Abstract
Fears persist despite compelling evidence refuting associations between vaccines and autism spectrum disorder (ASD). We compared vaccine hesitancy (VH) and beliefs about illness causes among parents of children in four groups: ASD, non-ASD developmental disorders, rheumatologic conditions, and the general pediatric population. VH was 19.9% overall; parents of children with ASD reported highest VH rates (29.5%) and more frequently attributed ASD to toxins in vaccines (28.9% vs. 15.7%, p=0.004). The odds of VH were increased among parents who attributed their child’s condition to diet or eating habits (aOR 4.2; 95% CI: 1.6, 11.2) and toxins found in vaccines (aOR 20, 95% CI: 7.1, 55.9). Parents who attributed the condition to chance or bad luck were less likely to be vaccine hesitant (aOR 0.1; 95% CI: 0.03, 0.5).
Introduction
Vaccines and the associated reductions in morbidity and mortality from vaccine-preventable diseases are widely recognized as one of public health’s greatest achievements.1,2 Despite this, a growing minority of parents have concerns about vaccine safety that manifests as vaccine hesitancy and/or refusal.3–5 Parental vaccine acceptance exists on a spectrum, ranging from parents who accept and actively advocate for vaccines to those who emphatically refuse all vaccines, with the majority of parents falling somewhere in between these beliefs.5–7 Vaccine-hesitant parents are unsure about one or more vaccines, which may result in the decision to delay or refuse vaccines for their children.6 While not all vaccine-hesitant parents delay or refuse vaccines, vaccine refusers often cluster geographically, which has been associated with outbreaks of vaccine-preventable diseases occurring among individuals susceptible to disease as a result of sub-optimal vaccine coverage.8,9
Despite compelling evidence refuting associations between vaccines and autism spectrum disorder (ASD),10–20 up to 40% of parents of children with ASD believe that vaccines contributed to or caused their child’s condition.21–26 Furthermore, recent studies have found that younger siblings of children diagnosed with ASD are underimmunized compared to their peers, even in the absence of vaccine refusal in the child with ASD,27–31 suggesting that concerns about vaccines causing autism persist and that receipt of an ASD diagnosis may result in the development of vaccine hesitancy among parents who previously accepted vaccines. Similarly, concerns that vaccines may cause other chronic health conditions, such as multiple sclerosis and diabetes, have been suggested in anecdotal case reports and uncontrolled observational studies. These potential links have been extensively investigated, and no evidence has been found to support a causal relationship.32–34 However, it is unclear how misinformation about an erroneous association between vaccines and chronic illness may impact vaccine hesitancy among parents of children with chronic illnesses and how vaccine beliefs in this population compare to those of parents of children with and without ASD.
As such, we examined vaccine hesitancy and beliefs about causes of their child’s diagnosis among parents of children with ASD, parents of children with other chronic medical or behavioral health conditions, including rheumatologic conditions and non-ASD developmental disorders, and parents of children from the general pediatric population seeking well-child care. The aims of this undertaking were to: determine the proportion of vaccine hesitant parents, overall and by diagnosis type; identify parental beliefs about the causes of chronic medical or behavioral health conditions that were associated with vaccine hesitancy; and examine differences in these beliefs by diagnosis type.
Methods
Study design and population
English-speaking parents of children 2–17 years of age were eligible to participate in this cross-sectional assessment if their child met one of three criteria: 1) enrolled in the Autism Center Research Database at the Texas Children’s Hospital Autism Centera and consented to being contacted about new studies; 2) diagnosed with a rheumatologic condition and receiving treatment at the Texas Children’s Hospital Rheumatology outpatient clinic; or 3) receiving well-child care at a Texas Children’s Pediatrics practice, a 58-practice network of pediatric primary care locations throughout the greater Houston area. Participants were recruited from these sources based on their child’s medical diagnosis to represent one of four groups: parents of children diagnosed with ASD, parents of children with non-ASD developmental disorders (non-ASD-DD), parents of children with rheumatologic conditions, and parents of children receiving well-child care in a general pediatric practice.
Survey instruments and data collection
Eligible parents were recruited in two stages. The first wave of parents of children with ASD and non-ASD developmental disorders received an invitation to participate by mail during January, 2015, with up to three subsequent reminders by email. A second wave of parents of children with ASD and non-ASD-DD received an identical invitation and reminder schedule in February, 2016. Parents of children with rheumatologic conditions and children from the general pediatric population seeking well-child care received invitations to participate in October, 2016, and January, 2017, respectively, with the same reminder schedule. The study ceased enrollment by November, 2017, for data analyses. These invitations included a cover letter introducing the study, questions about child and parent demographics, a self-addressed stamped envelope for return of measures, the Parent Attitudes about Vaccines (PACV) survey,35,36 and a modified version of the Revised Illness Perception Questionnaire (IPQ-R) specific to their group classification. Because diagnostic status (ASD versus non-ASD-DD) for those enrolled in the Autism Center’s research database was undetermined at the time invitations were sent, both the ASD and non-ASD-DD groups received the version for those with developmental delays (IPQ-R-DD).37 Those in the rheumatologic condition group received a version modified for children with a diagnosed rheumatoid disorder (IPQ-R-RC).37 Those in the group seeking well-child care received only the Cause subscale of the IPQ-R-ASD38, a version that had been adapted and validated for use in populations with ASD, as they were asked specifically for their perceptions about potential causes of ASD. Return of completed questionnaires indicated consent to participate.
