Abstract
Respiratory distress is one of the leading causes of neonatal morbidity and mortality. Factors such as gestational age at birth, pulmonary maturity, and congenital factors are peculiar to this demographic. Clinical evaluation accompanied by chest radiography is the standard protocol for evaluating the underlying causative factors. Knowledge of the radiographic appearances of various pathologies and associations with certain congenital factors is quintessential for radiologists and primary neonatal care providers to steer the management in the right direction.
Keywords: Chest radiograph, congenital factors, gestational age, neonates, respiratory distress
Introduction
Respiratory distress is a symptom, not a disease. There is increased work of breathing, which manifests as one or more of the following: nasal flaring, tachypnoea, grunting, and chest retractions. Not every neonate with respiratory symptoms has respiratory distress. Some may present due to a slightly delayed adaptation to the extra-uterine environment. However, respiratory distress is the most common cause of admission to neonatal care units. The frequency of preterm neonates having respiratory symptoms is higher than term neonates. Various factors predispose to respiratory distress including neonatal lung development, congenital disorders, perinatal stresses, intrauterine infections, and multisystemic pathologies. Chest radiography, being the most widely available imaging modality with a high degree of sensitivity and relatively limited radiation exposure, is commonly performed to assess the status of the neonatal pulmonary and cardiac systems. The following article classifies the major causes of neonatal respiratory distress and provides insights into their radiographic appearances.
Pediatric Airway Anatomy
Pediatric airway and respiratory system anatomy is different from its adult counterpart, which makes it prone to various pathologies and susceptible to infections. The differences are more pronounced at the time of birth and infancy. The neonatal head is disproportionately larger than the neck and the mandible. Newborn tongue is large and posteriorly placed and there is physiological enlargement of tonsils and adenoids. Pediatric trachea is shorter than that in adults and glottic opening is higher than that of adults. The narrowest part of the pediatric airway is at the level of cricoid cartilage, whereas in adults the narrowest part of upper airway is at the glottic opening. Normal pediatric subglottic airway has rounded shoulders (convex outwards) [Figure 1].
Figure 1.
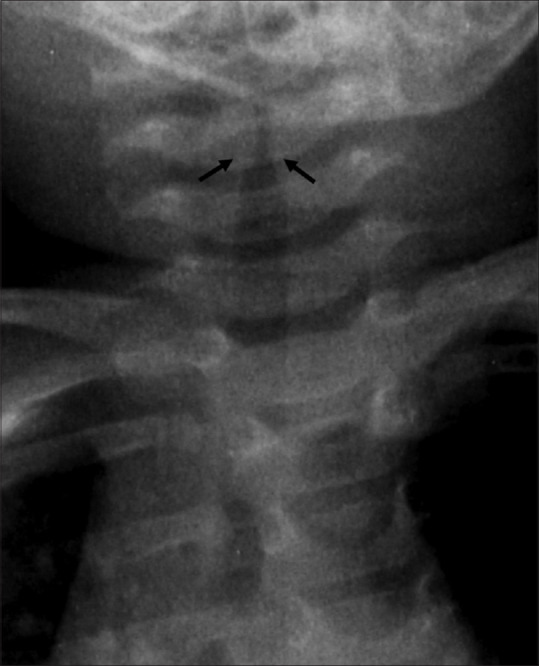
Normal appearance of pediatric subglottic airway. Note the convex margins (outward shoulders) (arrows) at the transition to larynx. Conditions such as croup occurring commonly in children between 6 months and 3 years of age lead to narrowing of this subglottic portion, giving an inverted V-shaped appearance known as steeple sign
Airways tend to be smaller, with poorly developed pores of Kohn and canals of Lambert and thus reduced collateral pathway of aeration. Pulmonary alveoli are thick-walled at birth and fewer in number as compared to adults.
Rules While Performing a Pediatric Chest Radiograph
It is essential to observe imaging guidelines while performing a pediatric chest radiograph to get diagnostic quality images. Most of these are also applicable to neonatal radiography. Rotation should be eliminated with an upright image as far as possible. Appropriate exposure factors should be used, keeping in mind the as low as reasonably achievable (ALARA) principle. Increasing kVp and decreasing mAs within the optimal range to allow adequate exposure to the image receptor is advisable for lowering patient dose. Exposure should be timed with full inspiration and the image should be centered between C3 vertebra and lower costal margin with proper film placement at the level of the upper lip to include upper airway. Care should be taken to avoid over-collimation, or else conditions such as diaphragmatic hernia may be missed. Appropriate lead side marker should be placed. Exposure precaution in the form of lead shielding for gonads is very important. Grid is not necessary for younger children as the soft tissue thickness is not adequate to warrant its use. Also, grids tend to increase the radiation dose to patients.
Basics of Interpreting a Neonatal Chest Radiograph
Standard chest radiograph is an anteroposterior projection. Additionally, lateral view may be occasionally taken to assess pleural effusion and pneumothorax or an oblique view for localizing a pathology in relation to adjacent structures. [1]
The cardiac silhouette may appear enlarged during the first few hours of birth due to the inflow of additional placental blood and bidirectional blood flow through arterial duct and foramen ovale prior to closure. [2] Thymus is large in neonates and may mimic a mediastinal mass. It can be identified due to the peculiar sail sign, which is seen more frequently on the right, and the notch sign, where the inferior border merges with the cardiac silhouette [Figure 2]. The presence of humeral head ossification can be considered as a relative indicator of neonatal maturity. Air is seen in stomach right after birth and further progresses up to rectum in the early hours of life. Appropriate position of tubes and lines [Figure 3] must be ascertained before looking for other pathologies, as a malpositioned nasogastric tube in the left hemithorax may raise a suspicion of diaphragmatic hernia whereas coiling in the upper esophagus may point towards esophageal atresia.
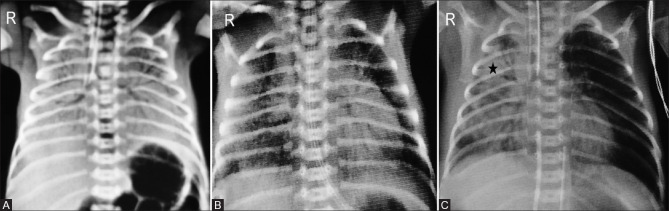
Figure 2.
