Abstract
Aims
Scapular notching is thought to have an adverse effect on the outcome of reverse total shoulder arthroplasty (RTSA). However, the matter is still controversial. The aim of this study was to determine the clinical impact of scapular notching on outcomes after RTSA.
Methods
Three electronic databases (PubMed, Cochrane Database, and EMBASE) were searched for studies which evaluated the influence of scapular notching on clinical outcome after RTSA. The quality of each study was assessed. Functional outcome scores (the Constant-Murley scores (CMS), and the American Shoulder and Elbow Surgeons (ASES) scores), and postoperative range of movement (forward flexion (FF), abduction, and external rotation (ER)) were extracted and subjected to meta-analysis. Effect sizes were expressed as weighted mean differences (WMD).
Results
In all, 11 studies (two level III and nine level IV) were included in the meta-analysis. All analyzed variables indicated that scapular notching has a negative effect on the outcome of RTSA . Statistical significance was found for the CMS (WMD –3.11; 95% confidence interval (CI) –4.98 to –1.23), the ASES score (WMD –6.50; 95% CI –10.80 to –2.19), FF (WMD –6.3°; 95% CI –9.9° to –2.6°), and abduction (WMD –9.4°; 95% CI –17.8° to –1.0°), but not for ER (WMD –0.6°; 95% CI –3.7° to 2.5°).
Conclusion
The current literature suggests that patients with scapular notching after RTSA have significantly worse results when evaluated by the CMS, ASES score, and range of movement in flexion and abduction.
Cite this article: Bone Joint J 2020;102-B(11):1438–1445.
Keywords: reverse total shoulder arthroplasty, scapular notching, meta-analysis
Introduction
Reverse total shoulder arthroplasty (RTSA) is one of the most effective ways of managing patients with an irreparable rotator cuff tear, a cuff tear arthropathy, or a fracture of the proximal humerus,1-4 as a a conventional shoulder arthroplasty cannot guarantee a reliable outcome. This is mainly because a conventional shoulder arthroplasty is compromised by a non-functioning rotator cuff.5 Since Grammont6 introduced the RTSA and its several subsequent modifications,5 it is now gaining in popularity7 and many mid-term follow-up studies have shown its effectiveness.8-17 Although its non-anatomical features make it both original and unique, these attributes are also associated with complications and limitations.
Scapular notching, in particular, is a unique phenomenon of RTSA, and is caused by mechanical impingement between the humeral component and the neck of the glenoid. This can cause wear of the polyethylene humeral component and consequent osteolysis of the surrounding bone.18,19 Many have predicted that scapular notching can also cause instability of the glenoid component; 20,21 others have expressed concern that scapular notching may have a negative effect on clinical outcome.14,22–30 The incidence of scapular notching after RTSA ranges from 10% to 96%.18,25,31 Due to its rising popularity and the alarmingly high reported incidence of scapular notching in some reports, it is important to have a better understanding of its complications.5 For this reason, many researchers have compared the clinical outcome of patients with scapular notching after RTSA to those of patients without scapular notching.8,14,16,22–30,32–44 However, the issue remains controversial.
Most studies on the topic have been underpowered25,29 because of the relatively small number of patients enrolled and an imbalance between the number of patients in notched and non-notched groups.25,29 Furthermore, the rate of scapular notching after RTSA is dependent on the implant design and its orientation.5,45,46 Consequently, we considered that a comprehensive review was needed which weighted the statistical powers of previous studies. Meta-analysis is the best available option for improving the level of evidence and provides a means of addressing the issue of underpowering. However, to the best of our knowledge, no meta-analysis has been previously been undertaken on the effect of scapular notching on patient outcome after RTSA.
The purpose of this meta-analysis was to compare the clinical variables of patients with and without scapular notching. Our hypothesis was that patients with scapular notching would have a worse clinical outcome in terms of functional score and range of movement.
Methods
Inclusion and exclusion criteria
A systematic review of the literature was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) protocol. Search criteria and review objectives were defined before the literature search was conducted. Following this protocol, a systematic search was undertaken using PubMed, EMBASE, and the Cochrane Database of Systematic Reviews. These electronic databases were searched from January 1980 to January 2020. Text searches used as ‘[{(notch*) OR (imping*) OR (abut*)} AND {(RTSA) OR (reverse arthroplasty) OR (reverse shoulder arthroplasty) OR (reverse total shoulder arthroplasty)}]’ in the three databases.
The inclusion criteria applied were that they were written in English, reported clinical outcomes and radiological assessments of scapular notching, and had a minimum two years of follow-up. The exclusion criteria were biomechanical studies, cadaver studies, reviews, and consensus meetings.
