Abstract
Aims
With recent progress in cancer treatment, the number of advanced-age patients with spinal metastases has been increasing. It is important to clarify the influence of advanced age on outcomes following surgery for spinal metastases, especially with a focus on subjective health state values.
Methods
We prospectively analyzed 101 patients with spinal metastases who underwent palliative surgery from 2013 to 2016. These patients were divided into two groups based on age (< 70 years and ≥ 70 years). The Eastern Cooperative Oncology Group (ECOG) performance status (PS), Barthel index (BI), and EuroQol-5 dimension (EQ-5D) score were assessed at study enrolment and at one, three, and six months after surgery. The survival times and complications were also collected.
Results
In total, 65 patients were aged < 70 years (mean 59.6 years; 32 to 69) and 36 patients were aged ≥ 70 years (mean 75.9 years; 70 to 90). In both groups, the PS improved from PS3 to PS1 by spine surgery, the mean BI improved from < 60 to > 80 points, and the mean EQ-5D score improved from 0.0 to > 0.7 points. However, no significant differences were found in the improvement rates and values of the PS, BI, and EQ-5D score at any time points between the two groups. The PS, BI, and EQ-5D score improved throughout the follow-up period in approximately 90% of patients in each group. However, the improved PS, BI, and EQ-5D scores subsequently deteriorated in some patients, and the redeterioration rate of the EQ-5D was significantly higher in patients aged ≥ 70 than < 70 years (p = 0.027).
Conclusion
Palliative surgery for spinal metastases improved the PS, activities of daily living, and quality of life, regardless of age. However, clinicians should be aware of the higher risk of redeterioration of the quality of life in advanced-age patients.
Cite this article: Bone Joint J 2020;102-B(12):1709–1716.
Keywords: Spinal metastasis, Advanced age, Elderly, Quality of life, Activity of daily living, Performance status, Palliative surgery, Surgical outcome
Introduction
Recent progress in cancer treatment has improved the survival of patients with advanced-stage neoplasia.1 As a result, more patients are at risk of developing bone metastases.2,3 Approximately 10% to 20% of patients with spinal metastases develop some degree of spinal destruction and epidural compression, leading to serious symptoms including neurological dysfunction, and intractable pain.3 These symptoms may impair the patients’ health state, including their activities of daily living (ADL) and quality of life (QoL).4-6 Because the final goal of cancer therapy is to improve and preserve independence QoL until the terminal phase, management of spinal metastases is essential.
Previous studies have shown that spinal surgery for symptomatic spinal metastases (SSM) can improve and maintain the performance status (PS) and ADL for at least six months after surgery.4,5 Additionally, spinal surgery for SSM has good cost utility.5
Japan had already become an aged society by 1970, at which time 7% of the population was of advanced age. Since then, the rate of ageing in Japan has remained 20% higher than that in any other country.7 Similar changes in the ageing population are being witnessed in the Western world.8,9 The older population requires more expensive medical interventions, resulting in a scarcity of healthcare resources.10 Thus, it is relevant to clarify the influence of advanced age on outcomes following surgery for spinal metastases. In general, ‘advanced age’ is defined as ≥ 65 years.9 However, this definition does not satisfactorily match the current age stratification of patients with spinal metastases. Therefore, the present prospective cohort study of surgical outcomes for spinal metastases involved patients aged ≥ 70 years. We placed a particular focus on health state values.
Methods
Ethics statement
The study was approved by the Institutional Review Board of Kobe University Hospital. Written informed consent was obtained from each patient in accordance with the principles of the Declaration of Helsinki,11 and the laws and regulations of our country.
Patients
In total, 110 consecutive patients who presented with SSM from January 2013 to December 2016 who had a surgical indication in our hospital were prospectively enrolled. Metastases were diagnosed by plain radiography, computerized tomography (CT), magnetic resonance imaging (MRI), bone scintigraphy, positron emission tomography, and histological evaluation of needle biopsy samples. The patients were divided into two groups: those aged < 70 years (Group Y) and those aged ≥ 70 years (Group O). We defined SSM as spinal metastases associated with progressive neurological deficits, spinal instability, or intractable pain resistant to conservative care. Consequently, all patients with SSM were also surgical candidates. The surgical indications were progressive neurological deficits, remarkable spinal instability (Spinal Instability Neoplastic Score of ≥ 13),12 or intractable pain resistant to conservative care, including the use of opioids. The interval to surgical intervention was decided based on surgical indication: patients with progressive neurological deficits underwent semi-urgent surgery (within 48 hours), whereas patients without neurological deficits underwent surgery within two weeks. The contraindication for surgery was impaired consciousness due to cerebral metastases. Additionally, we excluded patients with dementia who were unable to make decisions because evaluation of their subjective health state would have been inaccurate. To maintain case homogeneity, we excluded patients who underwent total en-bloc spondylectomy for curative treatment. All operations involved single-stage posterior decompression and stabilization with fixation using lateral mass screws for the cervical spine and pedicle screws for the thoracic and lumbar spine. Neither corpectomy nor an anterior approach was performed. All immobilization devices, including corsets and collars, were removed postoperatively. All patients underwent radiotherapy before or after surgery. If indicated, chemotherapy was managed by an oncologist.
