Abstract
Aims
Cardiac microRNA-132-3p (miR-132) levels are increased in patients with heart failure (HF) and mechanistically drive cardiac remodelling processes. CDR132L, a specific antisense oligonucleotide, is a first-in-class miR-132 inhibitor that attenuates and even reverses HF in preclinical models. The aim of the current clinical Phase 1b study was to assess safety, pharmacokinetics, target engagement, and exploratory pharmacodynamic effects of CDR132L in patients on standard-of-care therapy for chronic ischaemic HF in a randomized, placebo-controlled, double-blind, dose-escalation study (NCT04045405).
Methods and results
Patients had left ventricular ejection fraction between ≥30% and <50% or amino terminal fragment of pro-brain natriuretic peptide (NT-proBNP) >125 ng/L at screening. Twenty-eight patients were randomized to receive CDR132L (0.32, 1, 3, and 10 mg/kg body weight) or placebo (0.9% saline) in two intravenous infusions, 4 weeks apart in four cohorts of seven (five verum and two placebo) patients each. CDR132L was safe and well tolerated, without apparent dose-limiting toxicity. A pharmacokinetic/pharmacodynamic dose modelling approach suggested an effective dose level at ≥1 mg/kg CDR132L. CDR132L treatment resulted in a dose-dependent, sustained miR-132 reduction in plasma. Patients given CDR132L ≥1 mg/kg displayed a median 23.3% NT-proBNP reduction, vs. a 0.9% median increase in the control group. CDR132L treatment induced significant QRS narrowing and encouraging positive trends for relevant cardiac fibrosis biomarkers.
Conclusion
This study is the first clinical trial of an antisense drug in HF patients. CDR132L was safe and well tolerated, confirmed linear plasma pharmacokinetics with no signs of accumulation, and suggests cardiac functional improvements. Although this study is limited by the small patient numbers, the indicative efficacy of this drug is very encouraging justifying additional clinical studies to confirm the beneficial CDR132L pharmacodynamic effects for the treatment of HF.
Keywords: Heart failure, Clinical trial Phase 1b study, Cardiac remodelling, microRNAs
Graphical Abstract
Listen to the audio abstract of this contribution.
See page 189 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa967)
Introduction
Heart failure (HF) is a chronic progressive condition, to date largely irreversible, that continues to impose important global morbidity and cost burdens.1 Despite some progress in the therapy of HF reported in recent outcome trials, current management of HF remains largely symptomatic, focused principally on reducing cardiac load by shielding the heart against secondary neuro-hormonal overdrive and volume retention.2 A major unmet need exists for treatments addressing HF pathophysiology at the organ level that target fundamental pathophysiological processes.
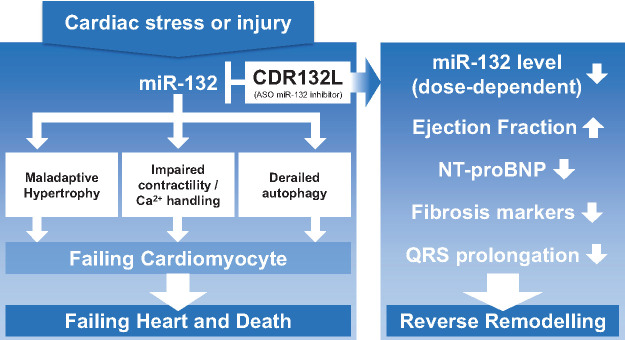
The microRNA-132-3p (miR-132) is a regulatory (noncoding) RNA that, in response to cardiomyocyte stress, is upregulated in cardiac tissue.3,4 Preclinical studies3,5–8 have shown miR-132 to affect signalling pathways involved in cardiomyocyte growth, autophagy, calcium handling, and contractility. In particular, it downregulates expression of the anti-hypertrophic, pro-autophagic transcription factor Forkhead box O3 (FOXO3) and also suppresses the expression of genes involved in intracellular calcium handling and contractility, e.g. Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2A (SERCA2A) leading to cardiac remodelling. Over-activation of miR-132 in cardiac tissue drives progressive adverse cardiac remodelling leading to HF. Thus, miR-132 appears as a potentially promising molecular pathophysiological target in HF treatment.9,10