Two authors (YHJ, JHL) independently reviewed the abstracts of each article and then jointly reviewed the full texts to determine whether they were suitable for inclusion in the study. When disagreements occurred, the third author (SHK) participated in discussions with the two authors to reach consensus. The methodological quality of each included study was appraised in accordance with the Oxford levels of evidence 2.47
Methodological quality assessment
Methodological quality was assessed using the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS),48 which consists of six components. The first is selection of participants, and evaluates the possibility of selection bias caused by the inadequate selection of participants; the second is confounding variables, which assesses selection bias caused by inadequate confirmation and consideration of confounding variables; The third is measurement of exposure, which evaluates performance bias caused by inadequate measurement of exposure; the fourth is blinding for outcome assessment, which assesses detection bias caused by inadequate blinding of outcome assessment; the fifth is incomplete outcome data, which evaluates attrition bias caused by inadequate handling of incomplete outcome data; the sixth is selective outcome reporting, which assesses reporting bias caused by selective outcome reporting. Two raters (YHJ and JHL) independently assessed each of the identified studies using this tool. The senior author (SHK) resolved disagreements. Final ratings were then discussed and agreed by all three authors. Results are given in detail in the online supplementary material.
Data extraction
A database was created from included studies using the following categories:
Study identification including author, journal, and year of publication.
References.
Study design.
Country in which the study was performed.
Study inclusion/exclusion criteria.
Number of patients included.
Patient age.
Minimum and length of follow-up.
Numbers of cases with and without scapular notching at final follow-up.
Grade of scapular notching as determined by the Nerot-Sirveaux classification14 . These grades were converted into a severity index, defined as notching grades 3 + 4 divided by grades 1 + 2.
Name of implant used.
Implantation features of the glenoid (diameter, tilting. eccentricity, lateralization), and humeral component (neck-shaft angle, version, lateralization).
Postoperative absolute Constant-Murley score (CMS).
Postoperative American Shoulder and Elbow Surgeons (ASES) score.
Postoperative range of movement (ROM): forward flexion (FF), abduction, external rotation (ER).
Statistical power, calculated using the one-tailed test using an α value of 0.05. As some of the studies did not report the CMS on each study group, we calculated the study power using the mean reported CMS for each study and assumed a difference in the CMS of 5 points and assumed standard deviation (SD) of 10 points for each group, as previously described by Mollon et al.25
Data synthesis
Available quantitative data were pooled in a statistical meta-analysis using Review Manager (RevMan) software (version 5.3; The Cochrane Collaboration, London, UK). For continuous data, effect sizes with 95% confidence intervals (CIs) were expressed as weighted mean differences (WMDs). A random-effects model or a fixed-effects model with an inverse variance method for WMDs was used for the meta-analyses. If the I2 was less than 50%, a fixed-effects model was used. If the I2 was 50% or more, a random-effects model was used. Statistical heterogeneity was assessed using the standard I2: values of less than 50% generally indicate consistent results and study homogeneity. Sensitivity analysis was carried out by the method of single elimination of individual studies. Funnel plots of effect size versus standard error (SE) were assessed by visual inspection to determine publication bias.
Missing SDs in the studies were dealt with as described by Walter et al49 and Ma et al.50 One study presented only ranges of the value, and in this study, the difference between minimum and maximum values was divided by four to estimate the SD.33 Four studies did not provide the SD; SDs were estimated using weighted averages of variances observed in other studies.34,37,39,44
Results
Systematic review and study properties
An initial search resulted in 764 articles (335 from PubMed, 412 from EMBASE, and 17 from Cochrane Database of Systematic Reviews). After deleting 268 duplicates, title reviews were carried out on 496 articles, which left 60 articles for abstract review. After abstract reviews, 23 articles were included for full-manuscript review.8,14,16,22–30,32–37,39,41–44 The properties of these 23 studies are detailed in Table I.
Table I.
Results of excluded and included studies for meta-analysis.
