Abstract
Social play consists of reciprocal physical interactions between conspecifics with many features conserved across species, including the propensity for males to engage in play more frequently and with higher physical intensity. Animal models, such as the laboratory rat, reveal that the underlying neural circuitry of play is subject to sexual differentiation during a critical period early in life. In this review, we discuss the developmental processes that produce distinct neural nodes which modulate both shared and sex-specific aspects of play with a focus on the medial amygdala, lateral septum, and prefrontal cortex. While the cellular mechanisms determining sex differences in play are beginning to be uncovered, the ultimate advantages of play continue to be debated.
Keywords: sexual differentiation, amygdala, prefrontal cortex, lateral septum, brain development, social behavior
Introduction
The playfulness of young animals, including children, is a fundamental and unique behavior filled with paradox. Largely limited to the narrow slice of life between weaning and reproductive maturity, play serves no obvious purpose, yet there are clear rules of engagement, patterns of reciprocity, and elements of reward. The consensus is that play is essential to the development of adult social behavior, as well as cognition and reproductive success (Blake & McCoy, 2015; Pellis et al., 2019; see also Stark & Pellis in this Special Issue), but why this is so remains a mystery. There are 3 recognized types of play: 1) locomotor play (also known as solitary play) by a lone individual involving jumps, kicks, etc., 2) object play such as carrying, flipping or flinging an inanimate object, and 3) social play between two or more similar-aged conspecifics, which includes chasing, wrestling, and play fighting, and sometimes called “rough and tumble” play or “play fighting” (Bekhoff & Byers, 1981; Martin & Caro, 1985). Each form of play is often presumed to have its roots in some other more “adaptive” behavior. Locomotor play can be considered rehearsal for escaping predators, while object play is the same for hunting. Rough and tumble play is akin to conspecific aggression or, conversely, to mating (Fagen, 1981; Wilson & Kleiman, 1974). But establishing the connections between play and later-life behavior—beyond the simple fact that preventing play impairs normal development—has as yet been elusive.
One fascinating feature of rough and tumble play is the presence of a reliable sex difference: males play more frequently and more intensely than females. This is interesting for several reasons. First, the sex difference is observable in many mammalian species ranging from rats, cats, and dogs, to humans, indicating that this sex difference in play (and play itself) is highly evolutionarily conserved, speaking to its importance (see Table 1). Second, the sex difference in behavior manifests during the stage of life when sexual differentiation of the brain is complete, but before the pubertal increase in circulating steroid hormones. This timing allows for targeted study of how early-life developmental processes establish sex differences in brain architecture which then manifest as differences in social behavior, without the confounds of sex-specific hormones later in life. Finally, the sex difference is manipulatable, providing an experimental wedge for mechanistic discovery. By altering the natural processes of brain sexual differentiation, basic researchers can determine how the development of specific brain regions contributes to later-life behavior.
Table 1.
Sex differences in the frequency and type of play across multiple mammalian species
| Species Studied | Sex with Higher Play Frequency | References | Additional Notes |
|---|---|---|---|
| Humans | Males | Whiting & Edwards, 1973; DiPietro, 1981; Humphreys & Smith, 1987 | |
| Rats | Males | Poole & Fish, 1976; Olioff & Stewart, 1978; Meaney & Stewart, 1981b | The sex difference is most robust in same-sex dyads and least robust when highly motivated, i.e. preceded by a period of social isolation |
| Cats (domestic) | Males | Caro, 1981 | Males from all-male groups play at higher frequency than females from all-female groups; male play frequency was not influenced by the number of opposite-sex playmates while female play is affected by the number of male playmates |
| Dogs (domestic) | Males | Pal, 2008; Ward et al., 2008 | In mixed-sex dyads, males also engaged in offensive behaviors and self-handicapped more than females (Ward et al., 2008) |
| Horses (domestic) | Males | Crowell-Davis et al., 1987 | |
| Pigs (domestic) | Males | Dobao et al., 1987; Brown et al., 2018; Weller et al., 2019 | |
| Cattle (domestic) | Males | Reinhardt et al., 1978 | Additionally, both sexes prefer to direct play behavior toward male calves |
| Sheep (domestic) | Males | Sachs & Harris, 1978 | |
| Sheep (wild) | Males | Hass & Jenni, 1993 | Male lambs also exhibit a larger repertoire of play behaviors than females |
| Siberian ibex | Males | Byers, 1980 | |
| Sea lions | Males | Gentry, 1974 | Male pups also exhibit a larger repertoire of play behaviors than females |
| Yellow-bellied marmots | Males | Jamieson & Armitage, 1987; Monclús et al, 2011 | Females with larger anogenital distances (i.e. masculinized females) engaged in play more frequently than females with smaller anogenital distances (Monclús et al., 2011) |
| Belding squirrels | Males | Holekamp et al., 1984 | |
| Rhesus monkeys | Males | Goy & Deputte, 1996 | Sex difference in frequency is based on a sex difference in play initiations (males > females) |
| Squirrel monkeys | Males | Biben, 2010 | Male play bouts are also longer than female play bouts |
| Lowland gorillas | Males | Meder, 1990 | |
| Baboons | Males | Owens, 1975 | |
| Spotted hyenas | Females | Pedersen et al., 1990 | Females of this species are dominant to males, are larger than males, and have external genitalia. Speaking to these observations, adult females also have higher levels of circulating testosterone relative to males than is typically seen in female mammals. |
Why is there a sex difference in rough and tumble play behavior? In this review, we address this question at both the proximate and ultimate level. Beginning with the proximate, we discuss our current understanding of the sexual differentiation of play at the cellular and molecular level. We then integrate this information into what is known about play’s ultimate causation, which may have its origins in a common reward circuitry and impart a lasting impact on sex-specific endpoints. Throughout this review, we emphasize research findings in the laboratory rat due to its robust and reliable play repertoire and the extensive and rich literature on play in this species.
Quantifying playfulness in rats
A variety of different approaches can be used to study rats at play. The testing environment may differ, ranging from random observation of animals and their cage mates in their home environment to social isolation-induced observation of animals paired with unfamiliar play partners in a play arena. The former can be thought of as spontaneous play, where the animals are being observed in a setting that is as unperturbed as possible. Under these conditions, any observed play is likely reflective of the animal’s spontaneous or natural desire to play. In contrast, the latter can be thought of as motivated play, defined primarily by the use of social isolation to increase the animal’s homeostatic drive for social interaction and increase the propensity for the isolated animal to engage in play. Both of these paradigms are useful and can be used to examine play from different perspectives. It is worth noting that sex differences in play are most robustly detected in spontaneous play paradigms (see discussion below).