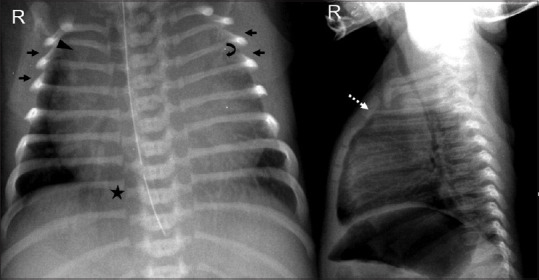
Standardizing the neonatal chest radiograph. Adequate aeration with right hemidiaphragm at the level of posterior arc of right 8th rib (asterisk). Cephalic orientation of anterior ribs (arrows) indicates X-ray tube misalignment. Apparent enlargement of the cardiac silhouette due to oblique orientation of the radiograph towards the left and prominent thymic shadow, which is causing superior mediastinal widening (dotted arrow) as seen on the lateral radiograph. The wave sign (curved arrow) and the notch sign (arrowhead) confirm this. Note that the positioning of nasogastric tube needs to be corrected
Figure 3.
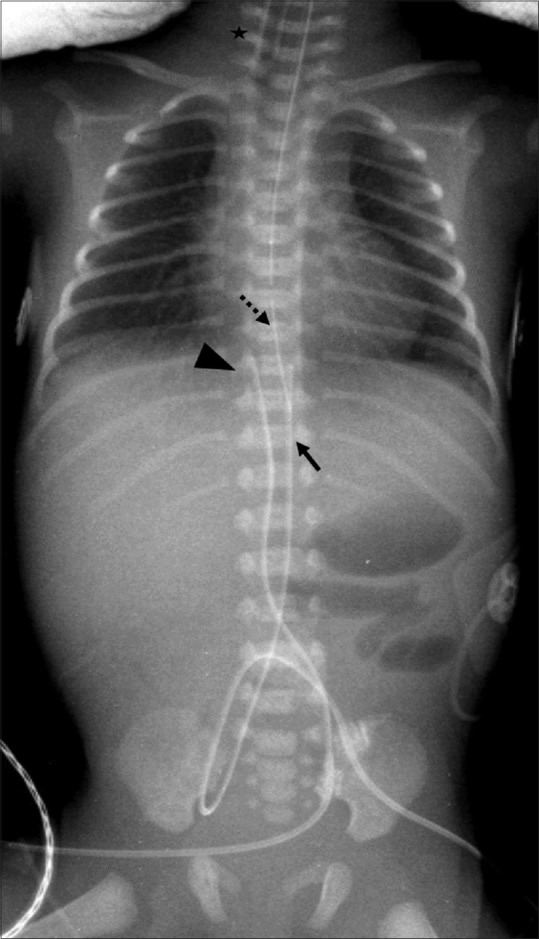
Positioning of lines and tubes. Babygram image shows high position of ET tube (asterisk), normal position of umbilical venous catheter (arrowhead) and umbilical arterial catheter (arrow). Nasogastric tube (dotted arrow) needs to be inserted further to reach stomach
Respiratory Distress in Neonates
The causes of respiratory distress can be classified into four main categories namely medical causes, surgical causes, congenital anomalies and diseases and lastly systemic causes.
A. Medical causes
These produce diffuse lung parenchymal changes. Radiographically, the prime suspects for low lung volumes and coarse reticular opacities are respiratory distress syndrome due to surfactant deficiency or pneumonia secondary to beta-hemolytic streptococci. If the radiograph depicts high lung volumes coupled with streaky perihilar opacities, possibilities to consider are transient tachypnoea of newborn, meconium aspiration syndrome, or neonatal pneumonia. However, an important criterion for distinguishing the underlying cause is the age of the neonate that is whether the infant is preterm/premature or is born at or after normal gestational age.
I] Preterm Infant
Pulmonary maturation occurs in five phases namely embryonic, pseudoglandular, acinar, saccular, and alveolar. [3] Alveolar ducts arise during the acinar phase, with the development of surfactant producing type II pneumocytes. This development of surfactant producing cells occurs predominantly in 24–34 weeks of gestation. Alveolarization, that is a process of increase in the number of alveoli and decrease in the size of air spaces occurs from 36 weeks of gestation and continues till 3 years of postnatal age. [4] High surface tension within the alveolus is diffused by surfactant. Multiple factors govern the structural and physiological lung maturation and thus can be responsible for disorders leading to respiratory distress.
1. Immature lung
It is seen in neonates of 23–26 weeks gestational age with birth weight below 1500 g or severe low birth weight below 1000 g. Their initial radiographs may appear unremarkable; however, towards the end of the first week, lungs become hazy and opacified on radiographs, not hypoaerated or hyperaerated and without air bronchograms [Figure 4]. They may develop congestive cardiac failure due to a patent ductus arteriosus and many proceed to develop bronchopulmonary dysplasia.
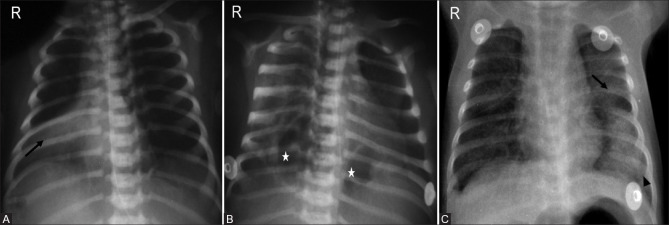
Figure 4(A and B).
Immature lung. (A) Initial radiograph of a premature neonate born at 24 weeks of gestation, weighing 540 grams shows mild coarsening of interstitial markings (arrowheads). (B) Repeat radiograph after 3 weeks reveals diffuse haziness in bilateral lung fields
The autopsy findings state edema and pulmonary hemorrhage rather than atelectasis as the primary pathology. A study done by Edwards et al. [5] found that such neonates had a mature pattern of surfactant phospholipids in the pulmonary effluent and hence, this is a separate radio-pathological entity compared to respiratory distress syndrome.
2. Respiratory distress syndrome
Respiratory distress syndrome (RDS) is an acute injury to immature lungs, affecting neonates typically in the 26–33 weeks gestational age group. However, it can also occur in term infants after cesarean sections, perinatal asphyxia, or in newborns of diabetic mothers. One aspect of this immaturity is surfactant deficiency, which leads to alveolar collapse, further reducing functional residual capacity and increasing dead space. This can lead to elevated pulmonary vascular resistance and thus right to left shunting, which in turn compound the hypoxia and hypoxemia. [6] Another aspect is defective surfactant quality.