The PACV is a validated, 15-item survey assessing 3 domains (behavior, vaccine safety and efficacy, and general attitudes) that can be self-administered in <5 minutes. Scores range from 0–100 and are dichotomized into vaccine hesitant (≥50) and non-hesitant (<50) groups, with hesitancy among parents predictive of vaccine delay in their children.39,40 The IPQ-R measures 9 subscales related to an individual’s perception of his or her diagnosis and how these perceptions influence treatment decisions and health-seeking behaviors. This scale was originally developed based on Leventhal’s Model of Illness Representation41,42 and measures thoughts and attributions (i.e., cognitive representations) of health-related conditions; information yielded from this scale is thought to provide insight into individuals’ (or their caregivers’) attempts to understand, predict, and control potential health threats related to such conditions.43–45 This instrument has been widely used to study cognitive attributions related to a variety of chronic health conditions (e.g., asthma, diabetes), for affected individuals and also for caregivers. The IPQ-R has been modified and validated for use with parents of children who have ASD, both in France 44,46 and in the U.S.26, 38, and parents’ attributions and beliefs regarding their own children’s ASD diagnoses are very similar to conceptualizations people have about other chronic diagnoses.38 The IPQ-R is designed to be adapted for use with whatever health condition is being studied with regard to the Identity (i.e., symptoms) and Cause (i.e., etiological attributions) subscales.37 The Cause subscale is considered a separate dimension of study,48 and its’ examination is often undertaken separately from other scales in order to understand the potential influence of causal attributions on various health-related behaviors. Because parents’ perceptions about what caused their children’s condition or what causes ASD may influence health-related decisions, like whether they will vaccinate their children,49 our focus in the current study was on how parents’ responses to items on the Cause subscale was potentially related to vaccine hesitancy. Importantly, the Cause subscale of the IPQ-R-ASD has been empirically investigated, with six causal belief factors (Personal Attributions, Parental Risk, Environmental Risk, Utero/Birth Stress, Biophysiological, and Metaphysical) explaining 61% of the variance in item scores.50
In the current study, we examined responses to the 21-item Cause subscale of the IPQ-R-DD, IPQ-R-RC, and IPQ-R-ASD.37,38 Items in the Cause subscale were identical across these measures, wherein parents of children with ASD, non-ASD-DD, and rheumatologic conditions were asked to indicate their level of agreement with 21 factors that may or may not have contributed to their child’s condition. Parents of children in the general pediatric population were asked to indicate their level of agreement with each of these same factors as a cause of ASD. Responses were documented using a 5-point Likert scale.
Return of completed questionnaires served as documentation of a participant’s consent to participate, and this information was shared in the cover letter introducing the study. Upon receipt of completed questionnaires, respondents were entered into a drawing for one of three $100 gift cards (one card per recruitment source) at the conclusion of the study period. This assessment was reviewed and approved by the institutional review board at Baylor College of Medicine.
Data Analysis
Demographic information (parent and child) was summarized using means with standard deviations and frequencies with percentages. Responses to the Cause subscale items were dichotomized (strongly agree and agree vs. strongly disagree, disagree, and neutral), summarized, and compared between diagnosis groups using Fisher’s exact tests. Vaccine hesitancy was calculated as hesitant (≥50) or non-hesitant (<50) from PACV responses using previously-described scoring methodology and categorized for participants overall and by child’s diagnosis (ASD, non-ASD-DD, rheumatologic condition, general pediatric population).40 Multiple logistic regression models estimated odds ratios with 95% confidence intervals for parental vaccine hesitancy in two stages. In the first stage, odds ratios for vaccine hesitancy by cohort were estimated, adjusting for parent age, child age, child gender, parent education, parent race, and parent ethnicity. The second stage included all predictors from the first stage, as well as all itemized causes. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
Results
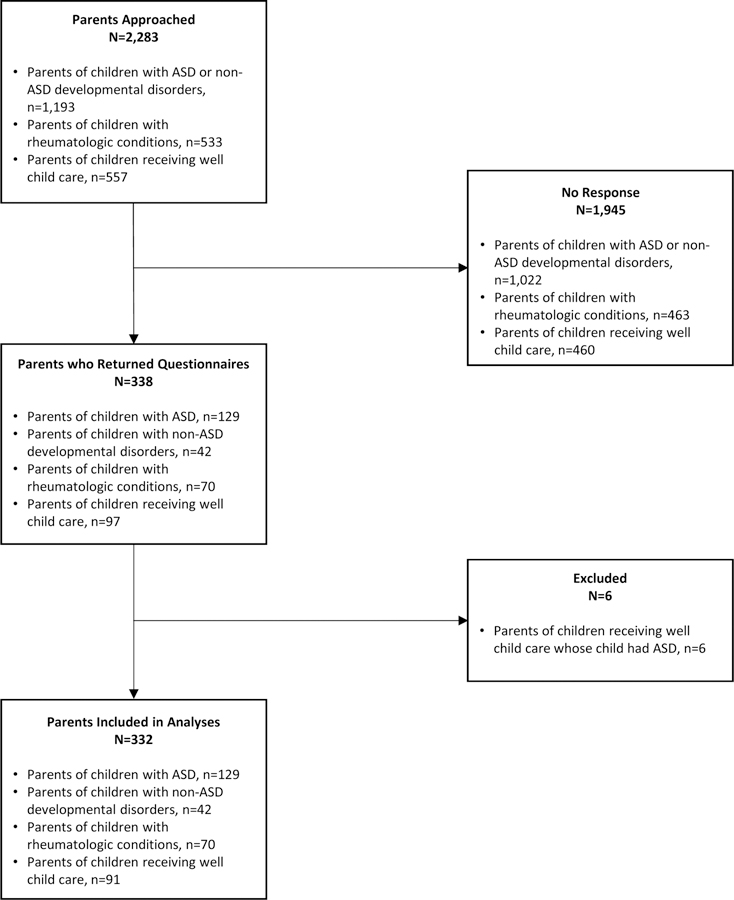
A total of 2,283 parents received an invitation to participate (n=1,193 parents of children with ASD and non-ASD-Db, n=533 parents of children with rheumatologic conditions, and n=557 parents of children in the general pediatric population) (see Figure 1). Of these, 338 parents [n=129 (10.8%) parents of children with ASD, n=42 (3.5%) parents of children with non-ASD-DD, n=70 (13.1%) parents of children with rheumatologic conditions, and n=97 (17.4%) parents of children in the general pediatric population] returned completed surveys, for an overall response rate of 14.8%. The parents of 6 children recruited from Texas Children’s Pediatrics practices were subsequently excluded because of a self-reported ASD diagnosis in their child; the remaining 332 participants were included in analyses.
