Abstract
Background:
Iliolumbar syndrome is a frequent cause of chronic nonspecific low back pain. The cornerstone of its treatment lies upon the specific diagnosis of the iliolumbar syndrome. The ultrasound guided interventions have the potential for the specific diagnosis and treatment of the iliolumbar syndrome.
Objective:
To assess the role of ultrasound-guided intervention for the diagnosis and treatment of the iliolumbar syndrome.
Materials and Methods:
The study comprised of fifty-seven patients of nonspecific low back pain with the clinically suspected iliolumbar syndrome. Two-staged ultrasound-guided interventions were performed: Primary diagnostic and secondary therapeutic interventions. Favorable response after the injection of local anesthetic agent in iliolumbar ligament (defined as VAS score to ≥3) was classified as confirmed Ilio-lumbar syndrome. Clinico radiological efficacy after platelet-rich plasma (PRP) injection in confirmed iliolumbar syndrome patients was done.
Results:
Out of 57 patients, 45 (78.95%) were diagnosed with confirmed Iliolumbar syndrome after primary diagnostic intervention. The mean value of VAS at presentation was 8.02 ± 0.72 which was decreased to 3.16 ± 1.63; P < 0.0001. All 45 patients underwent PRP injection in iliolumbar ligament and 42 patients (93.33%) showed reduction in mean VAS score from 8 ± 0.67 (at presentation) to 0.89 ± 1.23 after 6 weeks follow up; P < 0.0001. Iliolumbar ligament thickness was decreased from the day of presentation (2.66 ± 0.22) to 6 weeks after therapeutic intervention (0.91 ± 0.42); P < 0.0001.
Conclusion:
The ultrasound guided diagnostic and therapeutic intervention were found to result in a specific diagnosis and remarkable recovery in the iliolumbar syndrome group of nonspecific low back pain patients.
Keywords: Iliolumbar syndrome, nonspecific low back pain, ultrasound-guided local anesthetic injection, ultrasound-guided intervention, ultrasound-guided platelet-rich plasma injection
Introduction
Low back pain (LBP), a chronic debilitating condition, is one of the most common causes of disability and absence from work as well as one of the major reasons for physician visits.[1,2,3] Most cases of LBP fall into the category of nonspecific LBP for which no specific pathology can be detected on radiograph or cross-sectional imaging investigations. About half of these patients of nonspecific LBP have a clinical picture characterized by symptoms and signs localized to either iliac crest which is termed as iliolumbar syndrome.[4] Repetitive occupational microtrauma, acute strain, and/or poor posture have been postulated as possible etiologies for chronic iliolumbar syndrome.[5]
History and physical examination are important in the assessment of LBP but they lack sufficient specificity. US-guided targeted injection of small volumes of local anesthetic into the postulated pain generators (i.e., facet or sacroiliac joint injections and the iliolumbar ligament) has been successfully used to increase the specificity of the diagnostic workup in patients with chronic low backache apart from guiding various novel therapeutic injections like dextrose, local anesthetic agent, and corticosteroid.[6,7,8,9] The US is now being extensively used for the diagnosis of iliolumbar syndrome by detecting thickened iliolumbar ligament and also assessing response to the guided local anesthetic injection into the iliolumbar ligament.
Platelet-rich plasma (PRP), obtained by centrifugation, is autologous blood with platelet concentrations above the physiological baseline.[10,11] Although PRP injections have gained considerable attention as a treatment method for musculoskeletal conditions due to their ability to potentially enhance soft tissue healing, there is a paucity of literature on its role in iliolumbar syndrome.[12]
Our prospective interventional study was aimed to evaluate:
The role of US-guided intervention for the diagnosis of iliolumbar syndrome in nonspecific LBP patients.
The clinicoradiological efficacy of US-guided PRP injection in diagnosed cases of Iliolumbar syndrome.
Materials and Methods
This institutional review board and ethical committee approved US-guided interventional prospective study was conducted between March 2018 and March 2019.
The inclusion criteria
Patients of 30–50 years with nonspecific LBP without radicular symptoms for more than 3 months having visual analog score ≥7.
Lumbosacral and sacroiliac joints radiograph without significant findings: a) No osseous disease, b) No vertebral body height reduction, c) No osteophyte, d) No disc space reduction, e) No spondylolisthesis, f) No spondylosis, g) No facet joint hypertrophy, h) No sacroiliac joint space reduction, i) No sacroiliac juxta articular sclerosis, j) No sacroiliac juxta articular erosion.