Radiographic findings include small lung volumes and vertically oriented ribs leading to a bell-shaped thorax, fine granular (ground-glass) appearance of lung parenchyma, air bronchograms extending from hilum to periphery [Figure 5A–C]. In severe cases there may be a white out lung appearance [Figure 5D]. A normal radiographic film at 6 hours of birth excludes respiratory distress syndrome.
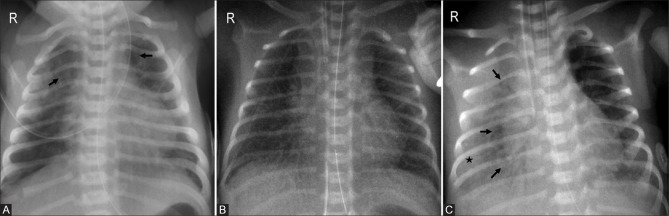
Figure 5(A-D).
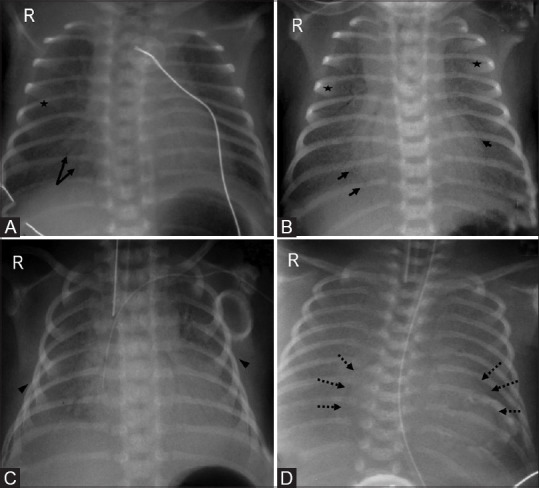
Progression of radiographic findings in respiratory distress syndrome. (A) Stage 1: Fine granularity (asterisk) with few air bronchograms (arrows). (B) Stage 2: Coarse, distinct granularity (asterisks) with extensive air bronchograms (arrows). (C) Stage 3: Increasing opacity with decreasing air bronchograms and granularity. Note the vertical orientation of ribs (arrowheads) with bell shaped thorax. (D) Stage 4: “Whiteout lung”-Diffuse bilateral opacification with lack of apparent heart borders (dotted arrows) and loss of all air bronchograms
Respiratory support and exogenous surfactant therapy are the common treatment modalities for RDS. Administration of surfactant changes the radiographic picture of RDS. There may be excellent response which radiographically appears as clearing of granular opacities and increased lung volumes [Figure 6A and B]. On occasion, there can be asymmetric or partial response leading to heterogenous appearance of granular opacities in untreated areas and expansion of treated areas [Figure 6c]. [7] No radiographic findings of response are associated with poor prognosis.
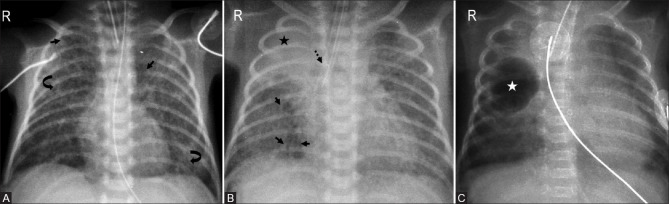
Figure 6(A-C).
Post surfactant therapy changes. (A) Extensive bilateral opacities and air bronchograms (arrows) prior to initiation of surfactant treatment. (B) Increased lung volumes with clearing of pulmonary opacities bilaterally. (C) Asymmetric surfactant response with clearing of left lung and expanded left lung volume in a different patient. Persistent granular opacities (asterisk) are seen involving right lung with air bronchograms (arrows)
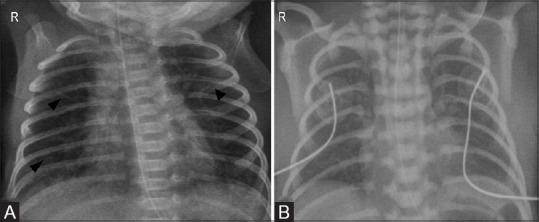
Positive pressure ventilation with or without surfactant therapy has been found to have several complications, particularly in preterm lungs with poor alveolar compliance. The adverse effects of barotrauma can lead to air leak, which manifests as one or more of the following: pulmonary interstitial emphysema (PIE) [Figure 7], pneumothorax [Figure 8A], pneumomediastinum, pneumopericardium [Figure 8B], pneumoperitoneum, air embolism. Other treatment-related complications include pneumonia, pulmonary hemorrhage, bronchopulmonary dysplasia, and systemic air embolism, a rare complication of ventilatory support.
Figure 7(A-C).
Pulmonary interstitial emphysema. (A) Frontal radiograph of the chest in a 30-week-old premature infant post surfactant therapy and positive pressure ventilation reveals bilateral cystic lucencies (arrows) and granular opacities (curved arrows) suggesting evolving pulmonary interstitial emphysema. (B) Another preterm infant with development of tubular bubbly lucencies in right lower zone with atelectasis (asterisk) in right upper zone. Note the malpositioned endotracheal tube in right main bronchus (dotted arrow). (C) A localized form of PIE with thin walled pseudocyst (asterisk), commonly seen in right parahilar regio
Figure 8(A and B).
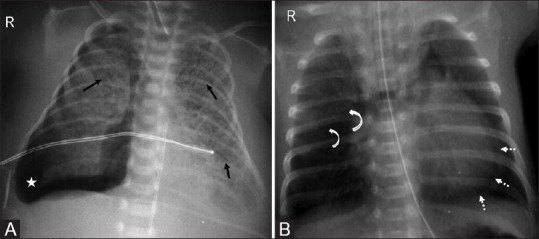
Barotrauma causing air leak. (A) Linear cystic lucencies (arrows) depicting pulmonary interstitial emphysema. There is right-sided pneumothorax (asterisk) with mediastinal shift to the left. (B) Upward and lateral displacement of thymic lobes (curved arrows) depicting angel wing sign of pneumomediastinum associated with pneumopericardium (dotted arrows)
Untreated form of respiratory distress syndrome presents with recovery towards the end of the first week. Respiratory decompensation can occur after initial recovery due to secondary surfactant deficiency [Figure 9]. Inflammatory mediators released during processes such as pneumonia, pulmonary edema, or hemorrhage damage surfactant producing cells. [8] Secondary surfactant deficiency can be treated by administering surfactant.