CDR132L, a synthetic locked nucleic acid antisense oligonucleotide (ASO) inhibitor with a fully phosphorylated backbone, is a first-in-class miR-132 inhibitor.7 Preclinical investigations,5–7 including large randomized, placebo-controlled studies in validated porcine models of subacute5 and chronic HF6 after myocardial infarction, found a favourable safety profile, efficient drug delivery to cardiac tissue, and potent, dose-dependent target reduction in myocardium and plasma. The results suggested a strong effect of CDR132L against adverse cardiac remodelling, a dose-dependent improvement in left ventricular ejection fraction (LVEF), and a reduction in the amino terminal fragment of pro-brain natriuretic peptide (NT-proBNP) concentrations. In addition, positive effects on relevant echocardiographic, electrophysiological, and biochemical parameters of disease severity showed improved cardiac systolic and diastolic function.3,5,6
These promising preclinical observations and the elaborated toxicological profile of ASOs led us to conduct this first-in-human study straight in patients with stable chronic HF of ischaemic origin. Main objectives were to evaluate safety, pharmacokinetics of ascending single and repeated intravenous doses of CDR132L and exploratorily analysing target engagement as well as HF relevant pharmacodynamic parameters. Here, we report the results of the first clinical trial of an ASO therapy targeting a specific microRNA as a major culprit for HF pathophysiology at the cellular level.
Methods
Study design, patients, and ethics
This FiH Phase 1b, prospective, randomized, double-blind, placebo-controlled, dose-ranging study (NCT04045405) of intravenous CDR132L was performed from June 2019 to March 2020 at Richmond Pharmacology Ltd research unit, London, UK. We recruited patients 30–80 years old with New York Heart Association class 1–3 chronic HF with LVEF between ≥30% and <50% or NT-pro BNP >125 ng/L at screening, and the body mass index of 18–28 kg/m2. Patients had to remain on guideline-directed standard-of-care therapy for HF,9,10 and on stable medication for comorbidities. Main exclusion criteria comprised previously decompensated HF or HF of non-ischaemic origin, i.e. hypertensive heart disease, myocarditis, alcoholic cardiomyopathy, or cardiac dysfunction due to atrial fibrillation with rapid ventricular rate. A full list of in-/exclusion criteria is found in the Supplementary material online. The study was approved by the concerned ethics committee and regulatory agency [Medicines and Healthcare products Regulatory Agency (MHRA)]. No patient underwent any study-related activity before giving written informed consent to study participation. This trial followed the precepts of Good Clinical Practice11 and the Declaration of Helsinki.
Study treatment, randomization, and blinding
Patients were randomized 5:2 to CDR132L or placebo (0.9% saline). Under a guideline-recommended sentinel dosing strategy,12 consecutive seven-patient cohorts received one of the four pre-specified ascending dosages, 0.32, 1, 3, or 10 mg/kg body weight, contingent on safety review. Study treatment was given twice, via one 15 min intravenous (i.v.) infusion each on Days 1 and 28. For an abundant safety margin, the initial CDR132L dosage, 0.32 mg/kg, was selected to be approximately one-tenth of the ‘no observed adverse events level (NOAEL)’ (20 mg/kg) in animal toxicology studies of repeated CDR132L dosing, considering allometric scaling of factor 6.2 according to the United States Food and Drug Administration (FDA) guidance.13 Consecutive dosages ascended by factors of 3, based on regulators’ recommendations. Participants, investigators, laboratory staff, employees of the sponsor, and additional clinical research organization staff were blinded to treatment assignments. The patient individual study medication was prepared by non-blinded pharmacists according to the random list and neutrally labelled (details appear in the Supplementary material online).
