on the distribution of the risk of transmission of trypanosomiasis, in sites/areas of this kind.
Supplementary Material
Acknowledgments
Funding for the field sample and data collection work was provided by the Ministry of Fisheries and Livestock, through the Department of Veterinary Services of Zambia – and this manuscript is submitted with the permission of the Director of Veterinary Services.
References Cited
- Akoda, K, Van den Bossche P, Marcotty T, Kubi C, Coosemans M, De Deken R, and Van den Atbbeele J. 2009. Nutritional stress affects the tsetse fly’s immune gene expression. Med. Vet. Entomol. 23: 195–201. [DOI] [PubMed] [Google Scholar]
- Alsan, M. 2015. The effect of the tsetse fly on african development. Am. Econ. Rev. 105: 382–410. doi: 10.1257/aer.20130604. [DOI] [Google Scholar]
- Allsopp, R. 1985. Variation in the rates of increase of Glossina morsitans centralis and their relevance to control. J. Appl. Ecol. 22: 91–104. [Google Scholar]
- Anderson, N E, Mubanga J, Machila N, Atkinson P M, Dzingirai V, and Welburn S C. 2015. Sleeping sickness and its relationship with development and biodiversity conservation in the Luangwa Valley, Zambia. Parasit. Vectors. 8: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi, G, Mattioli R C, Slingenbergh J, and de la Rocque S. 2008. Land cover and tsetse fly distributions in sub-Saharan Africa. Med. Vet. Entomol. 22: 364–373. [DOI] [PubMed] [Google Scholar]
- Ducheyne, E, Mweempwa C, De Pus C, Vernieuwe H, De Deken R, Hendrickx G, and Van den Bossche P. 2009. The impact of habitat fragmentation on tsetse abundance on the plateau of eastern Zambia. Prev. Vet. Med. 91: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English, S, Cowen H, Garnett E and Hargrove J W. 2016. Maternal effects on offspring size in a natural population of the viviparous tsetse fly. Ecol. Entomol. 41: 618–626. [Google Scholar]
- Evison, G and Kathuria K D S. 1982. A Survey of the distribution of Glossina spp. and factors influencing their control in the territory of Northern Rhodesia (Zambia). Department of Veterinary and Tsetse Control Services, Zambia. [Google Scholar]
- FAO . 1982. Training manual for tsetse control personnel: Tsetse biology, systematics and distribution, techniques, vol. 1, pp. 280. Food and Agriculture Organisation of the United Nations, Rome. [Google Scholar]
- Hargrove, J W. 1994. Reproductive rates of tsetse flies in the field in Zimbabwe. Physiol. Entomol. 19: 307–318. [Google Scholar]
- Hargrove, J W. 1988. Tsetse: the limits to population growth. Med. Vet. Entomol. 2: 203–217. [DOI] [PubMed] [Google Scholar]
- Hargrove, J W. 1999. Lifetime changes in the nutritional characteristics of female tsetse Glossina pallidipes caught in odour-baited traps. Med. Vet. Entomol. 13: 165–176. [DOI] [PubMed] [Google Scholar]
- Hargrove, J W. 2001. Factors affecting density-independent survival of an island population of tsetse flies in Zimbabwe. Entomol. Exp. Appl. 100: 151–164. [Google Scholar]
- Hargrove, J W. 2004. Tsetse population dynamics. InMaudlin I, Holmes, P and Miles M A (eds). The Trypanosomiases. CABI Publishing. 113–137. [Google Scholar]
- Hargrove, J W and Brady J. 1992. Activity rhythms of tsetse flies (Glossina spp.) (Diptera: Glossinidae) at low and high temperatures in nature. Bull. Entomol. Res. 82: 321–326. [Google Scholar]
- Hargrove, J W, Muzari M O, and English S. 2018. How maternal investment varies with environmental factors and the age and physiological state of wild tsetse Glossinapallidipes and Glossina morsitans morsitans. R. Soc. Open Sci. 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove, J, English S, Torr S J, Lord J, Haines L R, van Schalkwyk C, Patterson J, and Vale G. 2019. Wing length and host location in tsetse (Glossina spp.): implications for control using stationary baits. Parasit. Vectors. 12: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, P. 2013. Tsetse-transmitted trypanosomes–their biology, disease impact and control. J. Invertebr. Pathol. 112 Suppl: S11–S14. [DOI] [PubMed] [Google Scholar]
- Kabayo, J P. 2002. Aiming to eliminate tsetse from Africa. Trends Parasitol. 11: 473–475. [DOI] [PubMed] [Google Scholar]
- Leak, S G. 1999. Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosomosis. London: CABI Publishing. [Google Scholar]