Figure 9(A-C).
Secondary surfactant deficiency. (A) Initial white out lung appearance prior to initiation of surfactant therapy. (B) Clearing of opacities on day 7 post surfactant therapy. (C) Radiograph taken on day 14 shows development of right lung opacity (asterisk) due to pneumonia
Chronic Neonatal Lung Disease/Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of prematurity due to prolonged high pressure mechanical ventilation and high inspired oxygen volumes. [9] It is uncommon in children >32 weeks of gestational age. The incidence is high in premature infants needing ventilatory support, affecting more than 50% premature children with birth weight <1000 g. These severely premature neonates tend to develop a variant form of bronchopulmonary dysplasia, with infection and pulmonary edema. The respiratory difficulties with preterm birth can be deemed to be a Catch 22 situation, where all events that cause lung damage increase the need of mechanical ventilation which again increases lung damage.
The radiographic manifestations of BPD can be classified into the following stages [Figure 10 and Table 1]: [10,11]
Figure 10(A-D).
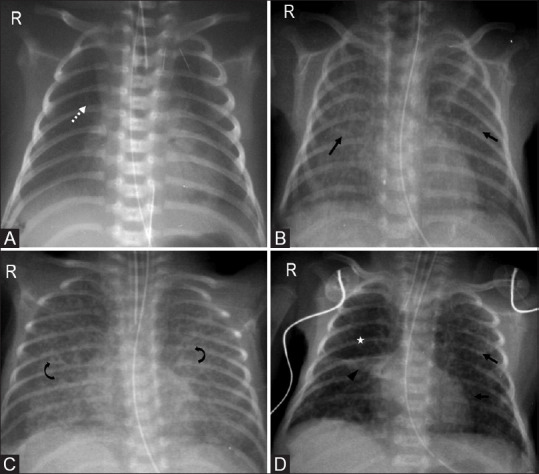
Progression from respiratory distress syndrome to bronchopulmonary dysplasia. (A) Stage 1: Initial radiograph depicting fine granular opacities (dotted arrow) of respiratory distress syndrome. (B) Stage 2: Radiograph taken on day 7 reveals bilateral increased opacities (arrows). (C) Stage 3: Day 10 radiograph with coarsening of markings (curved arrows) and honeycomb appearance. (D) Stage 4: Coarse interstitial markings (arrows), area of atelectasis (arrowhead) with hyperexpansion (asterisk) on day 30 radiograph are characteristic of bronchopulmonary dysplasia
Table 1.
BPD Classification
| Stage | Time | Radiographic appearance |
|---|---|---|
| 1 | 1-3 days | Ground glass opacities, similar to respiratory distress syndrome |
| 2 | 4-10 days | Bilateral increased density- “White out lung” |
| 3 | 10-20 days | Honeycombing: bubbly appearance interspersed by areas of irregular density |
| 4 | >1 month | Emphysematous changes i.e., hyper-expansion of bubbles and fibrotic strands |
10% of cases demonstrate complete resolution
II] Full-Term Infant
Neonates born at full term or post term have definitive alveoli and mature type II surfactant producing pneumocytes. Yet, these infants may suffer from respiratory distress due to various factors such as cesarean delivery, meconium-stained amniotic fluid, gestational diabetes, maternal chorioamnionitis amongst medical causes, and structural lung abnormalities amongst surgical causes. The common conditions manifesting as respiratory distress in full-term/post-term infants are:
1. Transient Tachypnoea of the Newborn (TTN)
Fetal lungs are fluid filled in utero. At the terminal stages of gestation as well as at the time of delivery, there is increased absorption of fluid from alveolar epithelium, a process which is enhanced by labor. [12] The absorption is governed by Rule of Threes that is, 1/3rd fluid is squeezed out during vaginal delivery, 1/3rd is resorbed by lymphatics and 1/3rd by capillaries. If delivery occurs before the onset of labor or by cesarean section, it can lead to a retained fetal lung fluid syndrome. The respiratory distress tends to first manifest around 6 hours postpartum, peaks at 24 hours and usually resolved by 72 hours, along with resolution of radiographic findings. Infants may require supplemental oxygen therapy. Classic radiographic presentation includes hyperinflated lungs with diffuse airspace opacification, coarse interstitial markings, fissural fluid, and cardiomegaly [Figure 11].
Figure 11(A and B).
Transient tachypnea of newborn. Term infant with respiratory distress. (A) Radiograph taken at 6 hours after birth reveals coarse interstitial markings (curved arrows) with mild cardiomegaly (dotted arrow). (B) Repeat radiograph after 3 days reveals clearing of opacities with normalization of cardiac size (dotted arrow)
2. Meconium Aspiration Syndrome (MAS)
Meconium-stained amniotic fluid is usually seen after 37 weeks of gestational age. Meconium causes inflammation and epithelial injury to the lungs. The incidence of meconium-stained amniotic fluid at delivery is nearly 10%; however, it is seen that only 10% of such neonates develop respiratory distress due to meconium aspiration syndrome. [13] Meconium aspiration syndrome manifests within a few hours of birth as respiratory distress that is tachypnoea, grunting, cyanosis, and chest retractions. On chest radiographs, the lung volumes appear increased. There is irregular aeration with coarse, perihilar densities representing scattered areas of atelectasis and consolidation mixed with focal areas of overinflation and peripheral air trapping [Figure 12A and B]. Of the various causes of respiratory distress in newborns, air leak in the form of pneumomediastinum and pneumothorax, as well as persistent pulmonary hypertension seen by reduced pulmonary vascularity are common in meconium aspiration syndrome [Figure 12C and D].
Figure 12(A-D).