Study outcomes
The primary outcome was safety evaluated in terms of treatment emergent adverse events (TEAE) and change in laboratory values, while the secondary outcome was pharmacokinetic profiles for single and repeated ascending CDR132L doses. Exploratory outcomes included CDR132L target engagement projected by plasma miR-132 levels, and pharmacodynamic effects on LVEF, blood biomarker levels, and electrocardiographic variables. A further important evaluation was the calculation of anticipated therapeutic dose ranges (ATD) to be used in upcoming clinical studies. These ATD calculations for monthly or quarterly HF therapy were based on a novel pharmacokinetic/pharmacodynamic modelling scheme combining human data from this clinical trial with plasma and cardiac tissue measurement from preclinical large animal studies.5,6 Details appear in the Supplementary material online.
Study schedule and procedures
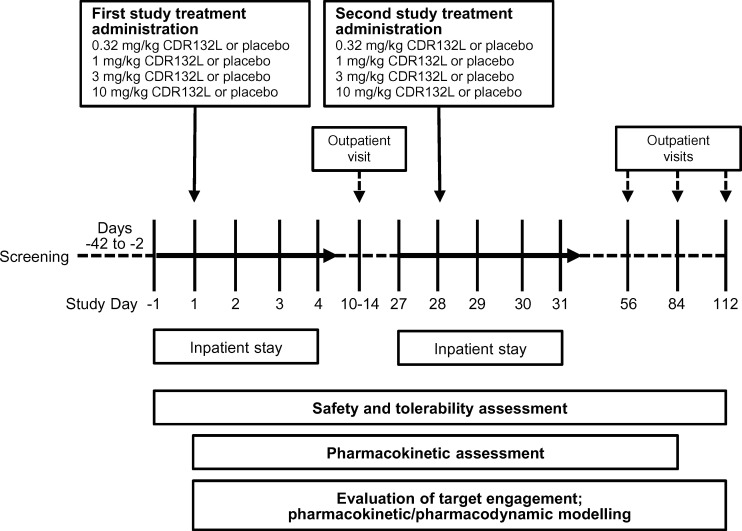
Overall study duration was 4 months for each patient (Figure 1). After a screening period of 6 weeks, patients were hospitalized on Day 1, discharged on Day 4, and re-admitted on Day 27 for another 3 days; they were monitored as outpatients on Days 10–14, 56, 84, and 112 (end of study).
Figure 1.
Study profile.
All patients underwent physical examination, vital signs, standard haematology, biochemistry, and coagulation determinations at pre-specified time points throughout the study. Adverse events were recorded at all study visits and graded using Common Terminology Criteria for Adverse Events version 4.0. In a blinded evaluation, investigators classified relationship of adverse events to study treatment (CDR132L or placebo).
A comprehensive echocardiographic examination, including LVEF measurement, was performed at screening, baseline, and Days 56 and 112 to assess the safety and explore pharmacodynamics of CDR132L. Furthermore, HF relevant blood biomarker were assessed at pre-specified time points: plasma miR-132, circulating concentrations of analytes of HF severity (NT-proBNP), heart injury (high-sensitivity troponin T, TnT), kidney injury (kidney injury molecule-1, KIM-1), HF mortality risk, cardiac remodelling, and cardiac fibrosis (galectin-3, Gal-3; lipocalin-2, NGAL; suppression of tumourgenicity-2, ST-2; matrix metallopeptidase-1, MMP-1) (details in the Supplementary material online).
Holter electrocardiograms (24 h) were recorded at screening, 12-lead real-time electrocardiograms (continuous telemetry), from 1 h pre-dose to 24 h post-dose, and additional 12-lead resting electrocardiograms at time points matching pharmacokinetic/pharmacodynamic sampling. QRS narrowing index was calculated as described in the Supplementary material online. Electrocardiographic data were reviewed by a blinded cardiologist, who provided expert manual adjudication of measured intervals. Blood for pharmacokinetic assessments of CDR132L was drawn shortly before treatment administration at 9 and 60 min and 3, 9, 24, and 48 h post-infusion.