Figure 1.
Flow diagram of participant inclusion
The majority of focal children were male (n=204; 61.6%) and not firstborn (n=170; 51.2%) with a mean age of 8.1 years (SD: 4.6 years), while the majority of parents were white (n=251; 75.6%) and not Hispanic/Latino (n=251; 75.6%) with a mean age of 38. 7 years (SD: 7.9 years) (see Table 1). Differences were observed across diagnostic groups in child gender, age, and birth order (firstborn vs. subsequent child) and in parent race (white vs. non-white), ethnicity (Hispanic/Latino vs. non-Hispanic/Latino), age, educational attainment (some college vs. no college), and household income.
Table 1.
Child and parent demographic characteristics by diagnosis group
| Well child care | Rheumatologic conditions | Non-ASD developmental disorders | ASD | p value | |
|---|---|---|---|---|---|
| N=91 | N=70 | N=42 | N=129 | ||
| 27.41% | 21.08% | 12.65% | 38.86% | ||
| Child Characteristics | |||||
| Age, years (standard deviation) | 6.45 (4.73) | 12.06 (5.04) | 7.96 (3.27) | 7.22 (3.31) | <0.001 |
| Male, n (%) | 51 (56.04) | 16 (22.86) | 27 (64.29) | 110 (85.94) | <0.001 |
| First born, n (%) | 49 (53.85) | 36 (51.43) | 12 (28.57) | 65 (50.39) | 0.042 |
| Parent Characteristics | |||||
| Age, years (standard deviation) | 37.34 (7.03) | 41.65 (7.27) | 39.94 (9.27) | 37.58 (7.89) | <0.001 |
| White, n (%) | 77 (84.62) | 56 (80.00) | 31 (73.81) | 87 (67.44) | 0.024 |
| Non-Hispanic/Latino, n (%) | 81 (89.01) | 59 (84.29) | 26 (61.90) | 85 (65.89) | <0.001 |
| Household income, n (%) | <0.001 | ||||
| ≤$50,000 | 17 (18.89) | 14 (20.29) | 19 (47.50) | 58 (45.31) | |
| $51,000-$100,000 | 21 (23.33) | 22 (31.88) | 15 (37.50) | 36 (28.13) | |
| >$100,000 | 52 (57.78) | 33 (47.83) | 6 (15.00) | 34 (26.56) | |
| Highest education, n (%) | 0.002 | ||||
| Less than HS/GED | 5 (5.49) | 4 (5.80) | 11 (26.19) | 20 (15.50) | |
| Some college or higher | 86 (94.51) | 65 (94.20) | 31 (73.81) | 109 (84.50) |
Vaccine Hesitancy
Overall vaccine hesitancy was 19.9% (95% CI: 15.7%, 24.6%), ranging from 8.6% among parents of children with rheumatologic conditions to 29.5% among parents of children with ASD. The median PACV score, regardless of child’s diagnosis, was 21.5 (minimum = 0, maximum = 100). Diagnostic cohort was significantly associated with parental vaccine hesitancy after adjusting for parent age, child age, child gender, parent education, parent race, and parent ethnicity (p=0.04). The odds of vaccine hesitancy among parents of children with non-ASD-DD, rheumatologic disorder, and the general pediatric population were less than the odds for parents of children with ASD. Respective, adjusted odds ratios were 0.4 (95% CI: 0.2, 1.2), 0.3 (95% CI: 0.1, 0.7), and 0.6 (95% CI: 0.3, 1.1) lower.
Factors Contributing to a Child’s Medical Condition or ASD
Overall, the proportion of parents who agreed with Cause items as having contributed to a child’s medical condition or ASD ranged from 6.8% for “mental attitude/negative views” to 76.3% for “genetics” (see Table 2). Across all parent groups, “genetics” was the most frequently identified cause, with 69.0%, 80.0%, 71.0%, and 88.9% of parents of children with ASD, non-ASD-DD, rheumatologic conditions, and the general pediatric population, respectively, agreeing or strongly agreeing that this item contributed to the identified condition. The least-frequently identified contributing causal beliefs varied by parent group. These included “my own tobacco consumption,” “my own alcohol consumption,” and “mental attitude/negative views” (see Table 2). Differences across parent groups were noted for all but five of the 21 items. Specifically, “diet or eating habits,” “chance or bad luck,” “poor medical care in the past,” “mental attitude/negative views,” and “will of God” did not vary by parent group.
Table 2.