| First author | Year | Statistical significance | Power* | Severity Index† | Reason of exclusion | Description of results |
|---|---|---|---|---|---|---|
| Studies which were excluded from meta-analysis | ||||||
| Sirveaux | 2004 | + | 67.2 | 0.36 | No specific data was presented | “The presence of the notch significantly affected the Constant score when the notch was either over the screw or extensive (p < 0.05).” |
| Werner | 2005 | + | 16.9 | 0.84 | No specific data was presented | “Inferior notching negatively correlated with the Constant score (r = 0.3184; p = 0.0311).” |
| Boileau | 2006 | - | 49.4 | 0.22 | No specific data was presented | ”Neither the presence nor the size of the notch had a negative effect on the Constant score, the adjusted Constant score, or the ASES score.” |
| Stechel | 2010 | - | 36.4 | 0.06 | No specific data was presented | “There were no statistically significant differences between the groups. No effect on the Constant score could be seen." |
| Sadoghi | 2011 | + | 18.8 | 0.20 | No specific data was presented | "We did not find any significant correlations at mid-term follow-up, ranging from 24 to 60 months. In long-term follow-up (60 months and more), we found significant positive correlations between infraglenoidal notching and the Constant pain score (p = 0.3), and active anteversion (p < 0.01) and active external rotation (p < 0.01).” |
| Sershon | 2014 | + | 29.1 | 0.00 | No specific data was presented | “There was no correlation between preoperative or postoperative radiological findings and clinical outcomes.” |
| Athwal | 2015 | - | 45.6 | 0.05 | Risk of selection bias due to study design | "There was no significant differences with respect to range of movement (p > .491) or functional scores (p > .556).” |
| Torrens | 2016 | - | 66.9 | 0.63 | Risk of selection bias due to study design | “Scapular notching did not significantly affect the total Constant score or range of movement.” |
| Erbstbbrunner | 2017 | + | 19.4 | 0.43 | Difference in grouping of comparison | “Patients with scapular notching of grade 2 or higher (n = 10) had a significantly lower mean relative Constant score (57% vs 81%; p = 0.006) ... at the time of final follow-up compared with patients with no or grade-1 notching (n = 11).” |
| Kirzner | 2018 | + | 46.2 | 0.05 | Risk of selection bias due to study design | ”Statistically significant differences could be seen; however, when comparing ASES, SSV, WOOS and pain scores between the two groups with the notching cohort showing worse outcomes.” |
| Pastor | 2018 | + | 50.7 | 0.09 | No specific data was presented | “Inferior notching negatively correlated with the Constant score (r = 0.3184; p = 0.0311). However, the Constant score did not significantly differ between each grade of notching.” |
| Torrens | 2019 | - | 62.3 | 0.05 | Risk of selection bias due to study design | ”The functional outcomes (Constant scores) were not significantly different between patients with and without a scapular notch.” |
| Studies which were included in meta-analysis | ||||||
| Simovitch | 2007 | + | 69.6 | 0.70 | Results are reflected in meta-analysis | |
| Levigne | 2008 | - | 99.7 | 0.53 | Results are reflected in meta-analysis | |
| Favard | 2011 | - | 34.2 | 1.25 | Results are reflected in meta-analysis | |
| Levigne | 2011 | - | 99.9 | 0.51 | Results are reflected in meta-analysis | |
| Mizuno | 2012 | - | 50.4 | 0.06 | Results are reflected in meta-analysis | |
| Torrens | 2013 | - | 40.8 | 0.29 | Results are reflected in meta-analysis | |
| Birgorre | 2014 | - | 85.2 | 0.05 | Results are reflected in meta-analysis | |
| Feeley | 2014 | - | 49.2 | 0.14 | Results are reflected in meta-analysis | |
| Katz | 2015 | - | 84.9 | 0.05 | Results are reflected in meta-analysis | |
| Mollon | 2017 | + | 94.9 | 0.09 | Results are reflected in meta-analysis | |
| Simovitch | 2019 | + | 93.5 | 0.21 | Results are reflected in meta-analysis | |
Statistical power was calculated from a one-tail test using an α = 0.05. As some of studies did not report any functional score on each study group, we calculated the study power using the mean of the Constant-Murley score (CMS) for each study and assumed a difference in CMS of 5 points and also assumed standard deviation of ten points for each group as Mollon et al.25
The severity index were defined as notching grade 3 + 4 divided by grade 1 + 2 by Nerot-Sirveaux classification.14
Overall, 17 of the 23 studies included in the full-manuscript review were sub-analyses of consecutive case series on outcomes after RTSA.8,14,16,22,24,26–28,30,33–37,39,41,44 Two were sub-analyses of randomized clinical trials on other topics,42,43 two were prospective or retrospective cohort comparative studies on other topics,23,32 and two were retrospective cohort comparative studies primarily on the effect of scapular notching on clinical outcomes after RTSA.25,29 Ten of the 23 studies concluded that effect of scapular notching was significant,14,16,22,23,25–30 and 13 failed to demonstrate significance.8,24,32–37,39,41–44 Other properties and the results of the 23 studies are summarized in Table I and in the online supplementary material.