MRI Lumbosacral spine and sacroiliac joints without significant findings: a) No disc disease, b) No disc-osteophyte complex, c) No vertebral body osseous lesion, d) No end plate disease, e) No epidural/paravertebral collection, f) No spinal canal narrowing, g) No nerve root disease, h) No ligamentum flavum hypertrophy, i) No facet joint space reduction less than 2 mm, or osteophytes, or hypertrophy or subchondral cyst, j) No posterior element osseous lesion, k) No synovial cyst/paravertebral collection.
Normal biochemical tests: Serum calcium, serum phosphate, serum Vitamin “D” level, blood sugar.
Iliolumbar ligament thickening ≥2 mm at posterosuperior iliac spine level on ultrasound imaging.
The exclusion criteria
Nonconsenting patients.
LBP with radicular symptoms.
Abnormality in lumbosacral, sacroiliac radiographs and in MRI, like bony abnormality, disc disease, etc.
History of recent trauma to the spine/lower back.
Deranged biochemical tests of serum calcium, serum phosphate, serum Vitamin “D” level, blood sugar.
Any operative history of the spine/paraspinous region.
Any previous intervention to spine/paraspinous region.
Bleeding diathesis, platelet disorder, patient on an anticoagulant.
Allergy to lidocaine.
Local infection on the lower back like cellulitis, abscess.
History of fever, leukocytosis, raised ESR.
Inability to visualize the iliolumbar ligament like morbid obesity.
Fifty-seven patients strictly meeting the inclusion criteria were included in this study after obtaining informed written consent. Under aseptic precaution, two-stage ultrasound-guided interventions were performed: a) Primary Diagnostic Intervention; b) Secondary Therapeutic intervention.
Primary diagnostic intervention
Procedure
The patient was placed in the prone position with a pillow placed under the abdomen to straighten the lumbar lordosis. A high frequency (12 MHz), linear transducer US transducer was inserted into a sterile sheath containing ultrasound gel. Sterile ultrasound gel was placed between the patient and the transducer. Under strict aseptic precaution, the US transducer was placed obliquely, having the lateral end of the transducer over the affected posterior superior iliac spine and medial end of the transducer over the spinous process of L5 vertebrae to focus the iliolumbar ligament. The soft tissue structures demonstrated in this plane from superficial to deep were skin, subcutaneous tissue, erector spinae muscle, and iliolumbar ligament extending from transverse process of L5 to posterior superior iliac spine [Figure 1]. After infiltrating the skin with 1% lidocaine, a 22 G spinal needle was advanced from the medial side of the transducer deep to erector spinae to iliac side of the iliolumbar ligament. Once the tip of the needle was within the thickened iliolumbar ligament, 3–4 mL of 0.25% plain bupivacaine was injected [Figure 2]. No complications occurred during or after the procedure.
Figure 1.
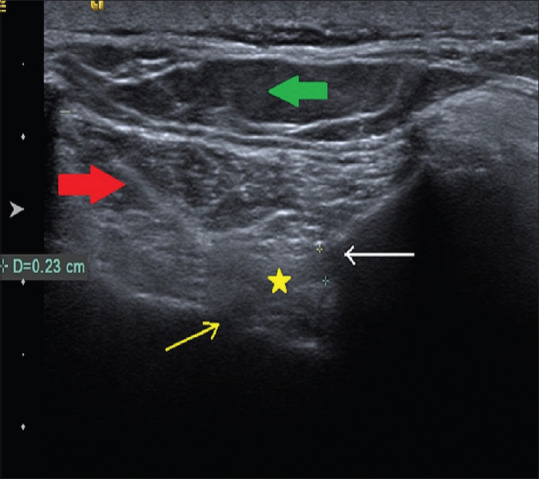
Ultrasound image of iliolumbar ligament [Yellow asterisk: Iliolumbar ligament; Yellow arrow: Transverse process of L5 vertebrae; White arrow: Posterior iliac crest; Red thick arrow: Erector spinae muscle; Thick green arrow: Subcutaneous tissue]
Figure 2.