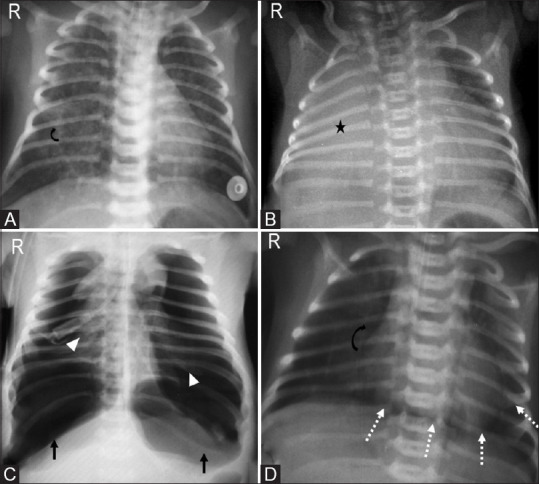
Meconium aspiration syndrome. (A) Neonate born with meconium stained amniotic fluid at 38 weeks of gestation with hyperinflated lungs and coarse perihilar reticular markings (curved arrow). (B) Another post-term infant with complete right lung atelectasis (asterisk). (C) Bilateral tension pneumothoraces (arrows) with collapsed bilateral lungs (arrowheads) and (D) pneumopericardium (dotted arrows) as seen by continuous diaphragm sign, reduced pulmonary vascularity (curved arrow) are commonly associated with meconium aspiration syndrome
3. Neonatal Pneumonia
Pneumonia in neonates occurs more often in preterm rather than term neonates. It can be early onset (less than or equal to 7 days of age) or late onset (more than 7 days of age). [13] Most common organisms associated with neonatal pneumonia are Group B streptococci, klebsiella pneumoniae, staphylococcus aureus. Chest radiographs can present as bilateral granular opacities with low lung volumes, thus difficult to differentiate from respiratory distress syndrome. However, diffuse parenchymal infiltrates with air bronchograms or areas of consolidation with lobar involvement is a more common radiographic appearance [Figure 13]. Pleural effusions are seen in 25–67% of the cases.
Figure 13(A-C).
Neonatal pneumonia. (A) Area of consolidation (arrow) with air bronchograms in right perihilar region. (B) Pneumatoceles (asterisks) are a common complication of staphylococcal pneumonia. This appearance may mislead to other diagnoses such as congenital pulmonary malformations. (C) Respiratory syncytial virus infection in a 27-day-old neonate with perihilar streakiness (arrow) and left lower lobe consolidation (arrowhead)
Overview of Medical Causes of Respiratory Distress [Table 2][10,11]
Table 2.
Overview of Medical Causes of Respiratory Distress
| Disease | Typical association | Time course | Lung volume | Characteristic feature |
|---|---|---|---|---|
| Respiratory distress syndrome (RDS) | Preterm | <6 hours | ↓ | Ground glass opacities/ fine granular infiltrates. |
| Transient tachypnoea of newborn (TTN) | Term, Cesarean section | 24-48 hours | ↑ | Coarse interstitial markings, interstitial edema. |
| Meconium aspiration syndrome (MAS) | Term, Post term | 12-24hoursh | ↑ | Coarse, nodular, asymmetric infiltrates, atelectasis. |
| Neonatal pneumonia | Premature rupture of membranes | <6 hours | ↑ | Perihilar streaking, consolidation, pleural effusion |
B. Surgical Causes
Respiratory distress may be secondary to certain focal pulmonary disorders which have more defined radiographic appearances [14] such as congenital diaphragmatic hernia, bronchopulmonary foregut malformation, congenital cystic adenomatoid malformation (now known as congenital pulmonary airway malformation), congenital lobar overinflation, pulmonary sequestration, and esophageal atresia.
1. Congenital Diaphragmatic Hernia (CDH)
Congenital diaphragmatic hernia is considered to be a syndrome rather than an individual entity, consistent of hernia, pulmonary hypoplasia and immaturity, left heart hypoplasia and persistent pulmonary hypertension. The most common site of defect is on the posterolateral aspect of the left hemidiaphragm, known as Bochdalek hernia. Other diaphragmatic hernias include- anterior (typically on the right) known as Morgagni hernia, and central tendon defect (septum transversum defect). Severe diaphragmatic eventration should be treated as CDH.
Radiographic findings [Figure 14] include cystic lucencies in hemithorax with indistinct diaphragmatic opacity, however the latter is not very reliable. Upturned nasogastric tube in left hemithorax is another imaging finding. Absence of bowel gases in abdomen confirm displacement of bowel loops into thoracic cavity. An important differential of CDH is congenital pulmonary airway malformation, which can be ruled out by passing a nasogastric tube into the stomach and injecting non-ionic contrast to demonstrate the position of bowel loops.
Figure 14(A and B).
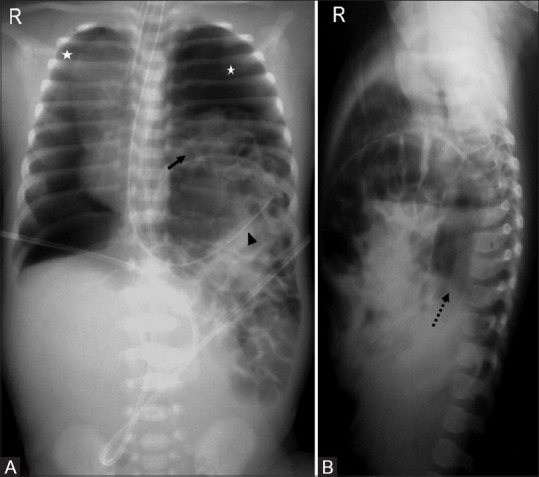
Congenital diaphragmatic hernia. Frontal (A) and lateral (B) babygrams with cystic lucencies (arrow) in left hemithorax, upturned nasogastric tube (arrowhead), mediastinal shift to the right, bilateral pneumothoraces (asterisks), and paucity of bowel gases in abdomen. Right hemidiaphragm is well seen on lateral radiograph (dotted arrow) with non-visualization of left hemidiaphragm
2. Congenital pulmonary airway malformation (CPAM)
Formerly known as congenital cystic adenomatoid malformation (CCAM), is a hamartomatous lesion due to adenomatoid proliferation of bronchial structures that form cysts and lack alveoli. It is associated with supplying airways which lack cartilage. Most often, CPAM is confined to a single lobe. [15] According to the modified Stocker classification, CPAM is classified into 5 types: [16]
Type 0- lethal, consistent of acinar dysgenesis or dysplasia.
Type 1 (about 50% of the cases) with single or multiple large cysts measuring 2–10 cm in size.
Type 2 (about 40% of the cases) with multiple small uniform cysts <2 cm in size.
Type 3 (nearly 10% of the cases) with solid appearance however consistent of microscopic cysts.