The postoperative survival duration was defined as the time from the date of surgery to the latest follow-up examination or death. At the start of the study (baseline), we documented age, sex, and the primary tumour type as preoperative-related factors. The Katagiri score,13 Tokuhashi score,14 and Frankel classification15 were used to evaluate the severity of spinal metastases. The Eastern Cooperative Oncology Group performance status (ECOGPS), Barthel index (BI), and EuroQol-5 dimension (EQ-5D)16 were used to evaluate the PS, ADL, and QoL, respectively. As surgery-related factors, we investigated the operative time, blood loss, number of fixed vertebrae, screw technique, and postoperative complications. To clarify the chronological changes, clinical follow-up was routinely performed at one, three, and six months postoperatively. Improvement or deterioration of each subjective health state value was defined as a ≥ one-level change in the PS, a ≥ ten-point change in the BI, and a ≥ 10% change in the EQ-5D score. Based on this definition, the improvement rate, deterioration rate, and redeterioration rate within six months were calculated. To investigate the outcome of all patients, patients who were alive and could not attend our department were contacted by telephone to obtain the latest follow-up information. For patients who died, we obtained information from the patient’s family or institution to which they had been transferred.
The Clavien-Dindo classification was used to investigate the rate and severity of postoperative complications.17 Complications were defined as Clavien-Dindo grade ≥ 2, and the proportion of patients with complications was calculated.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 (SPSS, Chicago, Illinois, USA) with significance set at a p value < 0.05. The overall survival rate was calculated by the Kaplan-Meier method, and the two groups were compared by the log-rank method. To compare the preoperative factors and surgery-related factors between the two groups, the Mann-Whitney U test was used for continuous variables, and chi-squared test or Fisher’s exact test for categorical variables. The chronological changes between the two groups were identified using the Kruskal-Wallis test and Scheffe’s post hoc test. The improvement rate, deterioration rate, and redeterioration rate were compared between the two groups using Fisher’s exact test. Finally, multivariate logistic regression analysis was performed to identify the associations between the preoperative related factors and redeterioration of the PS, BI, and EQ-5D score.
Results
Demographic data and multidisciplinary approach
Nine of 110 patients with SSM were excluded because of treatment contraindications (n = 6) and total en-bloc spondylectomy (n = 3). Consequently, 101 patients (mean age 65.4 years; 32 to 90) were enrolled. Group Y comprised 65 patients (mean age 59.6 years; 32 to 69), and Group O comprised 36 patients (mean age 75.9 years; 70 to 90). The patients’ age distribution is shown in Figure 1. No patients were lost to follow-up. The median follow-up period was 10.2 months (interquartile range 3.5 to 24.0). There were no significant differences between the two groups in preoperative demographic factors (Table I). Lung cancer was the most common type of primary neoplasm in both groups. The other primary malignant tumours are listed in Table II. Chemotherapy was performed in 50 patients (Group Y, n = 33; Group O, n = 17) at the start of the study. Postoperative chemotherapy was also performed in 48 patients (Group Y, n = 31; Group O, n = 18). The statistical analysis via Fisher's exact test showed no significant difference in postoperative chemotherapy (p = 0.835).
Fig. 1.
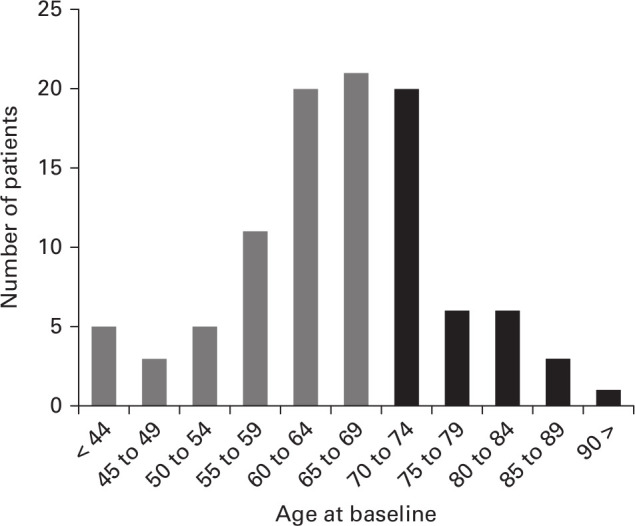
Age distribution of patients.
Table I.
Baseline demographics and clinical characteristics of patients according to age at surgery.