Additionally, the number and the sex of the animals being observed may differ. Most commonly animals are studied as same-sex pairs, which is particularly important for observing sex differences in play as mixed-sex pairs will play at an intermediate frequency (i.e. more frequently than female-female pairs but less frequent than male-male pairs) (Argue & McCarthy, 2015a). Far less common is the use of same or mixed-sex groups, as there is potential for “contagious” play which confounds the experimenter’s ability to isolate factors that may regulate behavioral sex differences (Pellis & McKenna, 1992). Lastly, the age at which the animals are tested can vary substantially. Play is most robustly expressed during the juvenile period, which in rats corresponds to postnatal days 26–40 (Panksepp, 1981). However, once the animal reaches puberty, playfulness dramatically decreases.
The components of play are generally well agreed upon and consist of several stereotypical behaviors. Locomotor play is characterized by high-energy behaviors like hops and darts (Pellis & Pellis, 1983). Rough and tumble play is comprised of pounces, pins, wrestling, chasing, and boxing (Thor & Holloway, 1984). Each of these behaviors represents an important aspect of social play. For example, a pounce occurs when one animal launches itself at the nape of the other—the target and goal of play fighting—in a highly rewarding attempt to gain a physical advantage (Pellis & Pellis, 1987). The intent of a pounce is clear: the pouncing animal is initiating a play event by engaging in this pseudo-attack behavior. In response, the animal being pounced on may adopt one of many defensive strategies. The animal may choose to evade by swerving or running away, or they may choose to rotate onto their side or back. In juvenile rats, the most common defense strategy is to rotate completely to a supine position, which typically results in the defensive animal becoming “pinned’ by the pouncer (Pellis & Pellis, 1998). This is thought to signal the intent of the animal to defend themselves from the pounce and prepare for a counter-attack of their own. Similarly, the animal may perform a partial rotation, an adult-like pattern of defense exhibited more frequently by juvenile males, but not females, around the onset of puberty (Pellis & Pellis, 1997). By rolling onto their side in this manner, the animal retains contact with the ground via its hindpaws and thus is able to rear into a defensive upright posture.
As a play bout continues, animals may trade pounces and pins while trying to gain an advantage over the other. If play becomes quite vigorous, the pair of animals may begin wrestling, which is characterized by the pair rolling and tumbling around the arena as they seamlessly exchange attack and counter-attack. Less common are boxing and chasing behaviors. Boxing occurs often during wrestling bouts or after a pin, in which both animals turn to face each other and rear up on their hind legs to bat at each other with their forepaws (Vanderschuren et al., 1995). Chasing occurs when one animal attempts to run away and the other quickly pursues, presumably in an attempt to continue the play bout. By analyzing which animal performs which behaviors, and identifying the sequence in which behaviors occur, investigators can gain more insight into the dynamic and reciprocal interactions of this complex social behavior.
Juvenile male rats are more playful
The greater playfulness of male rats is limited to rough and tumble play; no sex differences are observed in locomotor play (e.g. running, jumping, and leaping) or in object play (Pellis & Pellis, 1983). These types of play are more commonly observed in predatory species like cats and dogs (Burghardt, 1999), while social play is the most common type of play seen in rats (Poole & Fish, 1975). The male bias in play is largely attributed to increased rates of play initiation compared to females, which suggests an underlying difference in motivation (Meaney & Stewart, 1981; Thor & Holloway, 1986). Males are also more likely to counterattack, engage in boxing, and pin more frequently, all of which may reflect the initial difference in the rate of play initiation (Pellis & Pellis, 1990). There are also qualitative differences in the structure of male and female-typical play. After a pin, females are more likely to evade while males tend to rotate into wrestling. The tendency to evade is likely because females respond sooner to the approach of the attacker and therefore are able to execute this response more effectively (Pellis & Pellis, 1990, 1997).
Sex differences in play are reliably observed in experimental conditions that favor spontaneous play (VanRyzin et al., 2020). For example, animals may be group-housed and recorded undisturbed (Poole & Fish, 1976; Meaney & Stewart, 1981b), or paired as same-sex play partners and provided a “play date” each day (Krebs-Kraft et al., 2010; Argue & McCarthy, 2015a; VanRyzin et al., 2019). In these cases, the animals do not experience any form of social isolation to artificially increase their social motivation. In these paradigms, the sex difference in play behavior is remarkably consistent across the juvenile period. Male pairs consistently pin and box more frequently then female pairs and overall spend more time playing with one another (Argue & McCarthy, 2015b). When a male and female are partnered, the time spent playing is an intermediate of that of same-sex pairs (Argue & McCarthy, 2015b). Interestingly, when a new partner is introduced to same-sex pairs (meaning one member of the pair is replaced), males continue to engage in more frequent play, while in females play drops off considerably (Argue & McCarthy, 2015b).
When play is highly motivated, as is the case following periods of social isolation, and the play bout is short, there are no consistent sex differences in the structure of or time spent engaged in play (Panksepp & Beatty, 1980; Panksepp, 1981; Thor & Holloway, 1984). This appears to be because juvenile females increase to the same level of play as males if isolated long enough (Pellis, Field, Smith, & Pellis, 1997). This appears to be strictly a juvenile phenomenon, however, as the same isolation-induced changes in play are not observed in adult rats. Moreover, the neurochemical underpinnings of motivated play appear to be distinct in each sex (Bredewold & Veenema, 2018), which is discussed further below.
Sex differences in play are programmed during early life
How do sex differences in play behavior arise? As noted above, play is most robustly observed during the juvenile period (i.e. prior to the onset of puberty) which means that differences in behavior cannot be attributed to differences in circulating gonadal hormones (estrogens in females, androgens in males). The timing of this behavior makes it unique compared to most other sex differences in behavior, which are observed only in the adult animal (e.g. mating, parental behavior, aggression, etc.), and suggests that sex differences in play must have their origins in perinatal development.
