Abstract
Background
Hereditary antithrombin (AT) deficiency is an uncommon autosomal dominant thrombogenic disorder, which can cause venous thromboembolism (VTE). Although conservative treatment options for hereditary AT deficiency-associated VTE such as anticoagulation (warfarin, direct oral anticoagulant, or heparin), intravenous thrombolysis, and recombinant AT are well known, interventional treatment options have not been reported so far.
Case summary
A 19-year-old man with a family history of thrombogenic diseases, referred to our hospital with left leg pain, was diagnosed with AT deficiency-associated VTE. In the absence of symptomatic relief with intravenous thrombolysis and anticoagulation, he received venous intervention and catheter directed thrombolysis (CDT) for 4 days for left iliac venous thrombosis. Following a second venous intervention, venous thrombus disappeared almost entirely on cross-sectional imaging, and his symptoms improved. He was discharged on apixaban and has been recurrence-free for one and a half years.
Discussion
This case presents CDT and maintenance therapy with apixaban as possible treatment options for VTE in patients with hereditary AT deficiency, especially following failure of conservative therapy. Individual risks and benefits should be considered when CDT is performed for acute VTE in patients with AT deficiency.
Keywords: Case report, Hereditary antithrombin deficiency, Venous thromboembolism, Deep vein thrombosis, Catheter directed thrombolysis
Learning points
When a young patient developed venous thromboembolism (VTE), antithrombin deficiency should be considered as differential diagnosis.
Catheter directed thrombolysis and venous intervention should be considered for acute VTE irrespective of the underlying diseases when conservative treatments are not effective.
Introduction
Hereditary antithrombin (AT) deficiency is an uncommon autosomal dominant thrombogenic disorder, with an estimated prevalence in the general population of 0.02–0.2%.1 Individuals with hereditary AT deficiency are at an increased risk of venous thromboembolism (VTE) (odds ratio 16.3; 95% confidence interval 9.9–26.7).2 Owing to its low prevalence, developing a treatment strategy for VTE in patients with AT deficiency is challenging. Herein, we report a successful venous interventional treatment for a patient with AT deficiency and acute VTE.
Timeline
| Timeline | Description |
|---|---|
| Five days prior to admission | The patient, who had a family history of thrombogenic disease, developed acute left leg pain. |
| Day 0 | Vital signs were stable, but he complained of swelling, continuous pain, and tenderness of his left thigh to left groin. Pulmonary embolism and thrombotic occlusion in left iliac to femoral vein were confirmed by contrast enhanced computerized tomography (CT) and duplex ultrasound. |
| Day 1 | Inferior vena cava (IVC) filter deployment and intravenous monteplase administration was performed, and apixaban 10 mg × 2 per day was prescribed. |
| Day 3 | Symptoms hadn’t changed, and follow-up CT showed large amount of thrombus in left iliac and left femoral vein. |
| Day 4 | First venous intervention was performed and FOUNTAIN INFUSION catheter™ (Merit Medical, USA) was deployed from left femoral to iliac vein. |
| Days 5–7 | Catheter directed thrombolysis (CDT) was performed for 3 days. The CDT infusion contents were continuous heparin and argatroban, with intermittent urokinase four times a day. |
| Day 8 | Second venous intervention, which included thrombo-aspiration in left femoral and iliac vein and balloon dilatation, was performed. |
| Day 11 | Follow-up CT showed that left iliac vein thrombus disappeared. |
| Day 12 | IVC filter was removed. |
| Day 17 | He discharged on apixaban 5 mg twice a day. |
| Two months after discharge | Genetic test for hereditary antithrombin deficiency proved to be positive. |
Case presentation
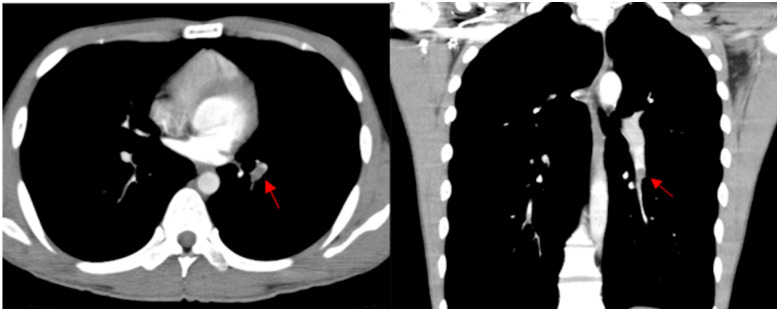
A 19-year-old man without any medical history was referred to our hospital, in August 2018, with acute left leg pain that developed over 5 days. His father and paternal relatives had thrombotic disease; however, genetic diagnosis hadn’t been performed. The patient did not report any other transient or reversible risk factors of venous thrombosis and did not take any medication. Contrast enhanced computed tomography (CT) performed at the referring hospital identified evidence of pulmonary embolus (Figure 1), and presence of venous thrombus extending from the left popliteal vein to the inferior vena cava (IVC).