| Variable | Total (n = 101) | < 70 years (n = 65) | ≥ 70 years (n = 36) | p-value |
|---|---|---|---|---|
| Mean age, yrs (range) | 65.4 (32 to 90) | 59.6 (32 to 69) | 75.9 (70 to 90) | < 0.001‡ |
| Male sex, n (%) | 67 (69.2) | 44 (63.9) | 23 (67.3) | 0.820* |
| Mean Katagiri score (range) | 4.9 (1 to 7) | 5.0 (1 to 8) | 4.9 (1 to 8) | 0.900‡ |
| Mean Tokuhashi score (range) | 6.4 (3 to 10) | 6.1 (3 to 11) | 7.0 (3 to 10) | 0.090‡ |
| Mean SINS (range) | 11.4 (5 to 16) | 11.3 (7 to 16) | 11.5 (5 to 16) | 0.726‡ |
| Mean operating time, mins (range) | 199.3 (73 to 373) | 197.1 (82 to 373) | 186.9 (73 to 370) | 0.440‡ |
| Mean blood loss, ml (range) | 355.2 (0 to 2,500) | 354.8 (10 to 2,500) | 355.8 (0 to 1,800) | 0.944‡ |
| Median fixed vertebrae, n (IQR) | 6.0 (5.0 to 7.0) | 6.5 (5.0 to 8.0) | 6.0 (5.0 to 7.0) | 0.215‡ |
| Screw technique, n (%) | > 0.999* | |||
| Open technique | 73 (72.3) | 47 (72.3) | 26 (72.2) | |
| Percutaneous technique | 28 (27.7) | 18 (27.7) | 10 (27.8) | |
| ECOGPS grade, n | 0.243* † | |||
| PS 1 | 1 | 0 | 1 | |
| PS 2 | 19 | 15 | 4 | |
| PS 3 | 40 | 26 | 14 | |
| PS 4 | 41 | 24 | 17 | |
| Mean BI (range) | 53.4 (0 to 100) | 55.7 (5 to 100) | 49.2 (0 to 100) | 0.270‡ |
| Mean EQ-5D, (range) | -0.037 (-0.594 to 1.000) | -0.020 (-0.594 to 0.796) | -0.130 (-0.594 to 1.000) | 0.110‡ |
| Frankel classification, n (%) | 0.668* | |||
| Grade A, B, and C | 38 (37.6) | 23 (35.4) | 15 (41.7) | |
| Grade D and E | 63 (62.4) | 42 (64.6) | 21 (58.3) |
Fisher’s exact test.
Chi-squared test.
Mann-Whitney U test.
BI, Barthel index; ECOGPS, Eastern Cooperative Oncology Group performance status; EQ-5D, EuroQol Five Dimension questionnaire; IQR, interquartile range; PS, performance status; SINS, Spinal Instability Neoplastic Score.
Table II.
Primary tumour types according to age at surgery.
| Tumour type, n (%) | Total (n = 101) |
< 70 years (n = 65) |
≥ 70 years (n = 36) |
|---|---|---|---|
| Lung | 19 (18.9) | 13 (20.0) | 6 (16.7) |
| Kidney | 12 (11.9) | 8 (12.3) | 4 (11.1) |
| Breast | 10 (9.9) | 7 (10.8) | 3 (8.3) |
| Liver | 8 (7.9) | 4 (6.2) | 4 (11.1) |
| Thyroid | 6 (5.9) | 1 (1.5) | 5 (13.9) |
| Lymphoma | 6 (5.9) | 5 (7.7) | 1 (2.8) |
| Colon | 6 (5.9) | 2 (3.1) | 4 (11.1) |
| Myeloma | 6 (5.9) | 6 (9.2) | 0 (0.0) |
| Unknown | 4 (4.0) | 2 (3.1) | 2 (5.6) |
| Others | 24 (23.8) | 17 (26.2) | 7 (19.4) |
Survival rate
One patient in each group died within one month due to primary cancer; therefore, the number of surviving patients at one month was 64 in Group Y and 35 in Group O (one-month survival rate, 98.5% and 97.2%; 95% confidence interval (CI) 95.5 to 101.3 and 91.8 to 102.7). The three-month survival rate in Groups Y and O was 81.5% (n = 53; 95% CI 73.9 to 92.3) and 80.6% (n = 29; 95% CI 64.0 to 91.6), respectively, and that at six months was 61.5% (n = 40; 95% CI 48.0 to 72.0) and 58.3% (n = 21; 95% CI 45.0 to 77.3), respectively. The median survival time after the start of the study was 10.2 months (95% CI 5.2 to 15.1; numbers at risk = 32) in Group Y and 11.2 months (95% CI 2.2 to 20.2; numbers at risk = 18) in Group O. There was no significant difference between the two groups (log rank, p = 0.439) (Figure 2).
Fig. 2.
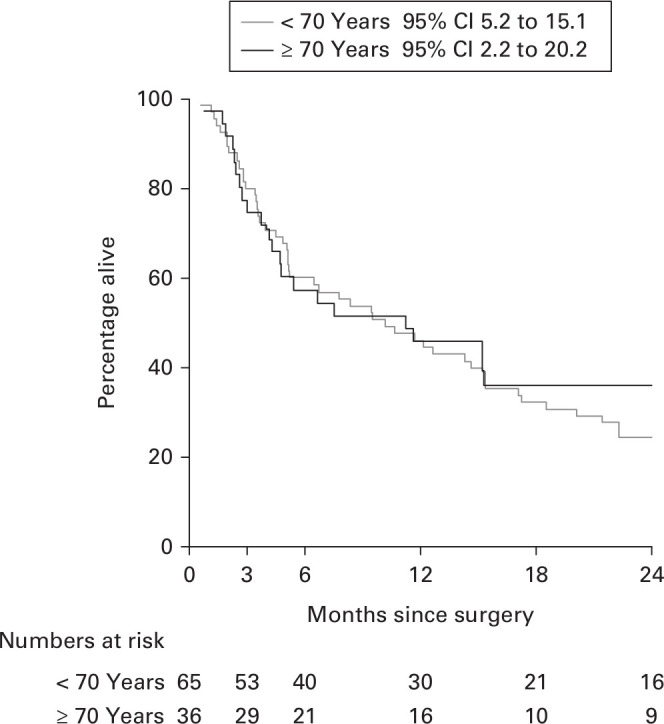
Kaplan-Meier survival curve. CI, confidence interval.