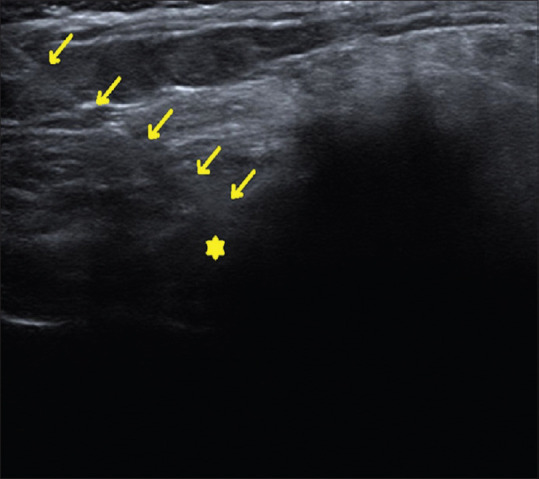
Ultrasound-guided injection of iliolumbar ligament [Yellow asterisk: Iliolumbar ligament; Yellow arrows: Needle trajectory]
Assessment
Clinical pain scoring was assessed both pre- and 72 h post procedure using visual analog score (VAS). The respondents to the primary intervention (reduction of VAS score to = 3) were considered to have a confirmed diagnosis of Ilio-lumbar syndrome.
Secondary therapeutic intervention
Procedure
The respondent group to the primary diagnostic intervention was subjected to ultrasound-guided therapeutic injection of autologous PRP. Under strict aseptic precaution with US transducer maneuver as described in primary diagnostic intervention, 4–5 mL of PRP (prepared in institutional blood bank using the standard protocol of PRP preparation and transportation to the ultrasound interventional room maintaining the cold chain) was injected into iliolumbar ligament after 5–7 gentle fenestrations by the tip of the needle. No complications occurred during or after the procedure. Patients were advised for follow-up US of iliolumbar ligament after 6 weeks.
Assessment
The VAS score and thickness of iliolumbar ligament on ultrasound were assessed 6 weeks after the PRP injection. The reduction in iliolumbar thickness less than 1 mm with reduced VAS to ≥3 was considered to have responded to US-guided PRP intervention [Figure 3].
Figure 3(A and B).
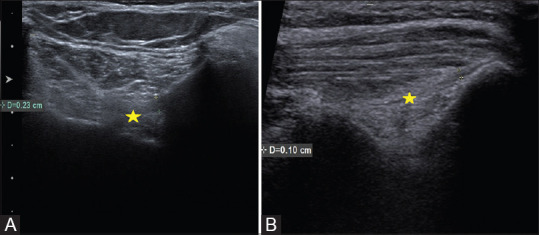
(A and B): Ultrasound images of iliolumbar ligament at presentation (A) and at 6 weeks post PRP follow up (B), demonstrating regression in thickness of iliolumbar ligament from 2.3 mm to 1.0 mm. [Yellow asterisk: Iliolumbar ligament]
Statistical analysis
Categorical variables were presented in number and percentage (') with 95’ CI and continuous variables were presented as mean ± SD and median. Normality of data was tested by Kolmogorov-Smirnov test. If the normality was rejected, then non parametric test was used. Quantitative variables were compared using Wilcoxon signed rank Test (as the data sets were not normally distributed) between pre and post. A P value of <0.05 was considered statistically significant. The data was entered in MS EXCEL spreadsheet and analysis was done using Statistical Package for Social Sciences (SPSS) version 21.0.
Results
A total of fifty-seven patients (n-38 male and n-19 female) ranging from 30 to 50 years of age (mean, 41.04; standard deviation, 6.26; median, 41; interquartile range, 35.75–47) were included in our study [Tables 1 and 2].
Table 1.
Sex distribution
| Frequency | Percentage | 95% CI | |
|---|---|---|---|
| Female | 19 | 33.33% | 20.71% to 45.95% |
| Male | 38 | 66.67% | 54.05% to 79.29% |
| Total | 57 | 100.00% |
Table 2.
Age distribution
| Sample size | Mean±Sthev | Median | Min–Max | Inter quartile Range | |
|---|---|---|---|---|---|
| Age (in years) | 57 | 41.04±6.26 | 41 | 30-50 | 35.750-47 |
Out of fifty-seven patients, forty-five (78.95%) patients responded to primary diagnostic intervention (VAS ≥3 after 72 h) and were diagnosed with confirmed Iliolumbar syndrome and included for secondary therapeutic intervention. Twelve patients (21.05%) did not respond to primary diagnostic intervention and classified as a doubtful iliolumbar syndrome and excluded from the secondary therapeutic intervention [Table 3]. The mean value of VAS at presentation was 8.02 ± 0.72 with a median (interquartile range) of 8 (7.75–9) which was significantly decreased to 3.16 ± 1.63 with median (interquartile range) of 3 (2–3) after the primary diagnostic intervention, P < 0.0001 [Table 4].