Quality assessment and selection of studies for meta-analysis
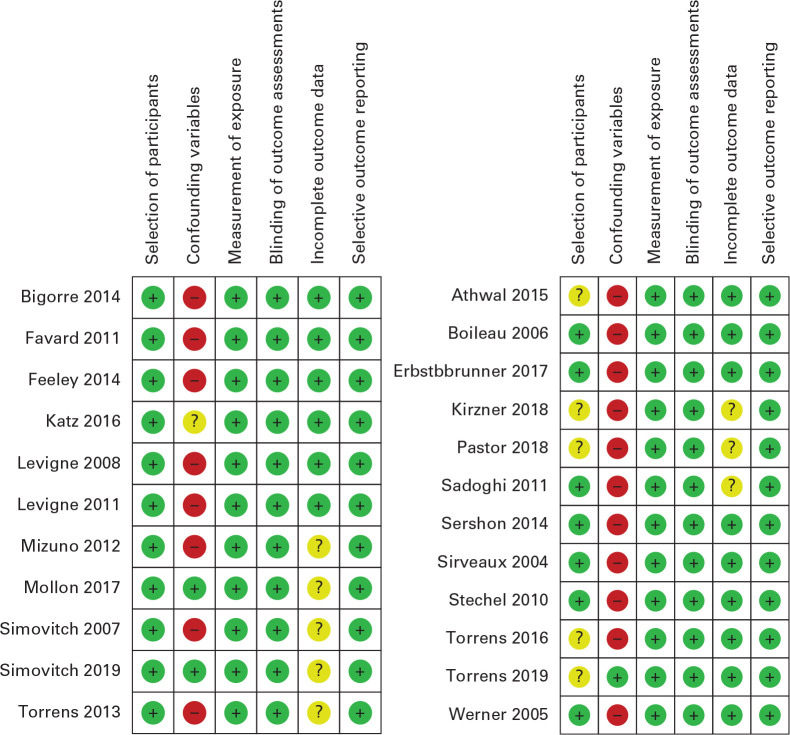
The results of quality assessments conducted using RoBANS are given in Figure 1. Six studies were found to have possible enrolment selection bias. Four of the six studies were comparative cohort studies23,32 or randomized clinical trials42,43 on other topics (e.g. implant type, size, or orientation), one study confined enrolment of the study group to a specific surgical indication (fracture sequalae),26 and the other study defined the grouping differently when comparing notching and non-notching group.22 Therefore, these six studies were excluded from the analysis.22,23,26,32,42,43
Fig. 1.
Methodological quality assessment using the Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS). ‘+’ refers to low risk, ‘-‘ refers to high risk, and ‘?’ refers to unclear risk, with showing assessment of the included studies for the meta-analysis (left), and showing the assessment of the excluded studies for the meta-analysis (right).
Most of the 17 remaining studies were assessed to lack confirming and considering confounding variables, which were known to influence the occurrence of scapular notching and clinical outcomes, and this, can possibly cause selection bias in the analysis.8,14,16,24,27,28,30,33–35,37,39,41,44 However, we decided to include studies with unclear confounding variable risks because the aim of this meta-analysis was primarily to describe phenomena and not to investigate specific cause and effect relationships. Moreover, eight studies did not disclose follow-up losses, and thus present unclear risks with respect to incomplete outcome data.23,25–27,29,30,39,44 Nevertheless, we decided to include these studies because the possibility of unbalanced follow-up losses between two groups (notching vs non-notching) which might cause selection bias was determined to be low.
Six of the 17 studies were excluded from the meta-analysis because they did not provide specific data for synthesis.8,14,16,27,28,40,41 Finally, 11 studies were eligible for the meta-analysis,24,25,29,30,33–37,39,44 that is, two retrospective cohort studies (Level III),25,29 and nine sub-analyses of pro- and retrospective case series (Level IV).24,30,33–37,39,44 As shown in Table I, the mean statistical power of the 11 studies included for meta-analysis was greater than that of the six excluded studies. (72.9 vs 42.4 respectively, p = 0.002), though the severity indices were similar (0.35 vs 0.30 respectively, p = 0.441).