Complications
Postoperative complications occurred in 24 patients (36.9%) in Group Y and 15 patients (41.6%) in Group O. The complication rate was not significantly different between the two groups (Table III). The total number of postoperative complications in Groups Y and O was 28 and 15, respectively (Table III). Grade 2 Clavien-Dindo complications (i.e. conditions requiring pharmacological treatment including blood transfusion) were the most common. The second most common grade was grade 3b (i.e. conditions requiring surgical intervention under general anaesthesia) (Table IV). Most such complications were wound problems due to radiation ulcers and surgical site infection. There was no significant difference in the severity of complications between the two groups (p = 0.522).
Table III.
Numbers and rates of postoperative complications.
| Variable, n | Total (n = 101) |
< 70 years (n = 65) |
≥ 70 years (n = 36) |
|---|---|---|---|
| Postoperative complication (%) | 39 (38.6) | 24 (36.9) | 15 (41.6) |
| Blood transfusion | 18 | 12 | 6 |
| Surgical site infection | 6 | 4 | 2 |
| Radiation-related disorders of the skin | 3 | 2 | 1 |
| Deep vein thrombosis | 2 | 1 | 1 |
| Hydrocephalus | 1 | 1 | 0 |
| Pneumonia | 1 | 1 | 0 |
| Ureteral stenosis | 1 | 0 | 1 |
| Acute renal failure | 1 | 1 | 0 |
| Acute myocardiac infarction | 1 | 0 | 1 |
| Peritonitis | 1 | 1 | 0 |
| Delayed wound healing | 1 | 1 | 0 |
| Cerebrospinal leakage | 1 | 1 | 0 |
| C5 palsy | 1 | 0 | 1 |
| Fixed end vertebral fracture | 3 | 2 | 1 |
| Rod breakage | 1 | 1 | 0 |
| Proximal junctional failure | 1 | 0 | 1 |
| Number of complications | 43 | 28 | 15 |
Table IV.
Severity of postoperative complications.
| Clavien-Dindo grade | Total, n | < 70 years, n | ≥ 70 years, n |
|---|---|---|---|
| 2* | 30 | 19 | 11 |
| 3a† | 2 | 1 | 1 |
| 3b‡ | 9 | 7 | 2 |
| 4a§ | 2 | 1 | 1 |
| 4b¶ | 0 | 0 | 0 |
Requires pharmacological treatment with drugs other than those allowed for grade 1 complications.
Requires intervention not under general anaesthesia.
Requires intervention under general anaesthesia.
Single organ dysfunction.
Multiple organ dysfunction.
PS
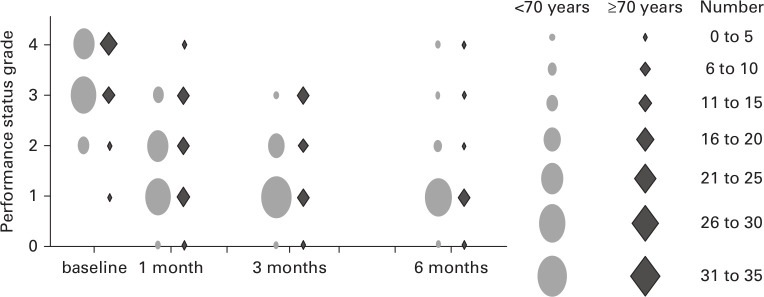
The median PS at the start of the study was PS3 in both groups. At one month postoperatively, it had improved to PS2 in both groups. In Group Y, it further improved and reached PS1 at three months postoperatively, and this was maintained until six months. In Group O, it was maintained at PS2 at three months postoperatively and reached PS1 at six months (Figure 3). The chronological course of the PS was not significantly different between the two groups at all postoperative intervals (one month, p = 0.426; three months, p = 0.07; six months, p = 0.834) (Table V).
Fig. 3.
Performance status preoperatively and at one, three, and six months postoperatively.
Table V.
Outcome at each time point according to age at surgery.
| Variable | < 70 years | ≥ 70 years | p-value* |
|---|---|---|---|
| Median ECOGPS (IQR), (number of PS 0, 1, 2, 3, 4) | |||
| Baseline | 3 (3 to 4), (0, 0, 15, 26, 24) | 3 (3 to 4), (0, 1, 4, 14, 17) | 0.243 |
| One month | 2 (1 to 2), (1, 26, 24, 14, 0) | 2 (1 to 2.75), (1, 11, 15, 7, 2) | 0.426 |
| Three months | 1 (1 to 2), (1, 32, 16, 4, 0) | 1 (1 to 3), (1, 13, 9, 8, 0) | 0.070 |
| Six months | 1 (1 to 2), (1, 27, 6, 3, 1) | 1 (1 to 2), (1, 13, 3, 2, 1) | 0.834 |
| Mean BI (range) | |||
| Baseline | 55.7 (5 to 100) | 49.3 (0 to 100) | 0.292 |
| One month | 83.1 (10 to 100) | 79.6 (15 to 100) | 0.460 |
| Three months | 88.4 (10 to 100) | 81.9 (30 to 100) | 0.236 |
| Six months | 91.8 (5 to 100) | 90.6 (15 to 100) | 0.849 |
| Mean EQ-5D (range) | |||
| Baseline | -0.020 (-0.594 to 0.796) | -0.130 (-0.594 to 1.000) | 0.110 |
| One month | 0.712 (-0.221 to 1.000) | 0.644 (-0.331 to 0.883) | 0.531 |
| Three months | 0.781 (-0.016 to 1.000) | 0.697 (-0.043 to 1.000) | 0.192 |
| Six months | 0.766 (-0.331 to 1.000) | 0.720 (-0.429 to 1.000) | 0.941 |
Kruskal-Walis test and Scheffe’s post hoc test.