Early in life, the brain undergoes the process of sexual differentiation, a process by which the brain develops to match the animal’s gonadal sex (McCarthy et al., 2017). In males, the maturation of the testes triggers the production of high levels of testosterone which will circulate throughout the body and reach the brain. It is this surge in steroid hormones that initiates the process of masculinization. In females, the gonads develop into ovaries and do not produce steroid hormones during this developmental period. In the absence of elevated hormone exposure, the brain is feminized. Ultimately, masculinization and feminization ensure that the brain (and later-life behavior) develops to match the animal’s reproductive physiology in order to ensure reproductive success.
In rodents, these processes occur during a critical period of life beginning just days prior to birth and ending shortly after parturition (McCarthy et al., 2018). For females, this time represents a “sensitive period” during which masculinization can be induced as a result of exposure to high levels of steroids, either naturally or experimentally (McCarthy, 2010). Once the sensitive period closes, by the end of the first week of the rodent’s life, females become impervious to the masculinizing impact of steroids. The existence of this sensitive period provides an excellent experimental tool to investigate the mechanisms underlying sexual differentiation of the brain (Figure 1).
Figure 1: Sexual Differentiation of Social Play.
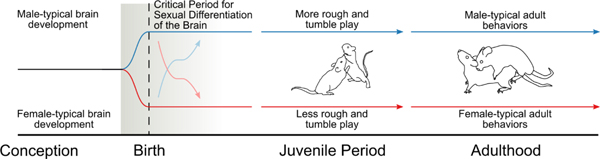
During the perinatal critical period for sexual differentiation of the brain (shaded region), the brain will undergo either the process of masculinization (dark blue line) or feminization (dark red line). Masculinization occurs in males as a result of fetal gonadal hormone production from the developing testes. Feminization occurs in females in the absence of gonadal hormone exposure. During this critical period, masculinization of brain and behavior can be experimentally blocked by preventing hormone signaling or their downstream effectors (light red line), resulting in a biological male with female-typical behavior. Similarly, females can be masculinized via exposure to high levels of gonadal hormones or their downstream effectors (light blue line). After the critical period closes, brain differentiation is largely permanent and results in the expression of sex-typical behaviors later in life.
The masculinizing effects of steroids are region-specific and quite diverse, ranging from epigenetic modifications to cell genesis, cell death, synaptogenesis, synaptic pruning, axonal and dendritic growth and branching, myelination, and neurochemical phenotype (McCarthy & Arnold, 2011). Early in the study of steroid-induced sexual differentiation, it was discovered that masculinization—initially attributed entirely to the effects of androgens—actually occurred through many estrogen-dependent mechanisms. Neurons in some regions of the brain express high levels of the enzyme aromatase, which converts testosterone into estradiol (Naftolin et al., 1975; McEwen et al., 1977). Together, the coordinated actions of both androgens and estrogens are necessary for complete masculinization of the brain and behavior in rodents (McCarthy, 2008; Zuloaga et al., 2008). However, this does not appear to be true in primates, where androgens mediate most, if not all, of the masculinization of the brain (Thornton et al., 2009).
Initial studies investigating the sexual differentiation of play discovered that the development of male-typical play is actually largely androgen-dependent. Treating female rat pups with testosterone or 5α-dihydrotestosterone (a testosterone analog that cannot be converted into estradiol) during the sensitive period in development increases their play to male-typical levels. Similarly, treating male rat pups with the androgen receptor antagonist flutamide to block the actions of testosterone during the critical period prevents the development of male-typical play. As a result, these males play at the same level as females (Meaney et al., 1983).
While the preponderance of evidence indicates androgens are central to sexual differentiation of playfulness, a role for estrogens cannot be discounted. Supporting this idea, male rats with nonfunctional androgen receptors, so-called “Tfm males”, engage in some aspects of play at male-typical levels (Meaney et al., 1983; Field et al., 2006). The Auger laboratory has consistently found a role for estrogens in the differentiation of play, including a convergence of dopamine signaling and the estrogen receptor (Olesen et al., 2005). Perinatal exposure to bisphenol A, an environmental estrogen, or diethylstilbestrol dipropionate, a synthetic estrogen, also increases play in female rats and rhesus macaques, respectively (Dessi-Fulgheri et al., 2002; Goy & Deputte, 1996). Lastly, we have found that disruption of estrogen synthesis within the developing cerebellum during a later sensitive period, the second postnatal week, reduces play by juvenile males (Dean et al., 2012).
One interpretation of the duality of steroid action is that play behavior could be a two-factor system in which androgens and estrogens impact the same circuit, but androgens are both necessary and sufficient while estrogens are necessary but not sufficient. In this sense, both androgens and estrogens are required for full masculinization of the entire underlying circuitry. Alternatively, the two classes of steroids may be modulating distinct processes or only parts of the circuit, but our level of understanding is not yet developed enough to discern precisely how. Furthermore, estrogens may have evolved to decrease rather than increase sex differences in play. For example, reducing the expression of a negative regulator of estrogen receptor alpha during sexual differentiation actually increases later playfulness of males but has no effect on females (Jessen et al., 2010). These data suggest that estrogen signaling might regulate a “ceiling” for male-typical levels of play.
The neural circuitry of play includes sexually differentiated modulatory nodes
The neural circuitry of play is ill-defined and diffusely embedded within the well-characterized social behavior and reward networks. Attempts to map the circuitry have thus far relied on lesions, site-specific pharmacology, and immediate early gene (IEG) expression. While advanced circuit mapping tools have recently been effective at identifying specific nodes critical for sex-specific behaviors like mating, parenting, and aggression (Hong et al., 2014; Wu et al., 2009), many of these techniques rely on the use of transgenic mice, which, in contrast to rats, do not engage in tertiary social play.
Complicating matters, many components of play are also components of other complex behaviors. The challenge of disentangling a given brain region’s contribution to play from other behaviors is highlighted by lesion studies. Rats with lesions to the medial prefrontal cortex (mPFC) initiate play more frequently but have a decreased likelihood of responding to play initiation from a partner (Bell et al., 2009). The same lesions, however, broadly impair social cognition and thus may not be specific to play (Bell et al., 2010). Following this trend, lesions of other play-associated brain regions also impair locomotor behavior, sensory processing, etc. (Neill et al., 1974; Sheehan et al., 2004; DiBenedictis et al., 2013) making it difficult to parse out the specific impact of each region on play itself.