Meta-analysis
Functional scores: CMS and ASES scores
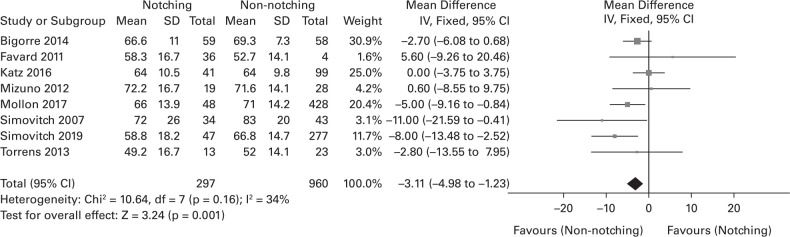
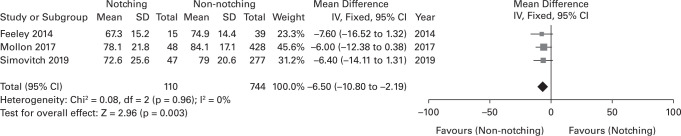
In all, eight of the 11 studies provided specific CMS data.25,29,30,33,34,36,39,44 The WMD of the CMS between notching and non-notching groups was 3.11, and the notching group had significantly lower scores than the non-notching group (Figure 2). Three studies gave specific data on the ASES scores (Figure 3).25,29,35 The WMD of the ASES score between notching and non-notching groups was 6.50; the notching group had a significantly lower score than the non-notching group.
Fig. 2.
Forest plot of weighted mean difference in postoperative Constant-Murley score (CMS) between notching and non-notching group. SD, standard deviation; IV, inverse variance; CI, confidence interval; DF, degrees of freedom.
Fig. 3.
Forest plot of weighted mean difference in postoperative American Shoulder and Elbow. Surgeons (ASES) score between notching and non-notching group. CI, confidence interval; DF, degrees of freedom; SD, standard deviation; IV, inverse variance.
Range of motion: forward flexion, abduction, and external rotation
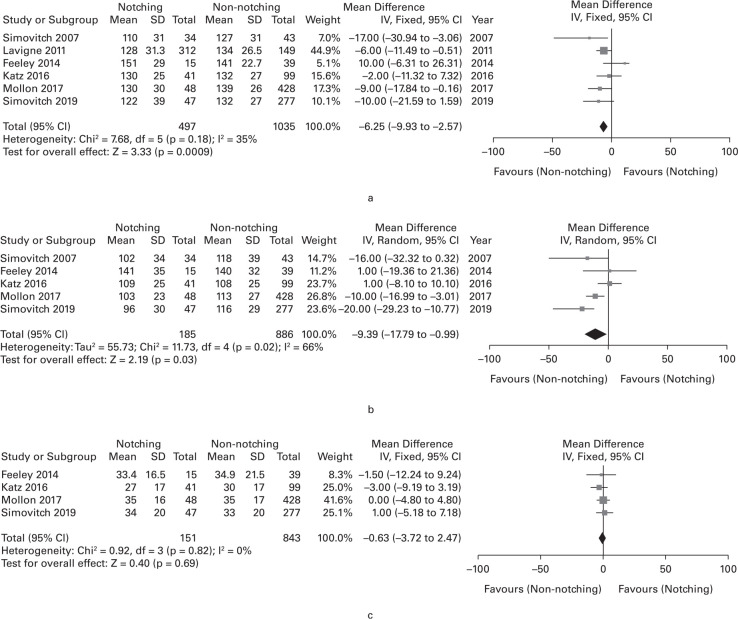
Six of the 11 studies provided specific data sufficient for meta-analysis of FF,25,29,30,35–37 five studies for meta-analysis of abduction,25,29,30,35,36 and four studies for meta-analysis of ER.25,29,35,36 Calculated WMD were 6.3° for FF (with significance), 9.4° for abduction (with significance), and 0.6° for ER (without significance), and all three results were lower in the notching group than the notching group (Figure 4).
Fig. 4.
Forest plot of weighted mean difference in postoperative range of movement between notching and non-notching group. (a) forward flexion; (b) abduction; and (c) external rotation at side. CI, confidence interval; DF, degrees of freedom; SD, standard deviation; IV, inverse variance.
Sensitivity analysis and publication bias
Single elimination of individual studies did not affect the overall results in terms of the analysis of any of the five variables of interest. The notching group maintained a statistically significant difference with respect to the CMS, the ASES score, and FF, but not with respect to abduction and ER. The funnel plots were symmetrical about the mean effect for all variables, indicating an absence of publication bias within the studies.
Discussion
This meta-analysis study shows that scapular notching has a significant negative effect on clinical outcome after RTSA. Although the studies included gave mixed results about whether scapular notching has an effect on clinical outcome, the results of this meta-analysis showed that patients with scapular notching after RTSA had significantly worse results when evaluated by the CMS, the ASES score, and the range of flexion and abduction.
Although the WMDs were rather small, the observed mean differences in CMS, flexion and abduction met or exceeded the minimal clinically important difference (MCID) thresholds previously established by Simovitch et al51 for RTSA for the CMS (MCID threshold 0.3; observed mean difference 3.11), flexion (MCID threshold 2.9°; observed mean difference 6.3°), and abduction (MCID threshold 1.9°; observed mean difference 9.4°), which confirms that these differences in outcome are clinically meaningful.