Type 4- multiple large peripheral cysts, indistinguishable from type 1 on imaging.
Antenatal ultrasound reveals heterogeneously hyperechoic areas which may be better characterised by fetal MRI. The appearance of CPAM is variable, and dependent on the clearance of fluid, can have a mass-like appearance, which may subsequently change to unilocular or multilocular cysts, dependent on the subtype. Chest radiographs may reveal cystic lucencies which may initially appear opaque due to fluid [Figure 15A]; however, CT chest is performed for confirmation [Figure 15B].
Figure 15(A and B).
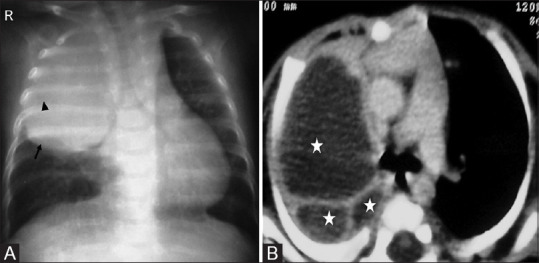
Congenital pulmonary airway malformation. (A) Rounded soft tissue density mass in right upper lobe (arrow) with subtle central cystic lucencies (arrowhead). (B) Axial post contrast CT reveals multiple large varying sized cysts (asterisks) consistent with Stocker type 1 CPAM
3. Congenital Lobar Overinflation (CLO)
Congenital lobar overinflation, also known as congenital lobar emphysema is not a specific disease, rather a constellation of clinical findings with specific imaging characteristics. It is a developmental malformation where lobar hyperinflation occurs due to bronchial obstruction. Left upper lobe involvement is seen in 40–50% cases, with 28–34% cases showing right middle lobe involvement and 20% involving right upper lobe. In majority of the patients, the etiology is unknown. Some treatable causes include (a) intrinsic bronchial structural abnormalities such as deficient bronchial cartilage, bronchial stenosis or atresia, (b) extrinsic factors such as pulmonary artery sling, mediastinal mass, bronchogenic cyst, esophageal duplication cyst, and (c) parenchymal disorders such as polyalveolar lobe or pulmonary alveolar glycogenesis. [17]
Imaging characteristics include hyperinflated lobe of lung (in first few days, it may be fluid-filled) with compressive atelectasis of the adjacent lobes [Figure 16A]. There is no evidence of septae or cyst walls within the expanded lobe. Hyperinflation may be associated with contralateral mediastinal shift. Further imaging with CT can help confirm the diagnosis and identify treatable causes [Figure 16B and C].
Figure 16(A-C).
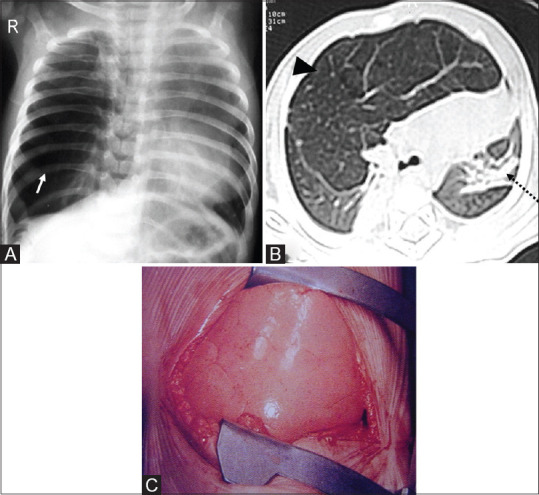
Congenital lobar overinflation. (A) Hyperinflated right lung (arrow) with persistent vascular markings, distinguishing it from pneumothorax. (B) Axial CT scan of the same patient confirms overinflation (arrowhead) with mediastinal shift to the left and lingular compressive atelectasis (dotted arrow). (C) Intra-operative image with spongy appearance of lung
4. Bronchopulmonary sequestration
This is a developmental abnormality where focal lung tissue does not demonstrate communication with bronchial tree. It is classified into intrapulmonary and extrapulmonary type, with extrapulmonary (extralobar) type presenting in neonatal period. Sequestration appears as a lower lobe opacity and is indistinguishable from congenital pulmonary airway malformation or neonatal pneumonia on radiographs.
C. Congenital Thoracic Anomalies and Diseases
Certain congenital anomalies may manifest as respiratory distress immediately after birth. These can be intrapulmonary or extrapulmonary. The predominant intrapulmonary causes have been discussed above. Extrapulmonary causes can be related to (a) central airways such as tracheomalacia, trachea-esophageal fistula, bronchopulmonary foregut malformations (b) congenital cardiac causes (c) chest wall abnormalities.
1. Tracheomalacia
It is one of the commonest congenital anomalies of central airways which results from weakness of tracheal wall and cartilage, leading to excessive collapsibility during expiration (>50% reduction in cross-sectional lumen). Congenital tracheomalacia can be diffuse or focal that is short segment funnel-like narrowing or focal stenosis. Diffuse form is commoner in premature infants and is commonly associated with laryngomalacia. [18] Frontal radiograph is usually normal. Lateral radiograph in inspiratory and expiratory phases may reveal reduction in tracheal caliber by more than 50% during expiration [Figure 17A]. Imaging modality of choice is paired inspiratory-dynamic expiratory CT scan. On expiratory imaging, crescentic frown-like configuration of the tracheal lumen with less than 6 mm AP diameter is characteristic. Bronchoscopic evaluation is indicated for confirmation of diagnosis [Figure 17B and C].
Figure 17(A-C).
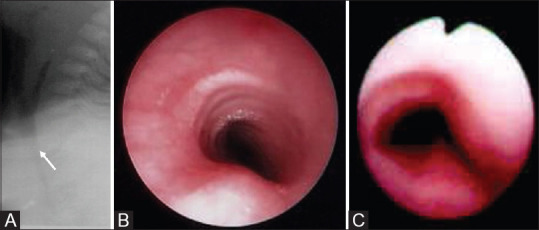
Tracheomalacia. (A) Lateral radiograph reveals reduced caliber of trachea (arrow). (B) Normal endoscopic appearance. (C) Fish-mouth appearance in tracheomalacia
2. Tracheo-esophageal fistula
Abnormalities of tracheo-esophageal separation lead to esophageal atresia with or without associated trachea-esophageal fistula. Neonates present with feeding difficulties and respiratory distress due to recurrent aspiration. Coiling of nasogastric tube in upper esophagus is typical for esophageal atresia without fistula. Air in stomach or bowel loops indicates presence of distal fistula [Figure 18]. Lung fields depict non-specific changes such as areas of atelectasis or consolidation secondary to recurrent aspiration of feeds.