- Loder, P M J. 1997. Size of blood meals taken by tsetse flies (Glossina spp.) (Diptera: Glossinidae) correlates with fat reserves. Bull. Entomol. Res. 87: 547–549. [Google Scholar]
- Matawa, F, Murwira A, and Atkinson P M. 2019. Evaluating the impact of declining tsetse fly (Glossina pallidipes) habitat in the Zambezi valley of Zimbabwe. Geocarto Int. 35(12): 1373–1384. doi: 10.1080/10106049.2019.1576780. [DOI] [Google Scholar]
- Munangandu, H M, Siamudala V, Munyeme M and Nalubamba K S. 2012. A review of ecological factors associated with the epidemiology of wildlife trypanosomiasis in the Luangwa and Zambezi valley ecosystems of Zambia. Interdiscip. Perspect. Infect. Dis. 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwanakasale, V, and Songolo P. 2011. Disappearance of some human African trypanosomiasis transmission foci in Zambia in the absence of a tsetse fly and trypanosomiasis control program over a period of forty years. Trans. R. Soc. Trop. Med. Hyg. 105: 167–172. [DOI] [PubMed] [Google Scholar]
- Potts, W H. 1930. A contribution to the study of numbers of tsetse flies (Glossina morsitans Westwood by quantitative methods. S. Afr. J. Sci. 27: 491–497. [Google Scholar]
- R Core Team . (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Reid, R S, Kruska R L, Deichmann U, Thornton P K and Leak S G A. 2000. Human population growth and the extinction of the tsetse fly. Agric. Ecosyst. Environ. 77: 227–236. [Google Scholar]
- Robinson, T, Rogers D, and Williams B. 1997. Mapping tsetse habitat suitability in the common fly belt of southern Africa using multivariate analysis of climate and remotely sensed vegetation data. Med. Vet. Entomol. 11: 235–245. [DOI] [PubMed] [Google Scholar]
- Rogers, D J. 2000. Satellites, space, time and the African trypanosomiases. Adv. Parasitol. 47: 129–171. doi: 10.1016/S0065-308X(00)47008-9. [DOI] [PubMed] [Google Scholar]
- Sala, O E, F SChapin, 3rd, Armesto J J, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke L F, Jackson R B, Kinzig A, . et al. 2000. Global biodiversity scenarios for the year 2100. Science. 287: 1770–1774. [DOI] [PubMed] [Google Scholar]
- Schowalter, T D. 2012. Insect responses to major landscape-level disturbance. Annu. Rev. Entomol. 57: 1–20. [DOI] [PubMed] [Google Scholar]
- Shereni, W. 1984. The use of cloth screens and acetone vapour as alternatives to a bait-ox. for sampling populations of tsetse flies (Diptera: Glossinidae). Trans. Zimbabwe Sci. Assoc. 62(1984):22–27. [Google Scholar]
- Tilman, D. 2001. Forecasting agriculturally driven global environmental change. Science. 292:281–284. [DOI] [PubMed] [Google Scholar]
- Timberlake, J and Chidumayo E. 2011. Miombo ecoregion vision report, vol. 20, pp. 1–76. Biodiversity Foundation for Africa, Famona, Bulawayo, Zimbabwe. [Google Scholar]
- Vale, G A. 1974. The responses of tsetse flies (Diptera, Glossinidae) to mobile and stationary baits. Bull. Entomol. Res. 64: 545–588. [Google Scholar]
- Vale, G A and Hall D R. 1985. The use of 1-octen-3-ol, acetone and carbon dioxide to improve baits for tsetse flies, Glossina spp. (Diptera: Glossinidae). Bull. Entomol.Res. 75: 219–232. [Google Scholar]
- Vale, G A and Phelps R J. 1978. Sampling problems with tsetse flies (Diptera: Glossinidae)’, J. Appl. Ecol. 15: 715. [Google Scholar]
- Van den Bossche, P and Hargrove J W. 1999. Seasonal variation in nutritional levels of male tsetse flies Glossina morsitans morsitans (Diptera: Glossinidae) caught using fly-rounds and electric screens. Bull. Entomol. Res. 89: 381–387. [Google Scholar]
- Van den Bossche, P, De la Rocgue S, Hendrickx G, and Bouyer J. 2010. A changing environment and the epidemiology of tsetse-transmitted livestock trypanosomiasis. Trends Parasitol. 21(4): 236–243. doi: 10.1016/j.pt.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Van den Bossche, P, and Staak C. 1997. The importance of cattle as a food source for Glossina morsitans morsitans Katete district, Eastern Province, Zambia. Acta Trop. 65: 105–109. [DOI] [PubMed] [Google Scholar]
- Willemse, L. 1991. A trial of odour baited targets to control the tsetse fly, Glossina morsitans centralis (Diptera: Glossinidae) in west Zambia. Bull. Entomol. Res. 81(3): 351–357. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.