Two studies, which failed to show significant results, were excluded because they did not provide specific data (Table I),8,41 and this exclusion may have caused errors in our results. However, these two studies were underpowered with statistical powers below 0.5, and it can therefore be inferred that the non-significant results obtained were probably due to insufficient statistical power.
Because of the nature of the subject, randomized controlled trials are not possible. The best possible study design would be a retrospective cohort comparison study with adjustment for major potential confounders. From this perspective, the conclusions of Mollon et al25 and Simovitch et al29 should be weighted more than others. These two studies had higher statistical power than other studies determined by post hoc analysis (Table I). Furthermore, their results were grossly consistent with ours, in that they agree that scapular notching has a negative effect on the clinical outcome of RTSA.
Mollon et al25 reviewed the minimum two-year outcome of consecutive patients who underwent RTSA at a single centre, and excluded patients with a history of previous shoulder surgery or arthroplasty, a diagnosis of infection or acute proximal humeral fracture, and arthroplasty with a constrained implant. They also concluded that patients with scapular notching had significantly poorer clinical outcomes, significantly less strength and ROM, and a significantly higher complication rate than patients without scapular notching at short-term follow-up. Simovitch et al29 reported on the impact of scapular notching on mid-term outcomes after RTSA by investigating the minimum five-year outcome of consecutive patients who underwent RTSA in a multicentre study after excluding patients with revision arthroplasty, a history of infection, or of acute fracture or fracture sequalae. They concluded that patients with scapular notching had a significantly worse clinical outcome and a significantly higher complication and revision rate compared to patients without scapular notching at mid-term follow-up.
The results of the current study should be interpreted carefully because most of the studies included did not consider various confounding factors. There is a possibility that other factors could cause notching and a worse outcome after RTSA.5,25,29,45,52 In particular, placing the glenoid component with an inferior overhang of the scapular neck during implantation may result in a low rate of scapular notching and a better functional outcome. Biomechanically, inferior overhang of the glenoid component can widen the impingement-free arc of rotation and distalize the centre of rotation, which might possibly give a better functional outcome after RTSA.18,31,45,46,53,54 Therefore, more studies are needed to verify how scapular notching causes a worse functional outcome after RTSA by stratifying factors related to the occurrence of scapular notching and clinical outcome. Nevertheless, we believe that the meta-analysis is valid regardless of this limitation. This is because this study confirmed the association of scapular notching with worse clinical outcomes regardless of the existence or not of confounders, and we can justify the various efforts to avoid scapular notching when performing RTSA.
The possible reasons for the mixed results of previous studies are insufficient statistical power due to small cohort size and inconstantly observed rate of scapular notching, which can cause sample size inconsistencies uncontrolled or poorly controlled confounding factors, such as various patient factors (obesity, body mass index, body weight and range of adduction, activity level) and implant factors (humeral or glenoid lateralization, glenosphere size and orientation), which can affect notching occurrence and clinical outcomes simultaneously.5,25,29,45,46,55 The limitations of this meta-analysis reflects the deficiencies in the current body of literature on scapular notching and clinical outcomes. Most of these studies are of short- or medium-term, non-randomized, and non-controlled. They also involve heterogeneous patient populations, a variety of implant types, and different surgical techniques.
Despite this lack of standardization, the present study gives an important perspective on the clinical relevance of scapular notching. The literature should be interpreted in the light of its statistical power. Studies of low power should be weighted less and those of high-power weighted more, and it should be recognized that simple reviews of the literature usually lack such a weighting procedure. Systemic review and meta-analysis by qualification and weighting procedures can provide conclusions that are more powerful. We believe that this meta-analysis contributes to our understanding by increasing the level of evidence and providing support to those who seek a means of avoiding scapular notching by refining the design of implants or their means of implantation. A meta-analysis of the available literature suggests that patients with scapular notching have significantly worse results after RTSA than those who do not.
Take home message
- The available literature suggests that patients with scapular notching after reverse total shoulder arthroplasty have significantly inferiorworse results when evaluated by the Constant-Murley scores, the American Shoulder and Elbow Surgeons score, and range of movement of forward flexion and abduction.
Author contributions
Y. H. Jang: Collected and analyzed the data, Wrote and edited the manuscript.
J. H. Lee: Collected and analyzed the data.
S. H. Kim: Analyzed the data, Edited the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
Special thanks should be given to Sungju Kim, PhD for statistical consultation.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Supplementary material
Tables showing risk of bias by the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS), and property of studies.
This article was primary edited by A. C. Ross.