Figure 1.
Enhanced computed tomography in the course of treatment. Filling defect in left pulmonary artery on axial and coronal imaging (red arrows) on admission day.
His vital signs were as follows: blood pressure, 133/68 mmHg; heart rate, 79/min, regular; oxygen saturation at room air, 99%; and respiratory rate, 22/min. Cardiac and pulmonary auscultation were normal. There was no elevated jugular venous pulse, and no peripheral oedema. The patient had swelling involving the left thigh and groin, which was tender to touch.
Laboratory investigations were as follows: D-dimer level, 23.1 μg/mL (normal < 1.0 μg/mL); AT activity, 49.3% (normal, 75–125%); protein C/S activity and lupus anticoagulant, normal range; and antibodies related to antiphospholipid antibody syndrome, negative. Echocardiogram showed no signs of right heart overload. Duplex ultrasound of his left leg showed thrombotic occlusion from the left femoral vein to left iliac vein.
Based on his family history and AT levels, a diagnosis of hereditary AT deficiency was made, and following consent, anticoagulant therapy was initiated. We prescribed apixaban 10 mg twice a day as loading dose for 7 days along with 275 000 units/kg of monteplase intravenously. Monteplase was administrated only once. Owing to the large size of the venous thrombus, a Günther Tulip IVC filter (Cook Medical, USA) was deployed on the first day of admission (Day 1). His symptoms failed to improve despite 2 days of treatment. Follow-up contrast enhanced CT on Day 3 showed no change in the size of the thrombus (Figure 2). Hence, we decided to perform venous intervention on Day 4 to reduce the risk of development of post-thrombotic syndrome (PTS).
Figure 2.
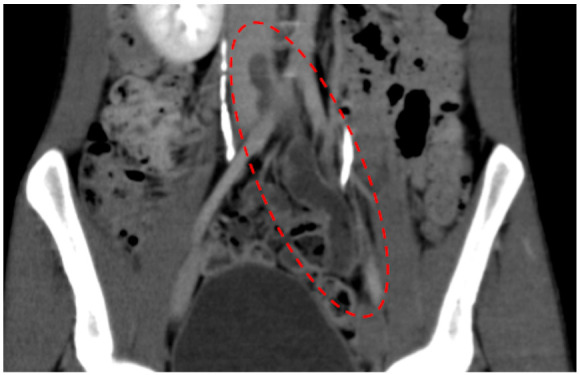
Enhanced computed tomography on Day 3. Thrombus in left iliac vein (in a red dotted line).
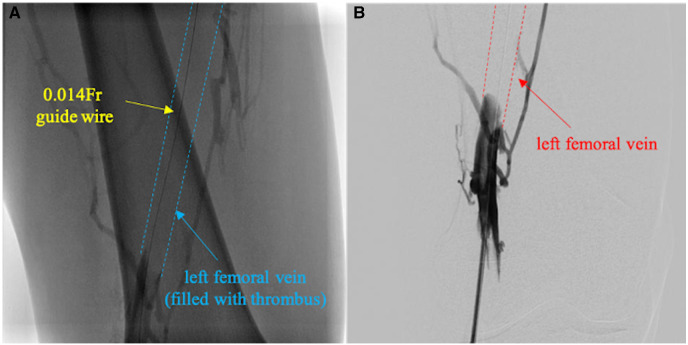
We employed a trans-popliteal vein approach with 6 Fr introducer sheath (Terumo, Japan). Preliminary angiogram identified total occlusion of the left femoral vein with thrombus (Figure 3A). Despite repeated thrombo-aspiration with 6 Fr Mach1 (Boston Scientific, USA), the deep femoral vein remained occluded with thrombus (Figure 3B). Subsequently, we deployed a Fountain infusion catheter (Merit Medical, USA) through his femoral vein into the iliac vein and injected 120 000 units of urokinase. This was followed by continuous injections of heparin (14 000 units per day) and argatroban (20 mg per day), and intermittent injection of urokinase (60 000 units four times a day) as catheter directed thrombolysis (CDT). On Day 8, after removing the Fountain infusion catheter, we performed thrombo-aspiration again and dilated the vein with 7.0/200 mm and 8.0/80 mm semi-compliant balloon, which resulted in improved venous flow (Video 1). We performed an intravascular ultrasound as the venous angiogram showed proximal iliac vein stenosis, which revealed that iliac vein compression between the lumbar spine and iliac artery (Figure 4).