Parental agreement with IPQ-R cause subscale items as factors contributing to chronic medical conditions
| Well child care | Rheumatologic conditions | Non-ASD developmental disorders | ASD | p valuea | All non-ASD conditions combined | p valueb | |
|---|---|---|---|---|---|---|---|
| N=91 | N=70 | N=42 | N=129 | N=203 | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| General life stress | 15 (16.7) | 18 (26.1) | 16 (41.0) | 44 (34.4) | 0.008 | 49 (24.7) | 0.060 |
| Genetics | 80 (88.9) | 49 (71.0) | 32 (80.0) | 87 (69.0) | 0.003 | 161 (80.9) | 0.014 |
| A germ or virus | 23 (25.6) | 19 (27.5) | 3 (7.7) | 16 (12.6) | 0.005 | 45 (22.7) | 0.023 |
| Diet or eating habits | 17 (19.1) | 25 (36.2) | 13 (33.3) | 29 (22.8) | 0.052 | 55 (27.9) | 0.308 |
| Chance or bad luck | 27 (30.0) | 13 (18.8) | 5 (12.8) | 25 (19.5) | 0.119 | 45 (22.7) | 0.493 |
| Poor medical care in the past | 13 (14.4) | 4 (5.9) | 7 (17.9) | 15 (11.7) | 0.209 | 24 (12.2) | 0.900 |
| Environmental pollution | 35 (38.9) | 14 (20.6) | 7 (17.9) | 36 (28.8) | 0.033 | 56 (28.4) | 0.953 |
| Own behavior or decisions | 16 (17.8) | 4 (5.8) | 9 (23.1) | 17 (13.3) | 0.044 | 29 (14.6) | 0.730 |
| In utero stress or accident | 39 (43.3) | 3 (4.3) | 18 (43.9) | 46 (36.2) | <0.001 | 60 (30.0) | 0.242 |
| Mental attitude/negative views | 4 (4.4) | 6 (8.7) | 2 (5.1) | 10 (8.0) | 0.677 | 12 (6.1) | 0.500 |
| Family worries about developmental delays | 5 (5.6) | 2 (2.9) | 9 (23.1) | 11 (8.6) | 0.005 | 16 (8.1) | 0.870 |
| Will of God | 29 (32.2) | 18 (26.1) | 11 (28.2) | 52 (40.6) | 0.170 | 58 (29.3) | 0.035 |
| My own emotional state (e.g. depression, anxiety) | 18 (20.0) | 4 (5.8) | 8 (21.1) | 27 (21.3) | 0.020 | 30 (15.2) | 0.164 |
| My or my partner’s age | 35 (38.9) | 2 (2.9) | 6 (15.4) | 15 (11.7) | <0.001 | 43 (21.7) | 0.021 |
| My alcohol consumption | 30 (33.3) | 0 (0.0) | 2 (5.1) | 8 (6.3) | <0.001 | 32 (16.2) | 0.008 |
| My tobacco consumption | 26 (28.9) | 0 (0.0) | 2 (5.1) | 6 (4.8) | <0.001 | 28 (14.2) | 0.029 |
| Accident or injury | 25 (27.8) | 5 (7.4) | 4 (10.3) | 14 (11.0) | 0.001 | 34 (17.3) | 0.123 |
| My child’s brain structure | 25 (27.8) | 59 (88.1) | 19 (48.7) | 57 (44.5) | <0.001 | 93 (47.4) | 0.158 |
| Deterioration of my child’s immunity | 21 (23.3) | 24 (35.3) | 8 (20.5) | 22 (17.3) | 0.048 | 53 (26.9) | 0.046 |
| Toxins found in vaccines/immunizations | 21 (23.3) | 3 (4.4) | 7 (17.9) | 37 (28.9) | <0.001 | 31 (15.7) | 0.004 |
| Stress at birth | 26 (28.9) | 7 (10.3) | 16 (41.0) | 46 (36.2) | <0.001 | 49 (24.9) | 0.029 |
Comparison across all groups
Comparison of ASD to all non-ASD conditions combined
Compared to parents of children with all non-ASD conditions combined, parents of children with ASD more frequently agreed that “toxins found in vaccines/immunizations,” “will of God,” and “stress at birth” contributed to a child’s diagnosis (see Table 2). Conversely, parents of children with ASD were less likely to agree that “genetics,” “a germ or virus,” “my or my partner’s age,” “my own alcohol consumption,” “my own tobacco consumption,” and “deterioration of my child’s immunity” contributed to a child’s condition compared with parents of children in all other groups.
Relationship between Vaccine Hesitancy and Beliefs about Causes of Chronic Developmental/Medical Conditions
Diagnostic cohort was no longer statistically significantly associated with vaccine hesitancy after adjusting for all previously identified confounding variables and all IPQ-R Cause subscale items (p=0.12). Compared with parents of children with ASD, the odds of vaccine hesitancy among parents of children with non-ASD-DD, rheumatologic conditions, and those seeking well child care were 0.6 (95% CI: 0.2, 2.0), 0.2 (95% CI: 0.03, 0. 8), and 0.9 (95% CI: 0.3, 2.7) lower, respectively.
Several Cause subscale responses were associated with vaccine hesitancy. The adjusted odds of vaccine hesitancy were 20 (95% CI: 7.1, 55.9) times greater among parents who strongly agreed or agreed that that “toxins found in vaccines/immunizations” contributed to their child’s condition compared with parents who were neutral or disagreed with this as a cause. Similarly, the adjusted odds ratios for vaccine hesitancy among parents who agreed that “diet or eating habits” contributed to developmental disabilities/chronic medical conditions was 4.2 (95% CI: 1.6, 11.2). Conversely, parents of children who believed that “chance or bad luck, contributed to a child’s condition were less likely to be vaccine hesitant (aOR 0.1; 95% CI: 0.03, 0.5).
Discussion
To our knowledge, this study is the first to directly compare vaccine hesitancy among parents of children with ASD to parents of children with other chronic medical conditions, such as rheumatologic conditions and non-ASD-DD, and to examine vaccine hesitancy in the context of parental beliefs about the causes of these conditions. In this study, almost 20% of parents were vaccine hesitant, although hesitancy varied widely across parent groups (well-child care = 17.6%, rheumatologic conditions = 8.6%, non-ASD-DD = 14.3%, ASD = 29.5%). Vaccine hesitancy among parents of children with rheumatologic disorders was similar to vaccine hesitancy previously noted among expectant mothers and parents of newborns in Houston, Texas (8% and 6% respectively), and is consistent with other assessments conducted across the United States using the PACV (6%℃9%).40,51–53 Vaccine hesitancy among all other parent groups was substantially higher than noted in prior studies, and was highest among parents of children with ASD, at almost 30%. Notably, vaccine hesitancy among parents of children from the general pediatric population seeking well-child care was almost twice as high as in comparable populations in prior research and warrants further investigation.
Each of the four parent groups included in this study represented parents of children with different developmental/medical conditions, or a lack of medical condition (i.e., well-child care group). Importantly, regardless of group, more than two-thirds of parents recognized the contribution of genetics to the medical condition in question. However, an important caveat to this finding is that parents of children with ASD had the lowest proportion endorsing genetics as a cause, despite the fact that genetic factors have more empirical support in the ASD literature than any other current cause. For parents of children with ASD, genetics, brain structure, will of God, in utero stress, and stress at birth were the most frequently identified beliefs about causes of their child’s conditions. A recent study of the IPQ-R-ASD Cause subscale found that genetics and brain structure loaded onto a common factor, and stress at birth and in utero stress loaded together on a separate Causal factor.50 This suggests that biophysiological and stress-related factors are among the most salient causal attributions for parents of children with ASD, yet belief in a higher power (i.e., will of God) is also an important potential explanation for ASD.