Statistics
No formal sample size calculation was performed for this Phase 1b study. All participants receiving at least one dose of CDR132L or placebo were included in safety analysis, while all patients receiving their planned study treatment were eligible for pharmacokinetic and exploratory analyses. No substitutions were made for missing or technically inevaluable data. Safety data are presented using descriptive statistics. Non-compartmental analysis was used to estimate the following pharmacokinetic variables: maximum concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration curve from time zero to the last quantifiable concentration or to infinity (respectively, AUC0–t and AUC0–inf), terminal elimination half-life (t½), terminal rate constant (λz), volume of distribution (Vd), and blood clearance (CL). Individual plasma concentration data and actual times of CDR132L administration and blood sampling were used to calculate these values. AUC0–t and AUC0–inf were calculated using the linear/log trapezoidal method; other pharmacokinetic variables were calculated using standard methodology. The underlying modelling assumption was that the logarithms of each pharmacokinetic variable and each respective dose were linearly related.14
All analyses of PD parameters were strictly exploratory mainly displaying results in a descriptive manner.
The analysis was performed according to the intention-to-treat principle.
Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA), Phoenix WinNonLin 8.2.0.4383 (Pharsight, St. Louis, MO, USA), and Prism 8 (GraphPad Software, San Diego, CA, USA).
Results
CDR132L treatment is safe and well tolerated
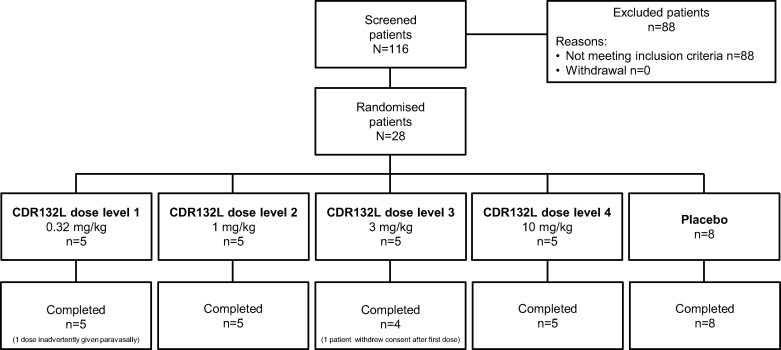
A total of 116 patients with HF were screened. Figure 2 summarizes patient disposition. Twenty-eight met eligibility criteria, of whom 20 were randomized to CDR132L and 8 to placebo. All patients completed the study as planned. One patient inadvertently received his first CDR132L, 0.32 mg/kg, administration paravasally. This patient was included in the study, except the first dosing that was not considered for the PK analysis. Another patient withdrew from study for personal reasons after Day 4 and received only the first administration of CDR132L, 3 mg/kg. This patient remained in the safety collective and with the first dosing in the PK collective, but was excluded for PD analysis since no relevant data could be obtained.
Figure 2.
Study participant flow chart.
Patients’ baseline characteristics by treatment group are displayed in Table 1. No relevant intergroup differences were apparent potentially affecting CDR132L safety or pharmacokinetics. Participants were predominantly elderly (mean age: 65.9 ± 8.8 years), male (27/28 patients), and Caucasian (25/28) and were on average overweight. Reflecting inclusion criteria, LVEF was reduced and NT-proBNP elevated and, as expected, showed heterogeneity among individuals and treatment groups. The mean time since HF diagnosis was 11.9 ± 8.2 years in CDR132L-treated patients and 8.8 ± 6.6 years in the placebo group; cardiac comorbidities, a history of cardiac procedures, hypertension, diabetes, and polypharmacy were frequent but heterogeneous.
Table 1.