References
- 1.Austin L, Zmistowski B, Chang ES, Williams GR. Is reverse shoulder arthroplasty a reasonable alternative for revision arthroplasty? Clin Orthop Relat Res. 2011;469(9):2531–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho C-H, Kim D-H, Song K-S. Reverse shoulder arthroplasty in patients with rheumatoid arthritis: a systematic review. Clin Orthop Surg. 2017;9(3):325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyun YS, Huri G, Garbis NG, McFarland EG. Uncommon indications for reverse total shoulder arthroplasty. Clin Orthop Surg. 2013;5(4):243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mata-Fink A, Meinke M, Jones C, Kim B, Bell J-E. Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: a systematic review. J Shoulder Elbow Surg. 2013;22(12):1737–1748. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson GP, Strauss EJ, Sherman SL. Scapular notching: recognition and strategies to minimize clinical impact. Clin Orthop Relat Res. 2011;469(9):2521–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grammont P, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16(1):65–68. [DOI] [PubMed] [Google Scholar]
- 7.Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(1):91–97. [DOI] [PubMed] [Google Scholar]
- 8.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I, Award N. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–540. [DOI] [PubMed] [Google Scholar]
- 9.Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697–1705. [DOI] [PubMed] [Google Scholar]
- 10.Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty. survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742–1747. [DOI] [PubMed] [Google Scholar]
- 11.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297–1304. [DOI] [PubMed] [Google Scholar]
- 13.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17–22. [DOI] [PubMed] [Google Scholar]
- 14.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–395. [DOI] [PubMed] [Google Scholar]
- 15.Wall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–1485. [DOI] [PubMed] [Google Scholar]
- 16.Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–1486. [DOI] [PubMed] [Google Scholar]
- 17.Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915–1923. [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. JAAOS-Journal of the American Academy of Orthopaedic Surgeons. 2009;17(5):284–295. [DOI] [PubMed] [Google Scholar]
- 19.Nyffeler R, Werner C, Simmen B, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86(8):1187–1191. [DOI] [PubMed] [Google Scholar]
- 20.Delloye C, Joris D, Colette A, Eudier A, Dubuc JE. [Mechanical complications of total shoulder inverted prosthesis]. Rev Chir Orthop Reparatrice Appar Mot. 2002;88(4):410–414. [PubMed] [Google Scholar]
- 21.Vanhove B, Beugnies A. Grammont's reverse shoulder prosthesis for rotator cuff arthropathy. A retrospective study of 32 cases. Acta Orthop Belg. 2004;70(3):219–225. [PubMed] [Google Scholar]
- 22.Ernstbrunner L, Suter A, Catanzaro S, Rahm S, Gerber C. Reverse total shoulder arthroplasty for massive, irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am. 2017;99(20):1721–1729. [DOI] [PubMed] [Google Scholar]
- 23.Kirzner N, Paul E, Moaveni A. Reverse shoulder arthroplasty vs BIO-RSA: clinical and radiographic outcomes at short term follow-up. J Orthop Surg Res. 2018;13(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2008;17(6):925–935. [DOI] [PubMed] [Google Scholar]
- 25.Mollon B, Mahure SA, Roche CP, Zuckerman JD. Impact of scapular notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg. 2017;26(7):1253–1261. [DOI] [PubMed] [Google Scholar]
- 26.Pastor MF, Kieckbusch M, Kaufmann M, et al. Reverse shoulder arthroplasty for fracture sequelae: Clinical outcome and prognostic factors. J Orthop Sci. 2019;24(2):237–242. [DOI] [PubMed] [Google Scholar]
- 27.Sadoghi P, Leithner A, Vavken P, et al. Infraglenoidal scapular notching in reverse total shoulder replacement: a prospective series of 60 cases and systematic review of the literature. BMC Musculoskelet Disord. 2011;12:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sershon RA, Van Thiel GS, Lin EC, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg. 2014;23(3):395–400. [DOI] [PubMed] [Google Scholar]
- 29.Simovitch R, Flurin PH, Wright TW, Zuckerman JD, Roche C. Impact of scapular notching on reverse total shoulder arthroplasty midterm outcomes: 5-year minimum follow-up. J Shoulder Elbow Surg. 2019;28(12):2301–2307. [DOI] [PubMed] [Google Scholar]
- 30.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89(3):588–600. [DOI] [PubMed] [Google Scholar]
- 31.Roche C, Flurin P-H, Wright T, et al. An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement, and stability. J Shoulder Elbow Surg. 2009;18(5):734–741 [DOI] [PubMed] [Google Scholar]
- 32.Athwal GS, MacDermid JC, Reddy KM, et al. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg. 2015;24(3):468–473. [DOI] [PubMed] [Google Scholar]