Our findings are similar to another analysis of parental beliefs about the causes of a child’s ASD conducted in France, which identified genetic susceptibility, brain damage or complications during pregnancy, and food intolerance as the main contributors to ASD. ASD was rarely attributed to vaccines (<5%) among this cohort of parents.47 In a prior analysis of parents who participated in the Simons Simplex Collection in Houston, Texas, genetics was the most frequently cited cause of a child’s ASD (76%), followed by child’s brain structure (59.7%), will of God (46.3%), and toxins in vaccines (41.8%).26 Although vaccines were more frequently identified as a cause of their child’s condition among parents of children with ASD compared with other parents included in the current study, vaccines were not among the top factors identified by parents, overall.
Our findings of high vaccine hesitancy among parents of children with ASD compared with other parent groups further suggest that parents of children with ASD differ in their beliefs about vaccines. Vaccine hesitancy is known to be associated with demographic factors, such as maternal age, maternal education, parental marital status and household income.54 Beyond this, physician trust, perceived disease risk, and governmental distrust also play important roles in parental vaccine hesitancy.54 Our findings suggest that additional factors, such as having a child with ASD, may influence vaccine hesitancy. These data emphasize the importance of understanding the entire milieu in which parents are making vaccine decisions. In adjusted analyses, parents of children with ASD had greater odds of hesitancy compared with parents in all other groups (non-ASD-DD, rheumatologic conditions, general pediatric population). Furthermore, parents who believed toxins in vaccines caused a child’s medical condition had a 20-fold increased odds of vaccine hesitancy. The strength of this association is not surprising. Parents who believe toxins in vaccines contributed to a child’s medical condition would be more likely to espouse vaccine-hesitant beliefs. Similarly, parents who did not believe that chance or bad luck caused a child’s medical condition were more likely to be vaccine hesitant.
It is important to point out that parents of children seeking well-child care (i.e., those in the general pediatric population) had the second highest proportion of vaccine hesitancy (17.6%) and also highest endorsement of a greater number of causes of ASD (n=8) compared to parents of children with ASD (n=3), non-ASD-DD (n=4), and rheumatoid disorder (n=4). This suggests elevated vaccine concerns among a group who does not have a child with ASD, as well as some confusion about what causes ASD. This underscores the need to improve communications about known causes of ASD through a variety of channels/platforms (e.g., social media, medical, government, education). For example, pediatric healthcare providers could initiate these conversations during the recommended ASD screenings (typically at 18- and 24-month well-child visits), particularly when children fail screens (i.e., ASD is suspected). Likewise, parents of children with ASD who refused vaccines as a way to avoid ASD could share their stories via social media about how their children developed ASD in the absence of vaccines. Our results further highlight the need for research that examines how ASD-associated vaccine concerns arise in the general pediatric population, where the public-health impact of vaccine delays/refusals has a larger footprint.
Despite overwhelming evidence that vaccines do not cause ASD, our findings and other assessments demonstrate that some parents continue to hold this belief.26–31,47 This is discouraging, as the medical community has worked diligently to dispel this myth among parents and the community at large. Parental decisions regarding a child’s medical treatment are influenced by physician recommendations as well as a variety of other factors, including emotion, faith, personal belief, prior experience, family, and other community members.55 Parents also seek and share medical information among peer networks and through in-person and online chat groups, support groups, and social media (i.e. Facebook and Twitter).56,57 Unfortunately, vaccine misinformation abounds online. Furthermore, Brunson and colleagues found that almost 75% of individuals who did not support childhood vaccination recommendations also advised others not to follow vaccination recommendations, compared with ~10% of people who conformed with vaccine recommendations.56 Therefore, it is understandable that a parent of a child with ASD may encounter other parents of children with ASD, either in person or online, who share their strongly-held vaccine concerns. This may then influence their own beliefs about vaccines as a cause of their child’s ASD and result in their becoming vaccine hesitant, possibly delaying and/or refusing vaccines for their children going forward. In fact, evidence supports changing vaccination behaviors among many parents of children with ASD, with increased delay/refusal following the ASD diagnosis for the affected child and his/her younger siblings.31
This study had several notable limitations. First, the overall response rate was low at 14.8%, and response rates in each parent group ranged from 3.4% to 17.4%. Moreover, participants self-selected and, therefore, may have had strongly-held beliefs about vaccines, which may have resulted in response bias affecting generalizability. Similarly, the majority of children included in this assessment were non-Hispanic/Latino, White, which impacts the generalizability of our findings. It is certainly conceivable that parents’ beliefs are influenced by cultural background, communities, and values; targeted investigation of parents’ attributions about ASD and their vaccine hesitancy among diverse families is much needed. Second, given the small numbers of participants in some parent groups (i.e. non-ASD-DD and rheumatologic conditions), it is possible that we lacked statistical power to detect differences between groups. While not ideal, difficulty in recruiting parents of children with each of the specific medical conditions resulted in unbalanced groups. Third, because parents of children from the general pediatric population seeking well-child care were not asked about the overall health of their children, it is possible that some may have had chronic medical conditions that would have impacted beliefs about vaccines. Finally, parents of children with ASD and non-ASD-DD completed the IPQ-R-DD and PACV before other parent groups. There may have been intervening events that impacted vaccine hesitancy among parents of children with rheumatologic conditions and those seeking well-child care, potentially resulting in hesitancy estimates that were lower in the ASD and non-ASD developmental disorder groups. However, as there were no nationwide or local outbreaks of vaccine-preventable disease that occurred in the interim, this is unlikely to have substantively impacted our results.
Implications for Practice
Our findings emphasize the need to address vaccine concerns among parents of children with ASD to reduce vaccine hesitancy in this population and prevent further subsequent vaccine hesitancy through the ongoing spread of misinformation. Several points are outlined below to contextualize our findings and highlight implications for practice and further research.