Table 3.
Response of primary diagnostic intervention
| Frequency | Percentage | 95% CI | |
|---|---|---|---|
| N | 12 | 21.05% | 10.14% to 31.97% |
| Y | 45 | 78.95% | 68.03% to 89.86% |
| Total | 57 | 100.00% |
N=Non respondents; Y=Respondents
Table 4.
VAS after primary diagnostic intervention
| Sample size | Mean± Sthev | Median | Min–Max | Inter quartile Range | P | |
|---|---|---|---|---|---|---|
| VAS at presentation | 57 | 8.02± 0.72 | 8 | 7-9 | 7.750-9 | <.0001 |
| VAS after primary diagnostic intervention | 57 | 3.16± 1.63 | 3 | 1-8 | 2–3 |
All forty-five patients who responded to primary diagnostic intervention underwent US-guided injection of PRP in iliolumbar ligament (secondary therapeutic intervention). Out of forty-five patients, forty-two patients (93.33') showed a reduction in iliolumbar ligament thickness <1 mm with a reduction of VAS to ≥3 after 6 weeks (Respondent to secondary therapeutic intervention). Three patients (6.67%) did not show a reduction in thickness of iliolumbar ligament or reduction in VAS score to ≥3 or both (Non-respondent to secondary therapeutic intervention) [Table 5]. The mean value of VAS at presentation was 8 ± 0.67 with median (IQR) of 8 (8-8) which was significantly decreased to 0.89 ± 1.23 with median (IQR) of 1 (0-1) after secondary therapeutic intervention, P < 0.0001. Significant decrease was seen in the value of Iliolumbar ligament thickness from day of presentation (2.66 ± 0.22) with median (IQR) of 2.7 (2.575–2.8) to 6 weeks after secondary therapeutic intervention (0.91 ± 0.42) with median (IQR) of 0.8 (0.7–0.9); P < 0.0001 [Table 6].
Table 5.
Response of secondary therapeutic intervention
| Frequency | Percentage | 95% CI | |
|---|---|---|---|
| N | 3 | 6.67% | 0% to 14.25% |
| Y | 42 | 93.33% | 85.75% to 100% |
| Total | 45 | 100.00% |
N=Non respondents; Y=Respondents
Table 6.
VAS and iliolumbar thickening after secondary therapeutic intervention
| Sample size | Mean±Sthev | Median | Min–Max | Inter quartile Range | P | |
|---|---|---|---|---|---|---|
| Iliolumbar ligament thickness at presentation | 45 | 2.66±0.22 | 2.7 | 2-3.1 | 2.575- 2.800 | <.0001 |
| ILL thickening after 6 weeks | 45 | 0.91±0.42 | 0.8 | 0.5-2.7 | 0.700- 0.900 | |
| VAS at presentation | 45 | 8±0.67 | 8 | 7-9 | 8-8 | <.0001 |
| VAS 6 weeks after secondary therapeutic intervention | 45 | 0.89±1.23 | 1 | 0-6 | 0-1 |
Discussion
This study probes into the clinical usefulness of US-guided intervention in the diagnosis of LBP due to iliolumbar syndrome and clinicoradiological efficacy of US-guided PRP injection in patients diagnosed with the iliolumbar syndrome. This usefulness of US hinges on the correct diagnosis of LBP due to iliolumbar syndrome done by evaluating the response to a trial of short-acting local anesthetic injection targeted with high-resolution US and also the response of the respondent patients to sonographically targeted injection of PRP into the diseased iliolumbar ligament.
Out of the fifty-seven patients with suspected Ilio-lumbar syndrome (Chronic nonspecific LBP having normal radiograph, MRI, and laboratory test but thickened iliolumbar ligament), forty-five patients (78.95%) responded positively to primary diagnostic intervention defined as a reduction in the Visual Analogue Scale score (VAS) less than or equal to three 72 h after the injection and were diagnosed with confirmed Iliolumbar syndrome and were included in the group for secondary therapeutic intervention. Twelve patients (21.05%) did not respond to primary diagnostic intervention and were classified as non-responders to the primary diagnostic intervention. The possible reasons for failed response could be contributed to co-existent gluteus medius tendinosis, myofascial trigger point as the pain generator missed on cross-sectional imaging or multi-factorial bio-mechanical etiologies.