BI, Barthel index; ECOGPS, Eastern Cooperative Oncology Group performance status; EQ-5D, EuroQol Five Dimension questionnaire; IQR, interquartile range.
BI (ADL)
The mean BI at the start was 55.7 (0 to 100) in Group Y and 49.2 (0 to 100) in Group O (p = 0.270, Mann-Whitney U test). There was no significant difference between the two groups (Table I). These scores indicated dependence in daily life. At one-month postoperatively, the mean BI had greatly improved in both groups sufficently to indicate independence in daily life. At three and six months, the BI was maintained with gradual additional improvement in both groups. The chronological course of the mean BI showed no significant differences between the two groups at all stages (Table V).
EQ-5D score (QoL)
The mean EQ5D score at the start was −0.020 (−0.590 to 0.796) in Group Y and −0.130 (−0.594 to 1.000) in Group O. There was no significant difference between the two groups (Table I). At one month postoperatively, the mean score had greatly improved to 0.712 (−0.021 to 1.000) in Group Y and 0.644 (−0.331 to 0.883) in Group O. Additionally, at three and six months, the score was maintained in both groups. There was no significant difference in the EQ-5D score between the two groups at all postoperative time points (Table V).
Chronological changes
Almost 90% of patients with SSM showed an improved PS (90 of 101, 89.1%), BI (91 of 101, 90.1%), and EQ-5D score (96 of 101, 95.0%) after spine surgery (Table VI). They also exhibited a similar chronological clinical course. The mean PS, BI, and EQ-5D scores improved after spinal surgery in both groups, with no significant difference between the groups at any point. Individual analysis showed that some patients’ health state deteriorated after the initial improvement. The redeterioration rate of the PS, ADL, and QoL among all patients was 15.6%, 14.3%, and 18.8%, respectively (Table VI). The multivariate analysis revealed that the Katagiri score was a significant independent risk factor for redeterioration of the PS (odds ratio (OR) 2.61), ADL (OR 1.90), and QoL (OR 2.26). Additionally, age of ≥ 70 years (OR 3.75) was a significant risk factor for deterioration of the EQ-5D score; it was identified as the strongest risk factor among all variables (Table VII).
Table VI.
Individual chronological changes according to age at surgery.
| Change | Total per score (n = 101) | < 70 years per score (n = 65) | ≥ 70 years per score (n = 36) | p-value* |
|---|---|---|---|---|
| ECOGPS, n (%) | ||||
| Improvement | 90 (89.1) | 60 (92.3) | 30 (83.3) | 0.193 |
| Unchanged | 9 (8.9) | 3 (4.6) | 6 (16.7) | |
| Deterioration | 2 (2.0) | 2 (3.1) | 0 (0.0) | |
| Redeterioration | 14 (15.6) | 8 (13.3) | 6 (20.0) | 0.538 |
| Barthel index, n (%) | ||||
| Improvement | 91 (90.1) | 58 (89.2) | 33 (91.7) | > 0.999 |
| Unchanged | 9 (8.9) | 6 (9.2) | 3 (8.3) | |
| Deterioration | 1 (1.0) | 1 (1.5) | 0 (0.0) | |
| Redeterioration | 13 (14.3) | 6 (10.3) | 7 (21.2) | 0.213 |
| EQ-5D, n (%) | ||||
| Improvement | 96 (95.0) | 61 (93.8) | 35 (97.2) | 0.653 |
| Unchanged | 4 (4.0) | 3 (4.6) | 1 (2.8) | |
| Deterioration | 1 (1.0) | 1 (1.5) | 0 (0.0) | |
| Redeterioration | 18 (18.8) | 7 (11.5) | 11 (31.4) | 0.027 |
Fisher's exact test.
BI, Barthel index;ECOGPS, Eastern Cooperative Oncology Group performance status; EQ5D, EuroQol-5 Dimension.
Table VII.
Multivariate analysis of risk factors for redeterioration of ECOGPS, BI, and EQ-5D.