Evidence suggests that addressing parental vaccine hesitancy with scientific information alone is rarely successful. Although there is a critical need to dispel vaccine myths and misinformation, little is known about the content and format of vaccine-information delivery that resonates most with parents who have concerns about vaccine safety. A strategy that may help families to feel more comfortable about and accepting of vaccines is engaging in conversations about vaccine concerns with trusted healthcare providers.58 To this end, providers should attempt to address vaccine concerns openly. One recommended method for discussing vaccine concerns is the C.A.S.E (Corroborate, About me, Science, Explain/Advise) method, an effective framework for counseling vaccine-hesitant parents.59,60
Nearly one in five parents in our sample was vaccine hesitant, with the highest proportions seen among parents of children with ASD and those in the general pediatric population. General pediatricians, developmental pediatricians, psychologists, and other providers should be mindful of the increased risk for vaccine hesitancy when questions of ASD arise and use these opportunities to initiate conversations about vaccine safety (e.g., during ASD-screening at well-child visits, during ASD-diagnostic feedbacks). Developmental pediatricians in particular may be influential in mitigating vaccine concerns in this population, given their role in caring for patients with ASD and supporting parents of children with ASD. This is particularly important to address with families affected by ASD who have multiple children, as the younger siblings of children with ASD are more likely to be unvaccinated or undervaccinated compared to their peers.27–31 Further research in this area should explore how information about an erroneous ASD-vaccine connection spreads both within the ASD community and between the ASD community and the general pediatric population.
Parents’ beliefs about what does or does not cause children’s conditions are associated with being vaccine hesitant (or not). Our findings revealed that vaccine hesitancy was particularly associated with the belief that toxins in vaccines may cause ASD, as well as diet/eating habits—both of which are considered modifiable factors that parents may acknowledge as being within their control (versus nonmodifiable factors, such as genetics or “bad luck”). This underscores the importance of clearly communicating known causes of medical and developmental conditions and directing families to trusted sources of information where they can learn more.
Tools are needed to support healthcare providers better (a) identify vaccine-hesitant parents or those at risk for becoming vaccine hesitant and (b) manage conversations with these families. Addressing provider time constraints is also critical, given the amount of time required for effective vaccine counseling.58 Research in these areas is key to developing evidence-based approaches that address misperceptions about vaccines and encourage vaccine acceptance, particularly within the ASD community.
Acknowledgments
Financial support: This work was supported in part by the William Stamps Farish Foundation (no number) and through in-kind funding from Baylor College of Medicine, Texas Children’s Hospital, and University of Houston. Dr. Goin-Kochel is also partially supported through the Intellectual and Developmental Disabilities Research Center [1U54 HD083092] at Baylor College of Medicine.
Footnotes
Potential conflicts of interest: Robin P. Goin-Kochel is contracted with Yamo Pharmaceuticals, LLC, to consult on clinical-trial research design.
All children presenting for clinical care at the Texas Children’s Hospital Autism Center are invited upon check-in to enroll in the Center’s research database, regardless of diagnosis.
Among those invited from the Autism Center’s research database to participate in the current study, final diagnosis (ASD or non-ASD-DD) was determined via review of the electronic medical record after survey submission; therefore, frequencies of ASD versus non-ASD-DD diagnoses were not examined for nonparticipants in the Center’s database.
References
- 1.Centers for Disease Control and Prevention. (1999). Ten great public health achievements—United States, 1900–1999. Morbidity and Mortality Weekly Report, 48(12), 241–243. [PubMed] [Google Scholar]
- 2.Roush SW, Murphy TV, and the Vaccine-Preventable Disease Table Working Group. (2007). Historical comparisons of morbidity and mortality for vaccine=preventable diseases in the United States. Journal of the American Medical Association, 298(18), 2155–2163. [DOI] [PubMed] [Google Scholar]
- 3.Hough-Telford C, Kimberlin DW, Aban I, Hitchcock WP, Almquist J, Kratz R, et al. (2016). Vaccine delays, refusals, and patient dismissals: A survey of pediatricians. Pediatrics, 138(3), e20162127. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui M, Salmon DA, & Omer SB (2013). Epidemiology of vaccine hesitancy in the United States. Human Vaccines & Immunotherapeutics, 9(12), 2643–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dube E, Vivion M, & MacDonald NE (2015). Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: influence, impact and implications. Expert Review of Vaccines, 14(1), 99–117. [DOI] [PubMed] [Google Scholar]
- 6.Gust D, Brown C, Sheedy K, Hibbs B, Weaver D, & Nowak G (2005). Immunization attitudes and beliefs among parents: Beyond a dichotomous perspective. American Journal of Health Behavior. 29(1), 81–92. [DOI] [PubMed] [Google Scholar]
- 7.Streefland P, Chowdhury AMR, & Ramos-Jimenze P Patterns of vaccination acceptance. (1999). Social Science & Medicine, 49(12), 1705–1716. [DOI] [PubMed] [Google Scholar]
- 8.Omer SB, Salmon DA, Orenstein WA, deHart MP, & Halsey N (2009). Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. New England Journal of Medicine, 360(19), 1981–1988. [DOI] [PubMed] [Google Scholar]