In contrast to lesion studies, which results in the ablation of a particular brain region, the neural circuitry of play can be studied by examining IEG expression. IEGs are expressed following neuron activation; thus, the brains of rats can by analyzed shortly after a playful experience and any neurons that were active during the play bout will highly express IEGs. Using this methodology, two leading play research groups demonstrated that play activates several distinct brain regions, including those involved in motivation, reward, sensory, and motor processing (Gordon et al., 2002; van Kerkhof et al., 2014). The activation patterns in many of these regions correlated with one another, suggesting that the coordinated activity, rather than individual activation, of specific brain circuits is necessary for proper play behavior.
The lesson learned from lesion studies, pharmacological manipulations, and network analyses is that play engages large and diverse regions of the brain, all of which engage in a variety of behaviors and physiological functions. Thus, attempting to distinguish neurons as “play” or “not-play” is confounded. However, the sex difference in frequency and intensity of play provides a contrast agent for visualizing differences within the circuit that are specific to play, whether they are differences in brain activation, connectivity and/or neurochemical phenotype. Importantly, the sex difference in play is not a distributed property of the play circuit but instead is determined by sex differences in specific nodes. Two nodes in particular, the medial amygdala (MeA) and lateral septum (LS), have been well characterized and are distinct in their influence on play. A third node, the prefrontal cortex (PFC), does not regulate sex differences but instead contributes generally to play (Bell, et al., 2009) (Figure 2). The PFC likely regulates play in a multifactorial manner, contributing to expression of the behavior through its roles in suppressing aggression, monitoring approach/avoid conflict, and modulating the stress response via its projections to the MeA and other stress-modulatory nodes (Aupperle & Paulus, 2010; McEwen & Morrison, 2013; Takahashi et al., 2014).
Figure 2. Distinct Nodes in the Neural Circuitry of Play.
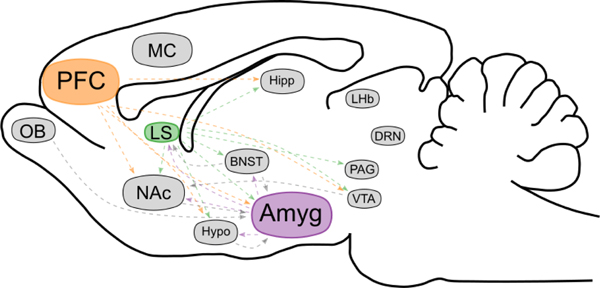
The sex-specific neural control of play is embedded within the larger neural circuitry of social behavior. Each node provides specific forms of regulatory control. The amygdala (purple) is critical for the control of the sex difference in play, whereas the lateral septum (green) exhibits sex differences in signaling pathways involved in play. The prefrontal cortex (orange) regulates the cognitive components of play, such as initiation and reciprocation, and seems to do so equally between the sexes. Other regions shown to be involved in social behavior are shown in grey. Hypothesized connectivity between social play nodes and individual brain regions is shown in grey and colored dotted lines. Abbreviations: Amyg, amygdala; BNST, bed nucleus of the stria terminalis; DRN, dorsal raphe nucleus; Hipp, hippocampus; Hypo, hypothalamus; LHb, lateral habenula; LS, lateral septum; MC, motor cortex; NAc, nucleus accumbens; OB, olfactory bulb; PAG, periaqueductal gray; PFC, prefrontal cortex; VTA, ventral tegmental area.
Medial Amygdala
The MeA is a region responsible for processing social information in a sex-specific manner (Bergan et al., 2014; Lischinsky et al., 2017), and has long been implicated as necessary for the sex differences in social play. Indeed, MeA lesions do not eliminate play but instead eliminate the sex difference via selectively lowering male play levels to that of females (Meaney et al., 1981). Conversely, implanting androgens directly into the MeA of developing females increases the frequency of their play to that of males (Meaney & McEwen, 1986).
How do sex differences in the MeA develop? And how does the MeA regulate the sex difference in play? Until recently, exactly how sexual differentiation of the MeA produced sex differences in play behavior was unknown. We discovered that during the critical period for sexual differentiation, males and females differ in the number of newborn cells in this region, suggesting differences in the developmental trajectory of the MeA. The sex difference in newborn cells is driven by a sex difference in a class of signaling molecules that are essential to brain development: endocannabinoids (Krebs-Kraft et al., 2010). The two primary endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), activate CB1 and CB2 receptors to affect numerous neurodevelopmental processes including cell proliferation, differentiation, connectivity, and synaptic transmission (Maccarrone et al., 2014).
Males have higher levels of endocannabinoids in the developing amygdala than females, and correspondingly produce fewer newborn cells during the critical period. These differences are due to the actions of testosterone; treating female rat pups with exogenous testosterone increases the endocannabinoid levels and decreases newborn cell number in the amygdala. Similarly, blocking testosterone by using an androgen receptor antagonist in females treated with testosterone completely prevents these effects (VanRyzin et al., 2019). If females are treated with CB1 and CB2 receptor agonists to “mimic” the higher endocannabinoid levels, their newborn cell number and eventual play behavior is masculinized, even in the absence of testosterone (Argue et al., 2017; VanRyzin et al., 2019).
The mechanism by which endocannabinoids regulate cell number in the developing amygdala was unexpected, as it involves the immune system. We found that in the developing amygdala, microglia, the innate immune cells of the brain, engulf and kill other brain cells which would otherwise survive. This microglial phagocytosis, primarily of newborn cells, is driven by the higher endocannabinoid levels, thereby leading to more engulfment of newborn astrocytes in males. Astrocytes, the primary non-neuronal cell of the brain, regulate neural communication (Chung et al., 2015) and the process by which microglia control astrocyte number is crucial to later playfulness. Lower astrocyte density in the MeA is correlated for male-typical levels of play (VanRyzin et al., 2019).
We hypothesize that by altering the astrocyte density in the MeA, the process of sexual differentiation changes the responsiveness of MeA neurons to social cues during play. In support of this idea, the lower astrocyte density in the MeA of juvenile males is associated with higher IEG expression following a play bout than females. Thus, by “tuning” the sensitivity of the MeA to social information, a common circuitry in males and females may produce behavioral sex differences. Such “tuning” may occur in other brain regions in the play circuitry as well, allowing behavioral differences to arise despite commonalities in the broader play network. Given the great diversity of neurons in the MeA (Cocas et al., 2009; Hirata et al., 2009), a better understanding of what cell types are active during play is needed in order to understand how their activity may modulate other nodes in the play circuit.