| Factors | OR (95% CI) | p-value* |
|---|---|---|
| ECOGPS | ||
| Age ( ≥ 70 years) | 1.96 (0.50 to 7.75) | 0.338 |
| Sex (male) | 2.19 (0.40 to 12.17) | 0.369 |
| Katagiri score | 2.61 (1.41 to 4.82) | 0.002 |
| Tokuhashi score | 1.23 (0.85 to 1.78) | 0.282 |
| ECOGPS at baseline | 0.96 (0.25 to 3.67) | 0.947 |
| BI at baseline | 1.02 (0.98 to 1.07) | 0.299 |
| EQ-5D at baseline | 0.39 (0.05 to 3.41) | 0.394 |
| Frankel classification at baseline | 0.88 (0.20 to 4.00) | 0.872 |
| Barthel index | ||
| Age ( ≥ 70 years) | 2.78 (0.71 to 10.81) | 0.141 |
| Sex (male) | 0.84 (0.18 to 3.82) | 0.816 |
| Katagiri score | 1.90 (1.09 to 3.31) | 0.024 |
| Tokuhashi score | 1.17 (0.81 to 1.71) | 0.408 |
| ECOGPS at baseline | 0.82 (0.21 to 3.12) | 0.768 |
| BI at baseline | 1.03 (0.98 to 1.08) | 0.201 |
| EQ-5D at baseline | 0.49 (0.06 to 3.82) | 0.449 |
| Frankel classification at baseline | 0.18 (0.04 to 0.87) | 0.032 |
| EQ-5D | ||
| Age ( ≥ 70 years) | 3.75 (1.05 to 13.41) | 0.042 |
| Sex (male) | 1.81 (0.41 to 7.96) | 0.433 |
| Katagiri score | 2.26 (1.31 to 3.91) | 0.003 |
| Tokuhashi score | 1.18 (0.83 to 1.67) | 0.365 |
| ECOGPS at baseline | 0.72 (0.20 to 2.67) | 0.625 |
| BI at baseline | 1.04 (1.00 to 1.08) | 0.085 |
| EQ-5D at baseline | 0.18 (0.02 to 1.48) | 0.111 |
| Frankel classification at baseline | 0.30 (0.07 to 1.23) | 0.094 |
Multiple logistic regression analysis.
BI, Barthel index; CI, confidence interval; ECOGPS, Eastern Cooperative Oncology Group performance status; EQ-5D, EuroQol-5 dimension.
On careful comparison of age and redeterioration, subsequent deterioration of an acquired PS and BI were observed in eight of 60 (13.3%) patients and six of 58 (10.3%) patients in Group Y, respectively, and in six of 30 (20.0%) patients and seven of 33 (21.2%) patients in Group O, respectively. These differences were not statistically significant (Table VII). Redeterioration of the EQ-5D score occurred in seven of 61 (11.5%) patients in Group Y and 11 of 33 (31.4%) patients in Group O. This difference was statistically significant (p = 0.027, Fisher's exact test) (Table VII). In addition, four out of seven patients (57.1%) in Group Y redeteriorated within three months postoperatively, while three out of seven patients (42.9%) in Group Y redeteriorated from three to six months postoperatively (Table VIII). Meanwhile, eight out of 11 patients (72.7%) in Group O redeteriorated within three months postoperatively (Table VIII). However, the QoL was maintained at more than the baseline level except in one of seven (14.3%) patients in Group Y and two out of 11 (18.2%) patients in Group O.
Table VIII.
Time points of redeterioration of EQ-5D score.
| Time | One to three months | Three to six months | Total |
|---|---|---|---|
| < 70 years | 4 | 3 | 7 |
| ≥ 70 years | 8 | 3 | 11 |
EQ-5D, EuroQol-5 Dimension.
Frankel classification (neurological status)
The Frankel classifications at the start and latest follow-up of the study are shown in Table IX. Almost all patients showed an unchanged or improved neurological status compared with their initial status; only two patients showed deterioration. Some patients (Group Y, n = 3; Group O, n = 4) developed neurological deterioration after improvement. However, there was no significant difference between the two groups (p = 0.420, Fisher's exact test).
Table IX.
Neurological status at the start and latest follow-up of the study based on Frankel classification, according to age at surgery.
| < 70 years (n = 65), n (%) | ≥ 70 years (n = 36), n (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A→A | A→B | A→C | A→D | A→E | Total | A→A | A→B | A→C | A→D | A→E | Total |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B→A | B→B | B→C | B→D | B→E | Total | B→A | B→B | B→C | B→D | B→E | Total |
| 0 | 0 | 1 (1.5) | 1 (1.5) | 1 (1.5) | 3 (4.6) | 0 | 1 (2.8) | 0 | 0 | 0 | 1 (2.8) |
| C→A | C→B | C→C | C→D | C→E | Total | C→A | C→B | C→C | C→D | C→E | Total |
| 0 | 0 | 2 (3.1) | 9 (13.8) | 9 (13.8) | 20 (30.8) | 0 | 0 | 4 (11.1) | 7 (19.4) | 3 (8.3) | 14 (38.9) |
| D→A | D→B | D→C | D→D | D→E | Total | D→A | D→B | D→C | D→D | D→E | Total |
| 0 | 0 | 2 (3.1) | 7 (10.8) | 16 (24.6) | 25 (38.5) | 0 | 0 | 0 | 1 (2.8) | 9 (25.0) | 10 (27.8) |
| E→A | E→B | E→C | E→D | E→E | Total | E→A | E→B | E→C | E→D | E→E | Total |
| 0 | 0 | 0 | 0 | 17 (26.2) | 17 (26.2) | 0 | 0 | 0 | 0 | 11 (30.6) | 11 (30.6) |
The first grade indicates the neurological status preoperatively. The second grade indicates the neurological status at the latest follow-up within six months. The Frankel classification: grade A, complete paralysis; grade B, sensory function only; grade C; incomplete motor function; grade D, fair to good motor function; and grade E, normal function.