- 9.Phadke VK, Bednarczyk RA, Salmon DA, & Omer SB (2016). Association between vaccine refusal and vaccine-preventable diseases in the United States: A review of measles and pertussis. Journal of the American Medical Association, 315(11), 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor LE, Swerdfeger AL, & Eslick GD (2014). Vaccines are not associated with autism: An evidence-based meta-analysis of case-control and cohort studies. Vaccine, 32(29), 3623–3629. [DOI] [PubMed] [Google Scholar]
- 11.Madsen KM, Hviid A, Vestergaard M, Schendel D, Wolfahrt J, Thorsen P, et al. (2002). A population-based study of measles, mumps, and rubella vaccination and autism. New England Journal of Medicine, 347(19), 1477–1482. [DOI] [PubMed] [Google Scholar]
- 12.Uchiyama T, Kurosawa M, & Inaba Y (2007). MMR-vaccine and regression in autism spectrum disorders: negative results presented from Japan. Journal of Autism & Developmental Disorders, 37(2), 210–217. [DOI] [PubMed] [Google Scholar]
- 13.Andrews N, Miller E, Grant A, Stowe J, Osborne V, & Taylor B (2004). Thimerosal exposure in infants and developmental disorders: A retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics, 114(3), 584–591. [DOI] [PubMed] [Google Scholar]
- 14.Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, et al. (2003). Safety of thimerosal-containing vaccines: A two-phased study of computerized health maintenance organization databases. Pediatrics, 112(5), 1039–1048. [PubMed] [Google Scholar]
- 15.Hviid A, Stellfeld M, Wohlfahrt J, & Melbye M (2003). Association between thimerosal-containing vaccine and autism. Journal of the American Medical Association, 290(13), 1763–1766. [DOI] [PubMed] [Google Scholar]
- 16.DeStefano F, Bhasin TK, Thompson WW, Yeargin-Allsop M, & Boyle C (2004). Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: A population-based study in metropolitan Atlanta. Pediatrics, 113(2), 259–266. [DOI] [PubMed] [Google Scholar]
- 17.Mrozek-Budzyn D, Kieltya A, & Majewska R (2010). Lack of association between measles-mumps-rubella vaccination and autism in children: A case-control study. The Pediatric Infectious Disease Journal, 29(5), 397–400. [DOI] [PubMed] [Google Scholar]
- 18.Smeeth L, Cook C, Fombonne E, Heavey L, Rodrigues LC, Smith PG, et al. (2004). MMR vaccination and pervasive developmental disorders: A case-control study. Lancet, 364(9438), 963–969. [DOI] [PubMed] [Google Scholar]
- 19.Uno Y, Uchiyama T, Kurosawa M, Aleksic B, & Ozaki N (2012). The combined measles, mumps, and rubella vaccines and the total number of vaccines are not associated with development of autism spectrum disorder: The first case-control study in Asia. Vaccine, 30(28), 492–498. [DOI] [PubMed] [Google Scholar]
- 20.Price CS, Thompson WW, Goodson B, Weintraub ES, Croen LA, Hinrichsen VL, et al. (2010). Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics, 126(4), 656–664. [DOI] [PubMed] [Google Scholar]
- 21.Fischbach RL, Harris MJ, Ballan MS, Fischbach GD, & Link BG (2016). Is there concordance in attitudes and beliefs between parents and scientists about autism spectrum disorder? Autism, 20(3), 353–363. [DOI] [PubMed] [Google Scholar]
- 22.Selkirk CG, McCarthy Veach P, Lian F, Schimmenti L, & LeRoy BS (2009). Parents’ perceptions of autism spectrum disorder etiology and recurrence risk and effects of their perceptions on family planning: Recommendations for genetic counselors. Journal of Genetic Counseling, 18(5), 507–519. [DOI] [PubMed] [Google Scholar]
- 23.Harrington JW, Patrick PA, Edwards KS, & Brand DA (2006). Parental beliefs about autism: Implications for the treating physician. Autism, 10(5), 452–462. [DOI] [PubMed] [Google Scholar]
- 24.Chaidez V, Fernadez y Garcia E, Wang LW, Angkustsiri K, Krakowiak P, Hertz-Picciota I, et al. (2018). Comparison of maternal beliefs about causes of autism spectrum disorder and association with utilization of services and treatments. Child: Care, Health, & Development, 44(6), 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercer L, Creighton S, Holden JAA, & Lewis MES (2006). Parental perspectives on the causes of an autism spectrum disorder in their children. Journal of Genetic Counseling, 15(1), 41–50. [DOI] [PubMed] [Google Scholar]
- 26.Goin-Kochel RP, Mire SS, & Dempsey AG (2015). Emergence of autism spectrum disorder in children from Simplex families: Relations to parental perceptions of etiology. Journal of Autism & Developmental Disorders, 45(5), 1451–1463. [DOI] [PubMed] [Google Scholar]
- 27.Abu Kuwaik G, Roberts W, Zwaigenbaum L, Bryson S, Smith IM, Szatmari P, et al. (2014). Immunization uptake in younger siblings of children with autism spectrum disorder. Autism, 18(2), 148–155. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg RE, Law JK, Anderson C, Samango-Sprouse C, & Law PA (2012). Survey of vaccine beliefs and practices among families affected by autism spectrum disorders. Clinical Pediatrics, 52(9), 871–874. [DOI] [PubMed] [Google Scholar]
- 29.Kuwaik G, Roberts W, Brian J, Bryson S, Smith IM, & Szatmari P (2008). Immunization uptake in siblings of children with autism. Pediatrics, 122(3), 684–685. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Marshall J, Buikema A, Bancroft T, Kelly JP, & Newschaffer CJ (2015). Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. Journal of the American Medical Association, 313(15), 1534–1540. [DOI] [PubMed] [Google Scholar]
- 31.Zerbo O, Modaressi S, Goddard K, Lewis E, Fireman BH, Daley MF, et al. (2018). Vaccination patterns in children after autism spectrum disorder diagnosis and in their younger siblings. JAMA Pediatrics, 172(5), 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Offit PA & Hackett CJ (2003). Addressing parents’ concerns: Do vaccines cause allergic or autoimmune disease? Pediatrics, 111(3), 653–659. [DOI] [PubMed] [Google Scholar]
- 33.Mailand MT & Frederiksen JL (2017). Vaccines and multiple sclerosis: A systematic review. Journal of Neurology, 264(6), 1035–1050. [DOI] [PubMed] [Google Scholar]