Lateral Septum
The LS is another sexually dimorphic region that is critical for sex-specific modulation of social behavior. It receives vasopressinergic inputs directly from the MeA (Caffe et al., 1987) and the bed nucleus of the stria terminalis (BNST) (De Vries & Buijs, 1983). The density of these inputs is substantially higher in males, as there are significantly more vasopressin-expressing neurons in the male MeA (De Vries et al., 1984; De Vries & Panzica, 2006). The boundaries of the LS are characterized by high expression of the vasopressin 1a (V1a) receptor (Allaman-Exertier et al., 2007), and pharmacological manipulation of the V1a receptor elicits a sex-dependent effect on social play. Infusions of V1a receptor antagonists directly into the LS reduce play in males, while increasing it in females (Veenema et al., 2013). The play-modulating effects of V1a receptor antagonism depends on social context and likely cannot be attributed to any effects on social recognition (Bredewold et al., 2014), demonstrating the specificity of this node in the play circuit in light of the profound role for vasopressin in social behavior and social memory in adults (Albers, 2012).
Given that the majority of LS neurons are GABAergic, it is likely that agonsim of V1a receptors on inhibitory neurons disinhibits projection neurons to downstream effectors of the social behavior network. Notably, there is little to no effect of manipulating oxytocin on play despite its widespread involvement in a myriad of social behaviors (Bredewold, et al., 2014; Bredewold & Veenema, 2018), implicating a sex-specific and region-specific role for vasopressin in promoting play.
Sex differences in the vasopressin system also have their origins in development and are androgen-dependent (De Vries & Panzica, 2006; Dumais & Veenema, 2016). Treating female or castrated male rat pups with testosterone increases the density of vasopressinergic inputs to the LS (De Vries et al., 1983), and these sex differences are maintained in adulthood by the actions of circulating gonadal hormones (Van Leeuwen et al., 1985; Miller et al., 1992). Despite our understanding of which hormones are responsible, very little is known about how hormones establish these sex differences. As such, elucidating these mechanisms remains a subject for future research.
Prefrontal Cortex
As noted previously, neonatal lesions of the mPFC impair play in a variety of ways, largely resulting in a disruption the animal’s play structure and ability to respond to play initiations (Panksepp et al., 1994; Bell et al., 2009). Moreover, pharmacological inactivation of the PFC, achieved by infusing GABA receptor agonists at the time of play, reduces the frequency and duration of play behavior (van Kerkhof et al., 2013). These data suggest that the PFC likely has a regulatory role and modulates the animal’s behavior in response to cues from a conspecific.
In support of this idea, play-induced activation patterns within many PFC regions correlate with activity in other connected regions within the striatum and amygdala (van Kerkhof et al., 2014). Corticostriatal circuitry is essential to processing reward, cognition, and movement (Haber, 2016) and dopamine depletion in the striatum produces alterations in play behavior sequence (Pellis et al., 1993). The amygdala, in addition to regulating the sex difference in play, contributes to processing the rewarding aspects of play (Trezza et al., 2012). The mPFC is also changed in response to play experience. The dendritic complexity of cortical pyramidal neurons is reduced in animals given multiple play partners over an extended period of time compared to those either socially deprived or given only limited access to the same partner (Bell et al., 2010). Thus, the PFC likely integrates information from several brain regions during play and modifies behavior accordingly.
Conclusions
Our understanding of the nature and origin of sex differences in play behavior has greatly advanced in the past few decades. However, many questions remain unanswered. First, what are the mechanisms by which sex differences in social behavior circuit nodes arise? How does coordinated activity of these nodes contribute to sex differences in play behavior? Finally, and perhaps most intriguingly, what ultimate purpose do sex differences in play serve in affecting the development of an animal as a whole?
Ultimate mechanisms mediating sexual differentiation of play
One hypothesis as to the purpose of play is that play functions to shape and refine neural circuitry facilitating expression of adult social behavior. This idea is supported by play deprivation studies, in which animals prevented from playing during the juvenile age exhibit reduced social behavior and inappropriate response to social challenge (Hol et al., 1999; van den Berg et al., 1999; Von Frijtag et al,. 2002; Stark & Pellis in this Special Issue). Notably, the two brain regions implicated in generating sex differences in play, the MeA and LS, are also fundamental to expression of adult social behaviors that also exhibit sex differences, such as parenting, sex behavior, and aggression (Chen et al., 2019; Gammie & Nelson, 1999; Gogate et al., 1995; Tsukahara et al., 2014; Wong et al., 2016). Thus, while play may facilitate the development of adult cognitive and social behaviors similarly in both sexes, the ultimate function of the sex difference in play may be to enable appropriate expression of sex-typical adult social behaviors. In this view, play is not purposeless; play broadly shapes circuitry common to both sexes, while refining the sex-specific nodes in the social behavior circuitry to optimally express sex-specific adult behaviors.
An additional component of play that may have an ultimate impact on its differential expression in males and females is the rewarding aspect of the behavior. Play is clearly rewarding to both participants (Vanderschuren et al., 2016), and animals readily seek out and engage in a variety of behaviors they find rewarding, including those we consider detrimental such as drug and alcohol ingestion to the point of addiction or dependency. Female rodents become addicted faster than males and this is believed to be due to a greater activation of the reward circuit (Becker & Koob, 2016). But if play is rewarding, and females have greater activation of the reward circuit, why would they play less than males? One explanation is that what starts as rewarding becomes aversive with repeated exposure, an inverted U-shaped curve of reward. This hypothesis has been empirically tested in hamsters with the incorporation of the social behavior-enhancing effects of oxytocin and supports the conclusion that females are more sensitive to the rewarding components and thereby more quickly find socialness aversive than males (Borland et al., 2019; Borland et al., 2019). The same may be true for play. Alternatively, play may be inherently less rewarding for females due to neurophysiological or intrinsic circuitry differences that cause the behavior to extinguish earlier than it does in males. Certainly, future research will need to directly test these hypotheses so that we may better understand the commonalities and differences in play behavior and circuitry in males and females.
Acknowledgments
Grant support: R01 DA039062 to MMM
Footnotes
Disclosure statement: The authors have nothing to disclose.