Figure 18.
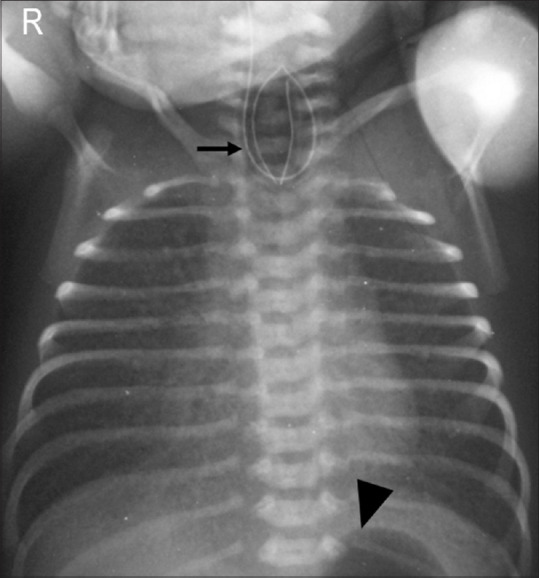
Tracheo-esophageal fistula. Coiling of nasogastric tube (arrow) in proximal esophagus with presence of fundic gas (arrowhead) indicative of esophageal atresia and distal trachea-esophageal fistula (type C)
3. Bronchopulmonary foregut malformations
Foregut duplication cysts such as bronchogenic cyst, esophageal duplication cyst, along with pulmonary sequestration and CPAM constitute this spectrum. Foregut duplication cysts are uncommon causes of mediastinal and pulmonary lesions which may manifest as neonatal respiratory distress due to mass effect. On plain radiographs they may appear as soft tissue density mass like lesions, with distal lobar overinflation due to mass effect.
4. Congenital cardiac causes
Congenital cardiac diseases lead to severe metabolic acidosis and pulmonary edema that are responsible for respiratory distress in newborns. While metabolic acidosis occurs due to hypoxia which is seen in multiple congenital cardiac diseases, pulmonary edema is seen in association with left-sided heart failure or obstructed pulmonary venous return. [19] The presence of cyanosis and cardiac murmurs with less severe respiratory distress points towards an underlying heart disease. Pulmonary vascular congestion secondary to cardiac causes presents with increased vascular markings, cardiomegaly, interstitial and alveolar edema. Patent ductus arteriosus is a common cause of respiratory distress in preterm newborns, presenting with prominent interstitial markings and cardiomegaly due to left to right shunting [Figure 19A]. Atrioventricular canal defect, ventricular septal defect cause respiratory distress in term- and post-term newborns [Figure 19B].
Figure 19(A and B).
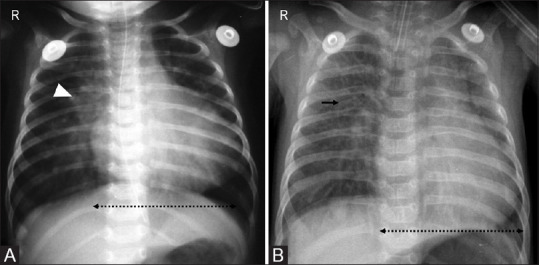
Cardiac causes of respiratory distress. (A) Respiratory distress in a newborn due to patent ductus arteriosus with radiographic features of mild cardiomegaly (dotted arrow) and increased pulmonary vascular markings (arrowhead) due to shunt vascularity. (B) Term neonate with changes of volume overload: cardiomegaly (dotted arrow) and interstitial edema (arrow) due to ventricular septal defect
5. Chest wall abnormalities
Chest wall hypoplasia, either idiopathic or secondary to various skeletal dysplasias may present as respiratory distress in newborns [Figure 20]. Other congenital chest wall disorders such as pectus excavatum, pectus carinatum and Poland syndrome, while clinically appreciable in neonates, may become symptomatic only in late childhood or adulthood.
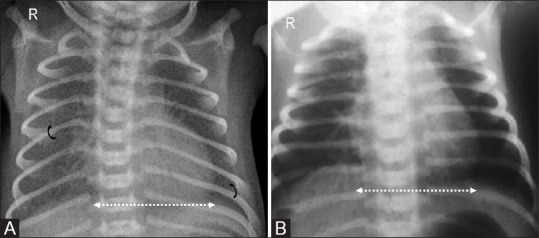
Figure 20.
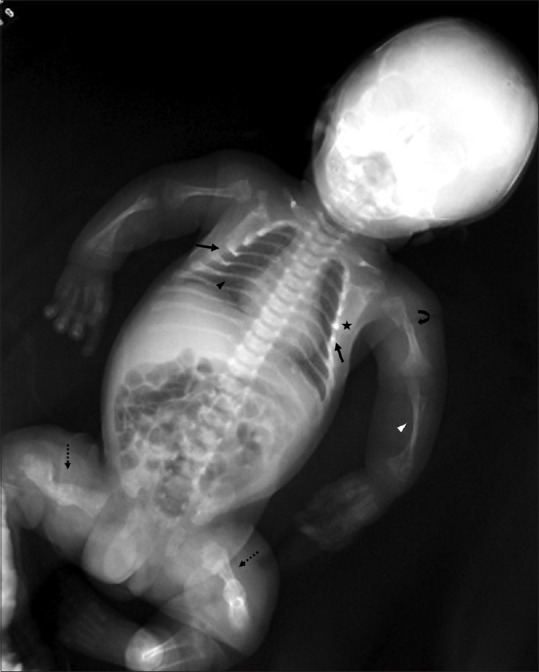
Chest wall abnormalities. Babygram of a patient with osteogenesis imperfecta. Note the small, bell shaped thorax (arrows). Also seen are skeletal manifestations such as beaded ribs (asterisk), thinning of posterior ribs (black arrowhead), gracile bones with thinning of diaphysis (white arrowhead), fractures (curved arrow), hypertrophic callus formation and bony angulation leading to thick, stubby appearance of bilateral femora (dotted arrows)
D. Systemic causes
Respiratory distress in newborns is not solely due to pulmonary causes. There are many systemic causes which may lead to respiratory distress such as:
Metabolic causes—acidosis, hypoglycemia
Infections—sepsis, neonatal TORCH infections
Anemia
Central nervous system disorders—hypoxic ischemic encephalopathy, vein of Galen malformation
Neuromuscular disorders—neonatal myasthenia gravis
Cardiac causes—cardiac tamponade, neonatal myocarditis.