- 34.Morgan E, Halliday SR, Campbell GR, Cardwell CR, & Patterson CC (2016). Vaccination and childhood type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia, 59(2), 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opel DJ, Mangione-Smith R, Taylor JA, Korfiatis C, Wiese C, Catz S, & Martin DP (2011). Development of a survey to identify vaccine-hesitant parents: The parents attitudes about childhood vaccines survey. Human Vaccines, 7(4), 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opel DJ, Taylor JA, Mangione-Smith R, Solomon C, Zhao C, Catz S, et al. (2011). Validity and reliability of a survey to identify vaccine-hesitant parents. Vaccine, 29(38), 6598–6605. [DOI] [PubMed] [Google Scholar]
- 37.Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, & Buick D (2010). The revised illness perception questionnaire (IPQ-R). Psychology & Health, 17(1), 1–16. [Google Scholar]
- 38.Mire SS, Tolar TD, Brewton CM, Raff NS, & McKee SL (2018). Validating the Revised Illness Perception Questionnaire as a measure of parent perceptions of autism spectrum disorder. Journal of Autism & Developmental Disorders, 48(5), 1761–1779. [DOI] [PubMed] [Google Scholar]
- 39.Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, & Mangione-Smith R (2013). The relationship between parent attitudes about childhood vaccines survey scores and future immunization status: A validation study. JAMA Pediatrics, 167(11), 1065–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams SE, Morgan A, Opel D, Edwards K, Weinberg S, & Rothman R (2016). Screening tool predicts future underimmunization among a pediatric practice in Tennessee. Clinical Pediatrics, 55(6), 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leventhal H, Nerenz DR & Steele DJ (1984). Illness Representations and Coping with Health Threats. In: Baum A, Taylor SE and Singer JE Eds., Handbook of Psychology and Health, Volume IV: Social Psychological Aspects of Health, Erlbaum, Hillsdale, NJ, 219–252. [Google Scholar]
- 42.Leventhal H, Leventhal EA & Cameron L (2001). Representations, procedures, and affect in illness self- regulation: A perceptual-cognitive model. In: Baum A, Revenson TA and Singer JE, Eds., Handbook of Health Psychology, Lawrence Erlbaum, Mahwah, 19–48. [Google Scholar]
- 43.Leventhal H, Leventhal EA, & Contrada RJ (1998). Self-regulation, health, and behavior: A perceptual-cognitive approach. Psychology and Health, 13(4), 717–733. [Google Scholar]
- 44.Taylor SE, Lichtman RR, & Wood JV (1984). Attributions, beliefs about control, and adjustment to breast cancer. Journal of Personality and Social Psychology, 46, 489. [DOI] [PubMed] [Google Scholar]
- 45.Wong PT, & Weiner B (1981). When people ask “why” questions, and the heuristics of attributional search. Journal of Personality and Social Psychology, 40, 650. [Google Scholar]
- 46.Al Anbar NN, Dardennes RM, Prado-Netto A, Kaye K, & Contejean Y (2010). Treatment choices in autism spectrum disorder: The role of parental illness perceptions. Research in Developmental Disabilities, 31, 817–828. [DOI] [PubMed] [Google Scholar]
- 47.Dardennes RM, Al Anbar NN, Prado-Netto A, Kaye K, Contejean Y, & Al Anbar NN (2011). Treating the cause of illness rather than the symptoms: Parental causal beliefs and treatment choices in autism spectrum disorder. Research in Developmental Disabilities, 32(3), 1137–1146. [DOI] [PubMed] [Google Scholar]
- 48.Dempster M, & McCorry NK (2012). The factor structure of the Revised Illness Perception Questionnaire in a population of oesophageal cancer survivors. Psycho‐Oncology, 21(5), 524–530. [DOI] [PubMed] [Google Scholar]
- 49.Yudell M, Tabor HK, Dawson G, Rossi J, & Newschaffer C (2013). Priorities for autism spectrum disorder risk communication and ethics. Autism, 17, 701–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewton C, Mire SS, Tolar T, Goin-Kochel RP, Keller-Margulis MA, Schoger K, & McNeel MM (Under review). Measuring parental perceptions about causes of autism spectrum disorder: An investigation using principal components analysis.
- 51.Cunningham RM, Guffey D, Minard CM, Opel DJ, & Boom JA (2019). Prevalence of vaccine hesitancy among parents of newborns in Houston, Texas. Poster Presentation. Pediatric Academic Societies Meeting, April 24-May 1, Baltimore, MD. [Google Scholar]
- 52.Cunningham RM, Minard CG, Guffey D, Swaim LS, Opel DJ, & Boom JA (2018). Prevalence of vaccine hesitancy among expectant mothers in Houston, Texas. Academic Pediatrics, 18(2), 154–160. [DOI] [PubMed] [Google Scholar]
- 53.Henrikson NB, Anderson ML, Opel DJ, Dunn J, Marcuse EK, & Grossman DC (2017). Longitudinal trends in vaccine hesitancy in a cohort of mothers surveyed in Washington State, 2013–2015. Public Health Reports, 132(4), 451–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozbroj T, Lyons A, & Lucke J (2019). Vaccine-hesitant and vaccine-refusing parents’ reflections on the way parenthood changed their attitudes to vaccination. Journal of Community Health. Doi: 10.1007/s/10900-019-00723-9 [DOI] [PubMed] [Google Scholar]
- 55.Lipstein EA, Brinkman WB, & Britto MT (2012). What is known about parents’ treatment decisions? A narrative review of pediatric decision making. Medical Decision Making, 2, 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunson EK (2013). The impact of social networks on parents’ vaccination decisions. Pediatrics, 131(5), e1397–e1404. [DOI] [PubMed] [Google Scholar]
- 57.Brunson EK (2013). How parents make decisions about their children’s vaccinations. Vaccine, 31(46), 5466–5470. [DOI] [PubMed] [Google Scholar]
- 58.Edwards KM, Hackell JM, The Committee on Infectious Diseases, and the Committee on Practice and Ambulatory Medicine. (2016). Countering vaccine hesitancy. Pediatrics, 138(3), e20162146. [DOI] [PubMed] [Google Scholar]
- 59.Boom JA & Cunningham RM (2014). Understanding and Managing Vaccine Concerns. Springer International Publishing, Switzerland. [Google Scholar]
- 60.Jacobson RM, Van Etta L, & Bahta L (2013). The C.A.S.E. approach: Guidance for talking to vaccine-hesitant parents. Minnesota Medicine, 96(4), 49–50 [PubMed] [Google Scholar]