References:
- Albers HE (2012). The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Hormones and Behavior, 61(3), 283–292. [DOI] [PubMed] [Google Scholar]
- Allaman-Exertier G, Reymond-Marron I, Tribollet E, & Raggenbass M (2007). Vasopressin modulates lateral septal network activity via two distinct electrophysiological mechanisms. European Journal of Neuroscience, 26(9), 2633–2642. [DOI] [PubMed] [Google Scholar]
- Argue KJ, & McCarthy MM (2015a). Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biology of Sex Differences, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, & McCarthy MM (2015b). Utilization of same- vs. mixed-sex dyads impacts the observation of sex differences in juvenile social play behavior. Current Neurobiology, 6(1), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, & McCarthy MM (2017). Activation of both CB1 and CB2 endocannabinoid receptors is critical for masculinization of the developing medial amygdala and juvenile social play behavior. eNeuro, 4(1), ENEURO.0344–16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger AP, Jessen HM, & Edelmann MN (2011). Epigenetic organization of brain sex differences and juvenile social play behavior. Hormones and Behavior, 59(3), 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, & Paulus MP (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues in Clinical Neuroscience, 12(4), 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJ, Counotte DS, O’Donnell P, & Vanderschuren LJ (2013). Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology, 38(8), 1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, & Koob GF (2016). Sex differences in animal models: focus on addiction. Pharmacological Reviews, 68(2), 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff M, & Byers JA (1981). A critical reanalysis of the ontogeny and phylogeny of mammalian social and locomotor play: an ethological hornet’s nest. In: Immelmann K, Barlow GW, Petrinovich L, & Main M (Eds). Behavioral development (pp. 296–337). London: Cambridge University Press. [Google Scholar]
- Bell HC, McCaffrey DR, Forgie ML, Kolb B, & Pellis SM (2009). The role of the medial prefrontal cortex in the play fighting of rats. Behavioral Neuroscience, 123, 1158–1168. [DOI] [PubMed] [Google Scholar]
- Bell HC, Pellis SM, & Kolb B (2010). Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behavioural Brain Research, 207(1), 7–13. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, & Dulac D (2014). Sex-specific processing of social cues in the medial amygdala. Elife, 3, e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biben M (1986). Individual- and sex-related strategies of wrestling play in captive squirrel monkeys. Ethology, 71, 229–241. [Google Scholar]
- Blake BE, & McCoy KA (2015). Hormonal programming of rat social play behavior: standardized techniques will aid synthesis and translation to human health. Neuroscience & Biobehavioral Reviews, 55, 184–197. [DOI] [PubMed] [Google Scholar]
- Borland JM, Aiani LM, Norvelle A, Grantham KN, O’Laughlin K, Terranova JI, Frantz KJ, & Albers HE (2019). Sex-dependent regulation of social reward by oxytocin receptors in the ventral tegmental area. Neuropsychopharmacology, 44(4), 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland JM, Rilling JK, Frantz KJ, & Albers HE (2019). Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology, 44(1), 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, & Veenema AH (2014). Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Behavioral Neuroscience, 8, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, & Veenema AH (2018). Sex differences in the regulation of social and anxiety-related behaviors: insights from vasopressin and oxytocin brain systems. Current Opinion in Neurobiology, 49, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Peters R, Nevison IM, & Lawrence AB (2018). Playful pigs: Evidence of consistency and change in play depending on litter and developmental stage. Applied Animal Behaviour Science, 198, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt GM (1999). Play. In: Greenberg G, & Haraway MM (Eds). Comparative psychology: a handbook (pp. 725–735). New York: Garland Publishing Co. [Google Scholar]
- Busquets-Garcia A, Bains J, & Marsicano G (2018). CB1 receptor signaling in the brain: extracting specificity from ubiquity. Neuropsychopharmacology, 43(1), 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers JA (1980) Play partner preferences in Siberian ibex, Capra ibex sibirica. Ethology, 53(1), 23–40. [Google Scholar]
- Caffe AR, van Leeuwen FW, & Luiten PG (1987). Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. Journal of Comparative Neurology, 261(2), 237–252. [DOI] [PubMed] [Google Scholar]
- Caro TM (1981). Sex differences in the termination of social play in cats. Animal Behavior, 29(1), 271–279. [Google Scholar]
- Chen PB, Hu RK, Wu YE, Pan L, Huang S, Micevych PE, & Hong W (2019). Sexually dimorphic control of parenting behavior by the medial amygdala. Cell, 176(5), 1206–1221 e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, & Eroglu C (2015). Astrocytes control synapse formation, function, and elimination. Cold Spring Harbor Perspectives in Biology, 7(9), a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell-Davis SL, Houpt KA, & Kane L (1987). Play development in Welsh pony (Equus caballus) foals. Applied Animal Behaviour Science, 18(2), 119–131. [Google Scholar]
- Cocas LA, Miyoshi G, Carney RS, Sousa VH, Hirata T, Jones KR, Fishell G, Huntsman MM, & Corbin JG (2009). Exm1-lineage progenitors differentially contribute to neural diversity in the striatum and amygdala. Journal of Neuroscience, 29(50), 15933–15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Best W, & Sluiter AA (1983). The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Developmental Brain Research, 8, 377–380. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, & Buijs RM (1983). The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Research, 273(2), 307–317. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, & van Leeuwen FW (1984). Sex differences in vasopressin and other neurotransmitter systems in the brain. Progress in Brain Research, 61, 185–203. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, & Panzica GC (2006). Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience, 138(3), 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SL, Knutson JF, Krebs-Kraft DL, & McCarthy MM (2012). Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. European Journal of Neuroscience, 35(8), 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, & Farabollini F (2002). Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environmental Health Perspectives, 110 Suppl 3, 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedictis BT, Ingraham KL, Baum MJ, & Cherry JA (2012). Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiology & Behavior, 105(2), 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA (1981). Rough and tumble play: A function of gender. Developmental Psychology, 17(1), 50–58. [Google Scholar]
- Dobao MT, Rodrigañez J, & Silio L (1985). Choice of companions in social play in piglets. Applied Animal Behaviour Science, 13(3), 259–266. [Google Scholar]
- Dumais KM, & Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology, 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen (1981). Animal Play Behavior. New York: Oxford University Press. [Google Scholar]