Discussion
Many studies have revealed the effectiveness of palliative surgery, but most have focused on objective indicators such as physical activity, neurological findings, walking ability, and bladder/rectal disturbance.18–20 In 2005, a Phase III trial involving patients who were non-ambulatory because of spinal metastases strongly suggested that decompressive surgery following postoperative radiotherapy was superior to radiotherapy alone in terms of the ambulatory status, regaining the ability to walk, ambulatory duration, and survival.19 With respect to surgical outcomes in patients of advanced age, a retrospective study of 92 patients aged ≥ 60 years (mean 68.0) by Liang et al18 showed that the neurological function improved in 78% of patients. They concluded that spinal surgery for older patients with spinal metastasis can achieve pain relief and neurological improvement. However, few studies have investigated the comprehensive surgical outcome for spinal metastasis in older patients.21
Accordingly, we designed the present prospective cohort study to reveal the surgical outcome for spinal metastases in older patients with a particular focus on health state values. Approximately 90% of patients experienced initial improvement by spinal surgery, and older and younger patients were equally affected. The surgical outcome of older patients was considered to compare favourably with that of younger patients. Regarding the surgical outcome of spinal surgery in older patients, Chen et al22 reported that improvement after surgical treatment of cervical spondylotic myelopathy was poor in patients aged ≥ 70 years. Elsewhere, Ethe clinical outcome of surgically treated spinal deformity in patients aged > 70 years has been reported to be worse than that in younger patients.23 However, Ragab et al24 concluded that the surgical outcome of lumbar spinal stenosis in patients aged ≥ 70 years was not worse than that in younger patients. The effect of spinal surgery for each disease in patients of advanced age remains controversial. Further studies with higher levels of evidence are needed.
The survival rate and the complication rate were similar among patients aged ≥ 70 years and younger patients. In a retrospective study of 92 cases by Liang et al,21 the surgical complication rate in patients aged > 60 years (mean, 68.0) with spinal metastases was 22.8%. Additionally, Jansson and Bauer20 reported that the surgical complication rate in 282 patients with spinal metastases (median age 66.0 years (23 to 93)) was 20.0%. In the current study, the survival rate was similar to these prior reports and was affected by the primary tumour type and lung metastasis rather than advanced age. The complication rate was higher than in these previous reports. However, those reports did not include the need of blood transfusion. With the exception of blood transfusion, the complication rate in our patients aged ≥ 70 years was 25.0%, which compares favourably with previous reports considering that our patients were older than those in previous reports. Interestingly, the current study showed that neither the complication rate nor the severity in the patients aged ≥ 70 years was significantly higher than that in the patients aged < 70 years. To minimize surgical stress, we performed posterior decompression and stabilization without using an anterior approach or resecting tumours that were located in front of the spinal cord; this might have been related to our favourable results.
The older patients in this study showed a trend toward deterioration of their heath status after the initial postoperative improvement, and the redeterioration rate of their QoL reached a statistically significant difference. In the multivariate analysis, the risk factors for redeterioration were the Katagiri score and age ( ≥ 70 years). This is because the Katagiri score, which reflects the primary tumour type and extent of metastatic progression, is associated with the prognosis. With respect to advanced age, Amelot et al21 also reported that surgery for spinal metastases significantly improved the QoL of patients in all age groups and maintained this improvement for at least one year; when limited to patients aged > 80 years; however, the two-year postoperative QoL deteriorated with greater variation. Because the QoL of older patients may be likely to deteriorate over time in general, this redeterioration can be considered the natural clinical course.
In the current study, advanced age did not have a significant effect on the chronological change in QoL using the Kruskal-Wallis test and Scheffe’s post hoc test with true value of the EQ5D score. However, we also analyzed these results from a critical viewpoint. According to Fisher’s exact test based on another definition (≥ 10% change in the EQ-5D score in each patient), the QoL of older patients with spinal metastases was more likely to redeteriorate after surgery than that of younger patients. However, even when the QoL redeteriorated after surgery, it was maintained at a higher level than that at baseline. Because the final goal of treatment for spinal metastases is to improve and maintain patients’ ADL and QoL, advanced-age patients with SSM should undergo decompression and stabilization surgery if they are surgical candidates, although the limitation of redeterioration of QoL in older patients still remains. When considering the treatment strategy for spinal metastases, advanced-age patients may need more attention and an individual tailored strategy through a multidisciplinary team discussion that includes the patient and his or her family.
This study has several limitations. First, the follow-up period varied and was relatively short. However, this limitation is inevitable because of the characteristics of patients with terminal-phase cancer. Actually, the overall median postoperative survival time was < one year in both groups. Another limitation is the bias of the age distribution. Consequently, the number of patients aged ≥ 70 years was relatively small, with a high number of patients aged 70 to 74 years (n = 20) and a low number of patients aged ≥ 75 years (n = 16). As the ageing of society progresses, the number of patients aged ≥ 75 years with spinal metastases is likely to increase. Therefore, future investigation will be required.
In conclusion, palliative posterior decompression and stabilization surgery for spinal metastases greatly improved the PS, ADL, and QoL of patients, regardless of age. Furthermore, the improvement rate in patients aged ≥ 70 years was as good as that in patients aged < 70 years without an increased risk of complications. However, it should be noted that the Katagiri score and advanced age ( ≥ 70 years) were risk factors for deterioration after improvement of the QoL.