Figure 3.
First venous intervention. (A) Initial venous angiogram via left trans-femoral approach in prone position showed total occlusion of left femoral vein (between blue dotted lines). (B) Final angiogram after several thrombo-aspirations showed persistent thrombotic occlusion in left femoral vein.
Figure 4.
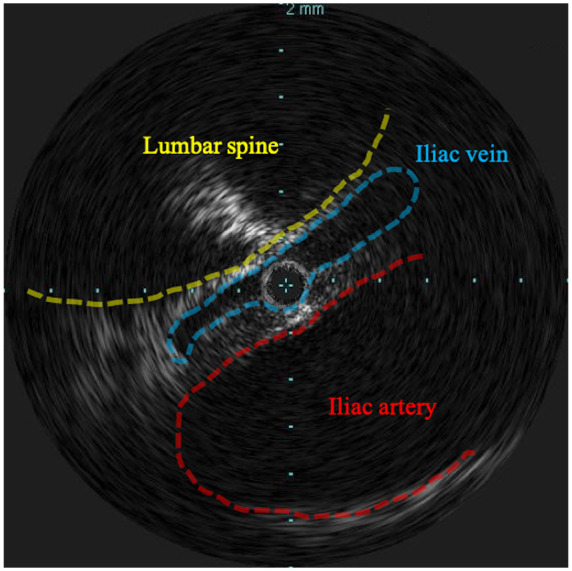
Intravascular ultrasound during the second venous intervention. Iliac vein (blue dotted line) was compressed between lumbar spine (yellow dotted line) and iliac artery (red dotted line).
Follow-up CT on Day 11 confirmed clearance of almost all venous thrombus (Figure 5). We removed the IVC filter on Day 12. The patient was discharged on Day 17 with apixaban 5 mg twice a day, which was considered lifelong prescription. Two months after his hospitalization, genetic testing confirmed hereditary AT deficiency (Exon 6, c.1171C>T, p.Arg359Ter). The patient has completed 18 months of follow-up without any recurrence of VTE or other treatment related complications.
Figure 5.
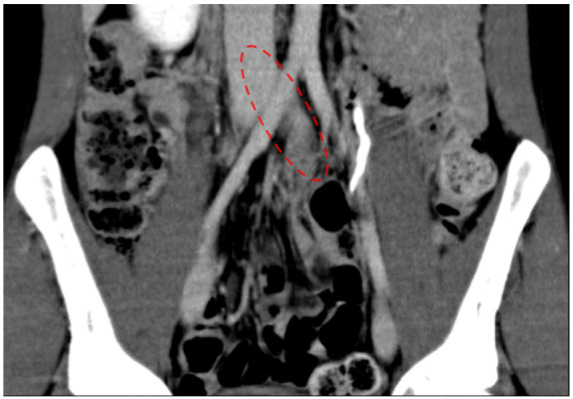
Follow-up computed tomography on Day 11. Thrombus in left iliac vein had disappeared (red dotted line).
Discussion
This case demonstrates successful CDT for ilio-femoral venous thrombosis in a patient with AT deficiency.
Management of acute VTE in patients with AT deficiency is not different from that of VTE in general.3 Thrombolytics, heparin or fondaparinux, and vitamin K antagonists are the conventional treatment modalities, although there is a theoretical risk of heparin resistance in patients with AT deficiency.4 Currently, direct oral anticoagulants (DOACs) are also prescribed for the treatment of VTE.5 A current consensus document for deep vein thrombosis (DVT) from the European Society of Cardiology (ESC) recommends anticoagulation for all patients with proximal DVT, and adjuvant CDT for selected patients.6 However, it does not provide any details regarding the management of patients with hereditary AT deficiency. Recent case reports described the successful use of rivaroxaban in the treatment of VTE in a patient with hereditary AT deficiency.7,8 DOACs are effective for VTE treatment regardless of anti-thrombin deficiency status, as they directly affect factor Xa. One randomized trial demonstrated decreased incidence of subsequent PTS but increased bleeding episodes with the use of CDT in addition to anticoagulation for patients with proximal DVT.9 In contrast, another randomized trial did not demonstrate any substantial benefit of pharmacochemical therapy, which was defined as catheter or device-mediated intrathrombus delivery of recombinant tissue plasminogen activator and thrombus aspiration or maceration, and CDT over anticoagulation.10 However, neither of these studies included patients with AT deficiency. Therefore, the strategy for acute VTE and DVT in patients with AT deficiency, especially with the advent of novel treatment options, can only be empirical owing to the small number of cases. In the current case, we performed CDT following the failure of anticoagulation therapy. Furthermore, the patient also satisfied the ESC criteria for CDT,6 and was at low risk of bleeding owing to his age and physical status.