- Field EF, Whishaw IQ, Pellis SM, & Watson NV (2006). Play fighting in androgen-insensitive Tfm rats: evidence that androgen receptors are necessary for the development of adult playful attack and defense. Developmental Psychobiology, 48(2), 111–120. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, & Auger CJ (2012). Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics, 7(3), 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, & Nelson RJ (1999). Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. Journal of Neuroscience, 19(18), 8027–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate MG, Brid SV, Wingkar KC, & Kantak NM (1995). Septal regulation of male sexual behavior in rats. Physiology & Behavior, 57(6), 1205–1207. [DOI] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, & Panksepp J (2002). Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Research Bulletin, 57(5), 651–659. [DOI] [PubMed] [Google Scholar]
- Goy RW, & Deputte BL (1996). The effects of diethylstilbestrol (DES) before birth on the development of masculine behavior in juvenile female rhesus monkeys. Hormones and Behavior, 30(4), 379–386. [DOI] [PubMed] [Google Scholar]
- Haber SN (2016). Corticostriatal circuitry. Dialogues in Clinical Neuroscience, 18(1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass CC, & Jenni DA (1993). Social play among juvenile bighorn sheep: structure, development, and relationship to adult behavior. Ethology, 93(2), 105–116. [Google Scholar]
- Hirata T, Li P, Lanuza GM, Cocas LA, Huntsman MM, & Corbin JG (2009). Identification of distinct telencephalic progenitor pools for neuronal diversity in the amygdala. Nature Neuroscience, 12(2), 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol T, Van den Berg CL, Van Ree JM, & Spruijt BM (1999). Isolation during the play period in infancy decreases adult social interactions in rats. Behavioural Brain Research, 100(1–2), 91–97. [DOI] [PubMed] [Google Scholar]
- Holekamp KE, Smale L, Simpson HB, & Holekamp NA (1984). Hormonal influences on natal dispersal in free-living Belding’s ground squirrels (Spermophilus beldingi). Hormones and Behavior, 18(4), 465–483. [DOI] [PubMed] [Google Scholar]
- Hong W, Kim DW, & Anderson DJ (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell, 158(6), 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys AP, & Smith PK (1987). Rough and tumble, friendship, and dominance in schoolchildren: evidence for continuity and change with age. Child Development, 58(1), 201–212. [Google Scholar]
- Jamieson SH, & Armitage KB (1987). Sex differences in the play behavior of yearling yellow-bellied marmots. Ethology, 74(3), 237–253. [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, & Auger AP (2010). The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology, 151(3), 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, & McCarthy MM (2010). Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proceedings from the National Academy of Sciences U S A, 107(47), 20535–20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischinsky JE, Sokolowski K, Li P, Esumi S, Kamal Y, Goodrich M, Oboti L, Hammond TR, Krishnamoorthy M, Feldman D, Huntsman MM, & Corbin JG (2017). Embryonic transcription factor expression in mice predicts medial amygdala neuronal identity and sex-specific responses to innate behavioral cues. Elife, 6, e21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Guzmán M, Mackie K, Doherty P, & Harkany T (2014). Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nature Reviews Neuroscience, 15(12), 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, & Caro TM (1985). On the functions of play and its role in behavioral development. Advances in the Study of Behavior, 15(59), 59–103. [Google Scholar]
- McCarthy MM (2008). Estradiol and the developing brain. Physiological Reviews, 88(1), 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2010). How it’s made: organisational effects of hormones on the developing brain. Journal of Neuroendocrinology, 22(7), 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, & Arnold AP (2011). Reframing sexual differentiation of the brain. Nature Neuroscience, 14(6), 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, de Vries GJ, & Forger NG (2017). Sexual differentiation of the brain: a fresh look at mode, mechanisms, and meaning. In: Pfaff DW, & Joels M (Eds). Hormones, brain, and behavior (pp. 3–32). Cambridge, MA: Academic Press. [Google Scholar]
- McCarthy MM, Herold K, & Stockman SL (2018). Fast, furious and enduring: sensitive versus critical periods in sexual differentiation of the brain. Physiology & Behavior, 187, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, & Krey LC (1977). Aromatization: important for sexual differentiation of the neonatal rat brain. Hormones and Behavior, 9, 249–263. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Morrison JH (2014). Brain on stress: vulnerability and plasticity in the prefrontal cortex over the life course. Neuron, 79(1), 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, & Stewart J (1981a) A descriptive study of social development in the rat (Rattus norvegicus). Animal Behaviour, 29(1), 34–45. [Google Scholar]
- Meaney MJ, & Stewart J (1981b). Neonatal androgens influence the social play of prepubescent rats. Hormones and Behavior, 15, 197–213. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, & Stewart J (1983). The influence of exogenous testosterone and corticosterone on the social behavior of prepubertal male rats. Bulletin of the Psychonomic Society, 21, 232–234. [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, & McEwen BS (1983). Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology, 37, 85–90. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, & McEwen BS (1986). Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Research, 398(2), 324–328. [DOI] [PubMed] [Google Scholar]
- Meder A (1990). Sex differences in the behaviour of immature captive lowland gorillas. Primates, 31(1), 51–63. [Google Scholar]
- Miller MA, De Vries GJ, al-Shamma HA, & Dorsa DM (1992). Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. Journal of Neuroscience, 12(8), 2881–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monclús R, Cook R, & Blumstein DT (2011). Masculinized female yellow-bellied marmots initiate more social interactions. Biology Letters, 8(2), 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ., Takaoka Y, & Wolin L (1975). The formation of estrogens by central neuroendocrine tissues. Recent Progress in Hormone Research, 31, 295–319. [DOI] [PubMed] [Google Scholar]
- Neill DB, Ross JF, & Grossman SP (1974). Effects of lesions in the dorsal or ventral striatum on locomotor activity and on locomotor effects of amphetamine. Pharmacology Biochemistry and Behavior, 2(5), 697–702. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, & Auger AP (2005). Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptors expression and juvenile social play behavior. Endocrinology, 146(9), 3705–3712. [DOI] [PubMed] [Google Scholar]
- Olioff M, & Stewart J (1978). Sex differences in the play behavior of prepubescent rats. Physiology & Behavior, 20(2), 113–115. [DOI] [PubMed] [Google Scholar]
- Owens NW (1975). Social play behavior in free-living baboons, Papio anubis. Animal Behaviour, 23(2), 387–408. [DOI] [PubMed] [Google Scholar]
- Pal SK (2008). Maturation and development of social behaviour during early ontogeny in free-ranging dog puppies in West Bengal, India. Applied Animal Behaviour Science, 111(1–2), 95–107. [Google Scholar]
- Panksepp J, Beatty WW (1980). Social deprivation and play in rats. Behavioral and Neural Biology, 30, 197–206. [DOI] [PubMed] [Google Scholar]