- 33.Bigorre N, Lancigu R, Bizot P, Hubert L. Predictive factors of scapular notching in patients with reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2014;100(7):711–714. [DOI] [PubMed] [Google Scholar]
- 34.Favard L, Levigne C, Nerot C, et al. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time? Clin Orthop Relat Res. 2011;469(9):2469–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feeley BT, Zhang AL, Barry JJ, et al. Decreased scapular notching with lateralization and inferior baseplate placement in reverse shoulder arthroplasty with high humeral inclination. Int J Shoulder Surg. 2014;8(3):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz D, Valenti P, Kany J, Elkholti K, Werthel JD. Does lateralisation of the centre of rotation in reverse shoulder arthroplasty avoid scapular notching? Clinical and radiological review of one hundred and forty cases with forty five months of follow-up. Int Orthop. 2016;40(1):99–108. [DOI] [PubMed] [Google Scholar]
- 37.Lévigne C, Garret J, Boileau P, et al. Scapular notching in reverse shoulder arthroplasty: Is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Dines JS, Waren RF, Craig EV, Dines DM. Inferior glenosphere placement reduces scapular notching in reverse total shoulder arthroplasty. Orthopedics. 2015;38(2):e88–e93. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno N, Denard PJ, Raiss P, Walch G. The clinical and radiographical results of reverse total shoulder arthroplasty with eccentric glenosphere. Int Orthop. 2012;36(8):1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee S-M, Lee JD, Park YB, Yoo JC, Oh JH. Prognostic radiological factors affecting clinical outcomes of reverse shoulder arthroplasty in the Korean population. Clin Orthop Surg. 2019;11(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stechel A, Fuhrmann U, Irlenbusch L, Rott O, Irlenbusch U. Reversed shoulder arthroplasty in cuff tear arthritis, fracture sequelae, and revision arthroplasty: outcome in 59 patients followed for 2–7 years. Acta Orthop. 2010;81(3):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torrens C, Guirro P, Miquel J, Santana F. Influence of glenosphere size on the development of scapular notching: a prospective randomized study. J Shoulder Elbow Surg. 2016;25(11):1735–1741. [DOI] [PubMed] [Google Scholar]
- 43.Torrens C, Miquel J, Martinez R, Santana F. Can small glenospheres with eccentricity reduce scapular notching as effectively as large glenospheres without eccentricity? A prospective randomized study. J Shoulder Elbow Surg. 2019;29(2):217-224. [DOI] [PubMed] [Google Scholar]
- 44.Torrens C, Santana F, Picazo B, Cáceres E. Retrospective study of scapular notches in reverse shoulder arthroplasties. Am J Orthop. 2013;42(8):362–365. [PubMed] [Google Scholar]
- 45.Lawrence C, Williams GR, Namdari S. Influence of glenosphere design on outcomes and complications of reverse arthroplasty: a systematic review. Clin Orthop Surg. 2016;8(3):288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner B, Chaoui J, Walch G. Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Joint J. 2018;100(9):1182–1186. [DOI] [PubMed] [Google Scholar]
- 47.Phillips B, Ball C, Sackett D, et al. Levels of evidence and grades of recommendation. 2009. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009 (date last accessed 27 August 2020).
- 48.Park J, Lee Y, Seo H, et al. editors. Risk of bias assessment tool for non-randomized studies (RoBANS): development and validation of a new instrument. 19th Cochrane Colloquium, 2011.
- 49.Walter SD, Yao X, XJJoce Y. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol. 2007;60(8):849–852. [DOI] [PubMed] [Google Scholar]
- 50.Ma J, Liu W, Hunter A, Zhang W, WJBmrm Z. Performing meta-analysis with incomplete statistical information in clinical trials. BMC Med Res Methodol. 2008;8(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simovitch R, Flurin P-H, Wright T, Zuckerman JD, Roche CP. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg. 2018;27(2):298–305. [DOI] [PubMed] [Google Scholar]
- 52.Lädermann A, Tay E, Collin P, et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res. 2019;8(8):378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grammont P, Trouilloud P, Laffay J, Deries X. Concept study and realization of a new total shoulder prosthesis. Rhumatologie. 1987;39(10):407–418. [Google Scholar]
- 54.Lädermann A, Walch G, Lubbeke A, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(3):336–341. [DOI] [PubMed] [Google Scholar]
- 55.Malhas A, Granville-Chapman J, Robinson P, et al. Reconstruction of the glenoid using autologous bone-graft and the SMR Axioma TT metal-backed prosthesis: the first 45 sequential cases at a minimum of two years’ follow-up. Bone Joint J. 2018;100(12):1609–1617. [DOI] [PubMed] [Google Scholar]