In our study, US-guided PRP was injected in forty-five confirmed patients of iliolumbar syndrome who responded to the primary diagnostic interventional challenge. Out of forty-five patients, forty-two patients showed significant clinico-radiological improvement defined as a reduction in iliolumbar thickness to less than 1 mm with reduced VAS score to ≥3. There was a statistically significant reduction in the VAS score to 0.89 ± 1.23 with a median (IQR) of 1 (0-1) after the secondary therapeutic intervention (P-value <0.0001). A significant reduction in the thickness of iliolumbar ligament was also noted post-therapeutic intervention of PRP injection to (0.91 ± 0.42) mm with median (IQR) of 0.8 (0.7–0.9) and P value <0.0001. The three out of forty-five patients who did not respond to either ultrasound-guided primary diagnostic and secondary therapeutic interventions were classified as nonspecific low back pain-not otherwise specified (NSLBP-NOS) and were subjected to rehabilitation, i.e., back muscle strengthening, posture corrections, hamstring and calf rehabilitation, and foot arch evaluation.
Traditional treatments for chronic nonspecific LBP include pharmacologic, steroid, or surgical interventions and the scientific evidence for their efficacy is variable. PRP is a noninvasive effective alternative treatment option for LBP centered on its immune-modulatory and angiogenic properties facilitating tissue healing. The exact mechanism of PRP is still evolving, but current research points to cytokines, growth factors, and other proteins as the main intermediary of action.[13]
There is relative paucity in the literature on the outcome of the use of US-guided PRP injection in iliolumbar syndrome. Statistically significant results from our study will help in formulating the selection, confirmation, and management strategy of patients with iliolumbar syndrome by the novel use of US-guided intervention.
The limitation of our study was the limited sample size from a single institute and no further workup of those patients, who fail the primary diagnostic challenge, despite the working diagnosis of the iliolumbar syndrome. The subjectivity of the VAS score may have introduced response bias and there was no control group to account for a placebo effect of the injections. Further randomized-controlled studies with larger sample sizes and longer follow-ups are warranted to further validate these results.
Conclusion
Ultrasound-guided diagnostic and therapeutic interventions using local anesthetic and PRP, respectively, were found to result in a specific diagnosis and remarkable recovery in the iliolumbar syndrome group of patients with nonspecific LBP. The potential of further exploiting this management strategy in the patients of nonspecific LBP living life in despair needs no further emphasis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976) 1999;24:1813–9. doi: 10.1097/00007632-199909010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Horvath G, Koroknai G, Acs B, Than P, Illes T. Prevalence of low back pain and lumbar spine degenerative disorders. Questionnaire survey and clinical-radiological analysis of a representative Hungarian population. Int Orthop. 2010;34:1245–9. doi: 10.1007/s00264-009-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–8. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschberg GG, Froetscher L, Naeim F. Iliolumbar syndrome as a common cause of low back pain: Diagnosis and prognosis. Arch Phys Med Rehabil. 1979;60:415–9. [PubMed] [Google Scholar]
- 5.Maigne JY, Maigne R. Trigger point of the posterior iliac crest: Painful iliolumbar ligament or cutaneous dorsal ramus pain? An anatomical study. Arch Phys Med Rehabil. 1991;72:734–7. [PubMed] [Google Scholar]
- 6.Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16:142–52. doi: 10.1179/jmt.2008.16.3.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naeim F, Froetscher L, Hirschberg GG. Treatment of the chronic iliolumbar syndrome by infiltration of the iliolumbar ligament. West J Med. 1982;136:372–4. [PMC free article] [PubMed] [Google Scholar]
- 8.El Khoury N, Zebouni SH, Revel M, Fayad F. AB0970 Ultrasonography in diagnosis and management of iliolumbar syndrome: Case series. Ann Rheum Dis. 2016;75:1232–3. [Google Scholar]
- 9.Langevin HM, Stevens-Tuttle D, Fox JR, Badger GJ, Bouffard NA, Krag MH, et al. Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet Disord. 2009;10:151. doi: 10.1186/1471-2474-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: Current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17:602–8. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Hsu WK, Mishra A, Rodeo SR, Fu F, Terry MA, Randelli P, et al. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–48. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 12.Mlynarek RA, Kuhn AW, Bedi A. Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop (Belle Mead NJ) 2016;45:290–326. [PubMed] [Google Scholar]
- 13.Dechellis DM, Cortazzo MH. Regenerative medicine in the field of pain medicine: Prolotherapy, platelet-rich plasma therapy, and stem cell therapy—Theory and evidence. Tech Reg Anesth Pain Manag. 2011;15:74–80. [Google Scholar]