- Panksepp J (1981). The ontogeny of play in rats. Developmental Psychobiology, 14(4), 327–332. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Normansell L, Cox JF, & Siviy SM (1994). Effects of neonatal decortication on the social play of juvenile rats. Physiology & Behavior, 56, 429–443. [DOI] [PubMed] [Google Scholar]
- Pedersen JM, Glickman SE, Frank LG, & Beach FA (1990). Sex differences in the play behavior of immature spotted hyenas, Crocuta crocuta. Hormones and Behavior, 24(3), 403–420. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1983). Locomotor-rotational movements in the ontogeny and play of the laboratory rat Rattus norvegicus. Developmental Psychobiology, 16(4), 269–286. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1987). Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggressive Behavior, 13(4), 227–242. [Google Scholar]
- Pellis SM, & Pellis VC (1990). Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Developmental Psychobiology, 23(3), 215–231. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & McKenna MM (1992). Intrinsic and extrinsic influences on play fighting in rats: effects of dominance, partner’s playfulness, temperament and neonatal exposure to testosterone proprionate. Behavioural Brain Research, 50(1–2), 135–145. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Castañeda E, McKenna MM, Tran-Nguyen LT, & Whishaw IQ (1993). The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neuroscience Letters, 158(1), 13–15. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, & Pellis VC (1997). Multiple differences in the play fighting of male and female rats: implications for the causes and functions of play. Neuroscience & Biobehavioral Reviews, 21(1), 105–120. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1997). The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus). Developmental Psychobiology, 31(3), 193–205. [PubMed] [Google Scholar]
- Pellis SM, & Pellis VC (1998). Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neuroscience & Biobehavioral Reviews, 23(1), 87–101. [DOI] [PubMed] [Google Scholar]
- Pellis SM, & Pasztor TJ (1999). The developmental onset of a rudimentary form of play fighting in C57 mice. Developmental Psychobiology, 34(3), 175–182. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, Pelletier A, & Leca JB (2019). Is play a behavior system, and, if so, what kind? Behavioural Processes, 160, 1–9. [DOI] [PubMed] [Google Scholar]
- Poole TB, & Fish J (1976). An investigation of individual, age, and sexual differences in the play of Rattus norvegicus (Mammalia: Rodentia). Journal of Zoology, 179(2), 249–259. [Google Scholar]
- Reinhardt C, Reinhardt A, & Reinhardt V (1986). Social behaviour and reproductive performance in semi-wild Scottish Highland cattle. Applied Animal Behaviour Science, 15(2), 125–136. [Google Scholar]
- Sachs BD, & Harris VS (1978). Sex differences and developmental changes in selected juvenile activities (play) of domestic lambs. Animal Behavior, 26(3), 678–684. [Google Scholar]
- Sheehan TP, Chambers RA, & Russell DS (2004). Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Research Reviews, 46, 71–117. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Nagayasu K, Nishitani N, Kaneko S, & Koide T (2014). Control of intermale aggression by medial prefrontal cortex activation in the mouse. PLoS One, 9(4), e94657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, & Holloway WR (1984). Developmental analyses of social play behavior in juvenile rats. Bulletin of the Psychonomic Society, 22(6), 587–590. [Google Scholar]
- Thor DH, & Holloway WR (1986). Social play soliciting by male and female juvenile rats: effects of neonatal androgenization and sex of cagemates. Behavioral Neuroscience, 100(2), 275–279. [DOI] [PubMed] [Google Scholar]
- Thornton J, Zehr JL, & Loose MD (2009). Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates. Hormones and Behavior, 55(5), 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, van Kerkhof LW, Pasterkamp RJ, Zhou Y, Compolongo P, Cuomo V, Di Marzo V, & Vanderschuren LJ (2012). Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. Journal of Neuroscience, 32(43), 14899–14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Kanaya M, & Yamanouchi K (2014). Neuroanatomy and sex differences of the lordosis-inhibiting system in the lateral septum. Frontiers in Neuroscience, 8, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, & Koolhaas JM (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology, 34(2), 129–138. [PubMed] [Google Scholar]
- van Kerkhof LWM, Damsteegt R, Trezza V, Voorn P, & Vanderschuren LJMJ (2013). Social play behavior in adolescent rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology, 38(10), 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof LWM, Trezza V, Mulder T, Gao P, Voorn P, & Vanderschuren LJMJ (2014). Cellular activation in limbic brain systems during social play behavior in rats. Brain Structure and Function, 219(4), 1181–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, & De Vries GJ (1985). Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Research, 325, 391–394. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Spruijt BM, Hol T, Niesink RJM, Van Ree JM (1995). Sequential analysis of social play behavior in juvenile rats: effects of morphine. Behavioural Brain Research, 72(1–2), 89–95. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Acterberg EJ, & Trezza V (2016). The neurobiology of social play and its rewarding value in rats. Neuroscience & Biobehavioral Reviews, 70, 86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, & McCarthy MM (2019). Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron, 102(2), 435–449.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, & McCarthy MM (2020). Assessing rough-and-tumble play in juvenile rats. Bio-protocol, 10, e3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, & De Vries GJ (2013). Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology, 38(11), 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Frijtag JC, Schot M, van den Bos R, & Spruijt BM (2002). Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Developmental Psychobiology, 41(1), 58–69. [DOI] [PubMed] [Google Scholar]
- Ward C, Bauer EB, & Smuts BB (2008). Partner preferences and asymmetries in social play among domestic dog, Canis lupus familiaris, littermates. Animal Behaviour, 76(4), 1187–1199. [Google Scholar]
- Weller JE, Camerlink I, Turner SP, Faris M, & Arnott G (2019). Socialisation and its effect on play behaviour and aggression in the domestic pig (Sus scrofa). Scientific Reports, 9(1), 4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting B, & Edwards CP (1973). A cross-cultural analysis of sex differences in the behavior of children aged three through 11. Journal of Social Psychology, 91(2), 171–188. [Google Scholar]
- Wilson SC, & Kleiman DG (1974). Eliciting play: A comparative study. American Zoologist, (14), 341–370. [Google Scholar]
- Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, Chang BC, Bernstein H, You X, Feng JE, Froemke RC, & Lin D (2016). Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Current Biology, 26(5), 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, & Shah NM (2009). Estrogen masculinizes neural pathways and sex-specific behaviors. Cell, 139(1), 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, & Breedlove SM (2008). The role of androgen receptors in the masculinization of brain and behavior: what we’ve learned from the testicular feminization mutation. Hormones and Behavior, 53, 613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]