Take home message
- Palliative surgery for spinal metastases improved the performance status, activities of daily living, and quality of life, regardless of age.
- In patients of advanced age, we should pay attention to the higher risk of redeterioration of quality of life.
Author contributions
Y. Kanda: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
K. Kakutani: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
Y. Sakai: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
T. Yurube: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
S. Miyazaki: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
T. Takada: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
Y. Hoshino: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
R. Kuroda: Conceptualized and designed the study, Acquired, analyzed, and interpreted the data, Drafted, critically revised, and approved the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
K. Kakutani reports grants/grants pending by Surgical Spine, and payment for lectures by Asahi Kasei Pharma, Eli Lilly Japan, Daiichi Sankyo, which are unrelated to this article. R. Kuroda reports consultancy from Medacta International, Arthrex, and Japan Tissue Engineering, grants/grants pending Stryker Japan, Zimmer Biomet, Smith & Nephew, and Johnson & Johnson, payment for lectures from Zimmer Biomet, Johnson & Johnson, Arthrex, and Japan Tissue Engineering, and payment for manuscript preparation by Stryker Japan, all of which are unrelated to this article. Y. Sakai reports Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science, payment for lectures from Asahi Kasei Pharma, Astellas Pharma, Eisai, Ono Pharmaceutical, Takeda Pharmaceutical, Chugai Pharmaceutical, Glaxosmithkline, MSD, Taisho Toyama Pharmaceutical, Eli Lilly Japan, AbbVie, and Daiichi Sankyo, and stock/stock options from CO2BE Medical Enginering, all of which are unrelated to this article. T. Yurube reports grants/grants pending from Japan Society for the Promotion of Science, which is unrelated to this article.
Acknowledgements
The authors thank Edanz (https://en-author-services.edanzgroup.com/) for English language editing.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
This article was primary edited by G. Scott.
References
- 1.Kimura T. Multidisciplinary approach for bone metastasis: a review. Cancers. 2018;10(6):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggermont F, Derikx LC, Verdonschot N, et al. Can patient-specific finite element models better predict fractures in metastatic bone disease than experienced clinicians?: towards computational modelling in daily clinical practice. Bone Joint Res. 2018;7(6):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J-S, Park S-J, Lee C-S. Incidence and prognosis of patients with spinal metastasis as the initial manifestation of malignancy: analysis of 338 patients undergoing surgical treatment. Bone Joint J. 2019;101-B(11):1379–1384. [DOI] [PubMed] [Google Scholar]
- 4.Kakutani K, Sakai Y, Maeno K, et al. Prospective cohort study of performance status and activities of daily living after surgery for spinal metastasis. Clin Spine Surg. 2017;30(8):E1026–E1032. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki S, Kakutani K, Sakai Y, et al. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: prospective cohort study. Int Orthop. 2017;41(6):1265–1271. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, He S, Liu T, et al. Quality of life of patients with spinal metastasis from cancer of unknown primary origin: a longitudinal study of surgical management combined with postoperative radiation therapy. J Bone Joint Surg Am. 2017;99(19):1629–1639. [DOI] [PubMed] [Google Scholar]
- 7.No authors listed . National Institute of population and social security research (2012). Population and projection for Japan: 2011 to 2060. http://www.ipss.go.jp/site-ad/index_english/esuikei/gh2401e.asp (date last accessed 8 October 2020).
- 8.Carreon LY, Puno RM, Dimar JR, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85(11):2089–2092. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Imagama S, Ando K, et al. Complications associated with spine surgery in patients aged 80 years or older: Japan association of spine surgeons with ambition (JASA) multicenter study. Global Spine J. 2017;7(7):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrow MJ, Goel V, Lemieux-Charles L, Black NA. The impact of context on evidence utilization: a framework for expert groups developing health policy recommendations. Soc Sci Med. 2006;63(7):1811–1824. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2195. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the spine oncology Study Group. Spine. 2010;35(22):E1221–1229. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3(5):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30(19):2186–2191. [DOI] [PubMed] [Google Scholar]
- 15.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7(3):179–192. [DOI] [PubMed] [Google Scholar]
- 16.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang T, Wan Y, Zou X, et al. Is surgery for spine metastasis reasonable in patients older than 60 years? Clin Orthop Relat Res. 2013;471(2):628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. [DOI] [PubMed] [Google Scholar]
- 20.Jansson K-A, Bauer HCF, Survival BHC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amelot A, Balabaud L, Choi D, et al. Surgery for metastatic spine tumors in the elderly: advanced age is not a contraindication to surgery. Spine J. 2017;17(6):759–767. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Liu Z, Zhong G, et al. Surgical treatment for cervical spondylotic myelopathy in elderly patients: a retrospective study. Clin Neurol Neurosurg. 2015;132:47–51. [DOI] [PubMed] [Google Scholar]
- 23.Yagi M, Fujita N, Okada E, et al. Clinical outcomes, complications, and cost-effectiveness in surgically treated adult spinal deformity over 70 years: a propensity score-matched analysis. Clin Spine Surg. 2020;33(1):E14–E20. [DOI] [PubMed] [Google Scholar]
- 24.Ragab AA, Fye MA, Bohlman HH. Surgery of the lumbar spine for spinal stenosis in 118 patients 70 years of age or older. Spine. 2003;28(4):348–353. [DOI] [PubMed] [Google Scholar]