Baseline patient characteristics by treatment group
| Cohort 1 (0.32 mg/kg CDR132L)(n = 5) | Cohort 2 (1 mg/kg CDR132L) (n = 5) | Cohort 3 (3 mg/kg CDR132L) (n = 5) | Cohort 4 (10 mg/kg CDR132L)(n = 5) | Placebo(n = 8) | |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age | 73.4 (3.5) | 69.0 (6.0) | 66.8 (5.4) | 61.0 (13.1) | 61.6 (8.6) |
| Sex ratio, men to women | 5:0 | 5:0 | 5:0 | 5:0 | 7:1 |
| Race, n (%) | |||||
| Caucasian | 5 (100) | 4 (80) | 5 (100) | 5 (100) | 6 (75) |
| Black African | 0 | 1 (20) | 0 | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 | 1 (13) |
| Others | 0 | 0 | 0 | 0 | 1 (13) |
| Body mass index (kg/m2) | 25.8 (1.9) | 25.6 (3.4) | 26.8 (2.6) | 26.0 (2.3) | 25.6 (2.9) |
| Relevant medical and surgical history, n (%) | |||||
| Myocardial infarction | 2 (40)a | 2 (40) | 2 (40) | 3 (60)b | 4 (50) |
| Myocardial ischaemia | 2 (40) | 1 (20) | 0 | 4 (80) | 2 (25) |
| Angina unstable | 0 | 0 | 1 (20) | 0 | 1 (13) |
| Atrial fibrillation or flutter | 2 (40) | 0 | 1 (20) | 2 (40) | 0 |
| Hypertension (essential or secondary) | 1 (20) | 3 (60) | 2 (40) | 2 (40) | 4 (50) |
| Type 2 diabetes mellitus | 2 (40) | 2 (40) | 1 (20) | 0 | 1 (13) |
| Coronary arterial stent insertion | 2 (40) | 0 | 0 | 1 (20) | 1 (13) |
| Coronary artery bypass | 2 (40) | 1 (20) | 2 (40) | 1 (20) | 2 (25) |
| Percutaneous coronary intervention including angioplasty | 2 (40) | 3 (60) | 1 (20) | 2 (40) | 7 (88) |
| Stent placement | 2 (40) | 0 | 0 | 0 | 1 (13) |
| LVEF, n (%) | |||||
| 31–39% | 1 (20) | 1 (20) | 1 (20) | 2 (40) | 0 |
| 41–45% | 2 (40) | 1 (20) | 1 (20) | 2 (40) | 3 (38) |
| 47–49% | 0 | 2 (40) | 3 (60) | 1 (20) | 2 (25) |
| 52–56% | 2 (40) | 1 (20) | 0 | 0 | 3 (38) |
| NT-proBNP, median (25th to 75th percentiles) (pg/mL) | 737 (576-926) | 125 (103-165) | 463 (319.5-1493.5) | 170 (122-632) | 125 (111-344) |
| Concomitant medication for HF, n (%) | |||||
| ACE inhibitors, plain | 3 (60) | 2 (40) | 2 (40) | 3 (60) | 4 (50) |
| Angiotensin II receptor blockers, plain or in combination with medication of other classes | 0 | 3 (60) | 1 (20) | 1 (20) | 2 (25) |
| Aldosterone antagonists | 2 (40) | 2 (40) | 1 (20) | 3 (60) | 3 (38) |
| Alpha-blockers and beta-blockers | 0 | 0 | 0 | 1 (20) | 0 |
| Beta blocking agents, selective or non-selective | 5 (100) | 5 (100) | 3 (60) | 4 (80) | 5 (63) |
| Dihydropyridine derivatives | 1 (20) | 1 (20) | 0 | 0 | 2 (25) |
| Platelet aggregation inhibitors (excl. heparin) | 4 (80) | 2 (40) | 4 (80) | 3 (60) | 7 (88) |
Data are mean (SD) or n (%). ACE, angiotensin-converting enzyme; LVEF, left ventricular ejection fraction; NT-proBNP, amino terminal fragment of pro-brain natriuretic peptide; SD, standard deviation.
One case of acute myocardial infarction.
Three cases of acute myocardial infarction.
Treatment with CDR132L, 0.32–10 mg/kg, was safe and well tolerated (Supplementary material online, Tables S1 and S2). No serious TEAE were reported; no patient died during the study. No adverse event occasioned any delay or cancellation of a study infusion or caused study withdrawal. Altogether, 53 adverse events were reported in 22/28 patients (79%). All events were mild in severity, except two rated moderate, neither considered study treatment related. No signs or symptoms of injection site reaction were reported. Notably, no local adverse reaction was seen in the patient inadvertently administered one dose of CDR132L, 0.32 mg/kg, paravasally.