According to the current guidelines, the indications for IVC filter placement are: anticoagulation is absolutely contraindicated in patients with proximal DVT, recurrent pulmonary embolism despite adequate anticoagulation, and as primary prophylaxis in patients with a high risk of VTE.6,11 However, the effect of IVC filter placement on CDT has never been discussed. We considered that CDT has a risk of destabilization of thrombus, which may develop pulmonary embolus. Our use of IVC filter was not based on existing guidelines. Although our patient did not develop any complications related to IVC filters, their use should be carefully considered. There is a need for further studies focusing on IVC filter placement and CDT.
The duration of anticoagulation is a potential area of difficulty for this patient. Since hereditary AT deficiency is neither transient nor reversible, the patient has a high estimated risk for long-term recurrence of VTE.11 This patient also had iliac venous compression syndrome (IVCS), which may have been an additional contributor to VTE. Left iliac vein stenting may appear to be optimal treatment for IVCS.12 However, we were reluctant to deploy a stent owing to his age and underlying thrombogenic condition. Our opinion was that this patient required lifelong anticoagulant therapy owing to the irreversible risk factor of AT deficiency, even in the absence of IVCS.
In conclusion, this case presents CDT as an effective option in treating acute DVT in patients with AT deficiency. As for any DVT, CDT should be considered in the event of failure of anticoagulant treatment alone. This strategy is important for prevent PTS. There is a need for further studies to standardize the treatment of DVT in patients with AT deficiency.
Lead author biography

Hirokazu Miyashita graduated from Kanazawa University in 2014. He was trained as a clinical resident in Shonan Kamakura General Hospital for 2 years, and joined department of cardiology at the same hospital in 2016. He has learned interventional cardiology, peripheral artery diseases, and structure heart diseases. Since 2020, he is studying abroad as a research fellow in Helsinki University Hospital.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: S.S. reports lecture fees from TERUMO Corporation, Japan. All other authors declare that there is no conflict of interest.
Funding: none declared.
Supplementary Material
References
- 1.Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, McCall F. et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol 1994;87:106–112. [DOI] [PubMed] [Google Scholar]
- 2.Di Minno MN, Ambrosino P, Ageno W, Rosendaal F, Di Minno G, Dentali F.. Natural anticoagulants deficiency and the risk of venous thromboembolism: a meta-analysis of observational studies. Thromb Res 2015;135:923–932. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik MM, Moll S.. Inherited antithrombin deficiency: a review. Haemophilia 2008;14:1229–1239. [DOI] [PubMed] [Google Scholar]
- 4.Schulman S, Tengborn L.. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost 1992;68:634–636. [PubMed] [Google Scholar]
- 5.Becattini C, Agnelli G.. Treatment of venous thromboembolism with new anticoagulant agents. J Am Coll Cardiol 2016;67:1941–1955. [DOI] [PubMed] [Google Scholar]
- 6.Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R. et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J 2018;39:4208–4218. [DOI] [PubMed] [Google Scholar]
- 7.Minami K, Kumagai K, Sugai Y, Nakamura K, Naito S, Oshima S.. Efficacy of oral factor Xa inhibitor for venous thromboembolism in a patient with antithrombin deficiency. Intern Med 2018;57:2025–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi J, Hara N, Yamaguchi T, Nagata Y, Nozato T, Miyamoto T.. Successful treatment of a massive pulmonary embolism using rivaroxaban in a patient with antithrombin III deficiency. J Cardiol Cases 2017;16:144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haig Y, Enden T, Grøtta O, Kløw NE, Slagsvold CE, Ghanima W. et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol 2016;3:e64–e71. [DOI] [PubMed] [Google Scholar]
- 10.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ. et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med 2017;377:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP. et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 12.Radaideh Q, Patel NM, Shammas NW.. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag 2019;15:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.