Accurate diagnosis requires detailed clinical history, physical examination, and laboratory evaluation. Few conditions such as neonatal sepsis and cardiac diseases warrant radiographic evaluation, while some cases may not demonstrate radiographic abnormalities. Neonatal sepsis may present as focal pulmonary infiltrates/consolidation [Figure 21].
Figure 21.
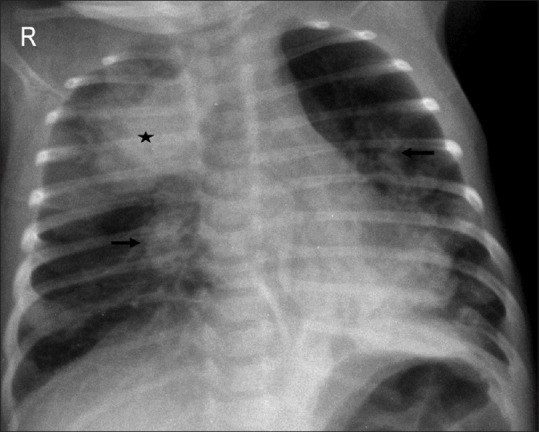
Systemic causes. Neonate in sepsis with an area of consolidation in right upper lobe (asterisk) and bilateral perihilar coarse granular opacities (arrows)
Conclusion
A thorough evaluation of neonatal chest radiographs is important for disease classification and management. Despite similar appearances in various pathologies, clinico-radiological correlation can help in accurate diagnosis and timely management. Due to limitations of utilization of other imaging modalities in this age group, sound knowledge of radiographic appearances forms the backbone of neonatal respiratory distress management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Goo HW, Drubach LA, Lee EY. Imaging techniques. In: Coley BD, editor. Caffey's Pediatric Diagnostic Imaging. Philadelphia, PA: Elsevier; 2013. pp. 506–18. [Google Scholar]
- 2.Álvares BR, Pereira IC, Araújo Neto SA, Sakuma ET. Normal findings on chest X-ray of the newborn. Brazilian Radiology. 2006;39:435–40. [Google Scholar]
- 3.Langston C. Prenatal lung growth and pulmonary hypoplasia. In: Stocker JT, editor. Pediatric Pulmonary Disease. Washington, DC: Hemisphere; 1989. pp. 1–3. [Google Scholar]
- 4.Copland I, Post M. Lung development and fetal lung growth. Paediatr Respir Rev. 2004;5(S2):59–64. doi: 10.1016/s1526-0542(04)90049-8. [DOI] [PubMed] [Google Scholar]
- 5.Edwards DK, Jacob J, Gluck L. The immature lung: Radiographic appearance, course, and complications. Am J Roentgenol. 1980;135:659–66. doi: 10.2214/ajr.135.4.659. [DOI] [PubMed] [Google Scholar]
- 6.Agrons GA, Courtney SE, Stocker JT, Markowitz RI. Lung disease in premature neonates: Radiologic-pathologic correlation. Radiographics. 2005;25:1047–73. doi: 10.1148/rg.254055019. [DOI] [PubMed] [Google Scholar]
- 7.Dinger J, Schwarze R, Rupprecht E. Radiological changes after therapeutic use of surfactant in infants with respiratory distress syndrome. Pediatr Radiol. 1997;27:26–31. doi: 10.1007/s002470050057. [DOI] [PubMed] [Google Scholar]
- 8.Bissinger R, Carlson C, Hulsey T, Eicher D. Secondary surfactant deficiency in neonates. J Perinatol. 2004;24:663–6. doi: 10.1038/sj.jp.7211154. [DOI] [PubMed] [Google Scholar]
- 9.Cherukupalli K, Larson JE, Rotschild A, Thurlbeck WM. Biochemical, clinical, and morphologic studies on lungs of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1996;22:215–29. doi: 10.1002/(SICI)1099-0496(199610)22:4<215::AID-PPUL1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Slovis T. Caffey's Pediatric Diagnostic Imaging. 11th ed. Elsevier Health Sciences; 2008. Congenital and acquired lesions (Most causing respiratory distress) [Google Scholar]
- 11.Ward R, Blickman H. Pediatric Imaging: Case Review Series. Elsevier Health Sciences; 2005. [Google Scholar]
- 12.Weisman LE, Hansen TN. Contemporary Diagnosis and Management of Neonatal Respiratory Diseases. 3rd ed. Newton, PA: Handbooks in Health Care Co; 2003. [Google Scholar]
- 13.Pramanik AK, Rangaswamy N, Gates T. Neonatal respiratory distress: A practical approach to its diagnosis and management. Pediatr Clin. 2015;62:453–69. doi: 10.1016/j.pcl.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Liszewski MC, Lee EY. Neonatal lung disorders: Pattern recognition approach to diagnosis. AJR Am J Roentgenol. 2018;210:964–75. doi: 10.2214/AJR.17.19231. [DOI] [PubMed] [Google Scholar]
- 15.Stocker JT. Cystic lung disease in infants and children. Fetal Pediatr Pathol. 2009;28:155–84. doi: 10.1080/15513810902984095. [DOI] [PubMed] [Google Scholar]
- 16.Stocker J, Mani H, Husain AN. The respiratory tract. In: Stocker JT, Dehner LP, Husain AN, editors. Stocker & Dehner's Pediatric Pathology. 3rd ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2011. pp. 441–515. [Google Scholar]
- 17.Demir OF, Hangul M, Kose M. Congenital lobar emphysema: Diagnosis and treatment options. Int J Chron Obstruct Pulmon Dis. 2019;14:921. doi: 10.2147/COPD.S170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Cascella M. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. [Last Updated on 2020 Jan 14]. Tracheomalacia. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553191/ [Google Scholar]
- 19.Rudolph AM. Diagnosis and treatment: Respiratory distress and cardiac disease in infancy. Pediatrics. 1965;35:999–1002. [PubMed] [Google Scholar]