TEAE were less frequent and affected a lesser percentage of patients in the CDR132L group, compared to the placebo group: 33 events in 15/20 patients vs. 20 events in 7/8 patients. Adverse events considered to be at least possibly CDR132L-related were observed in only 2/20 patients receiving CDR132L and comprised single mild episodes of dizziness (during infusion) and euphoric mood during the first dosing period in 1 patient given 3 mg/kg and of dizziness and diarrhoea during the second dosing period in 1 patient given 10 mg/kg (Supplementary material online, Table S2). Dizziness or euphoric mood during the first dosing period was observed in 1 patient each given placebo and, in blinded evaluation, was classified as study treatment related. All study treatment-related adverse events resolved without specific therapy.
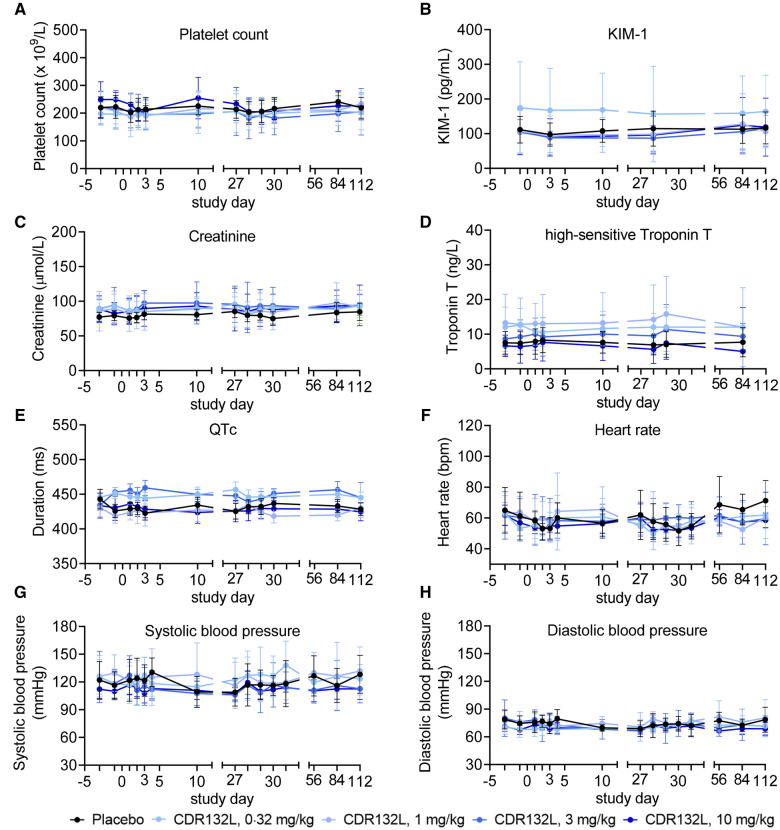
Thrombocytopenia, pre-specified as a potential adverse event of special interest (AESI) due to experience with certain first-generation ASOs,15–18 was not observed: platelet counts remained normal and stable throughout (Figure 3A). Laboratory assessments of liver and kidney function including KIM-1 and creatinine determinations (Figure 3B and C) revealed no signs of hepatic or renal toxicity for the tested doses. No clinically significant out-of-range values were recorded in all other laboratory values.
Figure 3.
Selected safety variables over time. Data are mean (95% confidence interval). KIM-1, kidney injury molecule-1; QTc, corrected QT interval; bpm, beats per minute.
No clinically significant changes from baseline values were seen in high-sensitivity troponin T concentration, monitored as a safety marker of ischaemic injury (Figure 3D). No clinically significant out-of-range values that could not be explained by an individual’s ischaemic HF were noted in any electrocardiographic variable. No participant had clinically relevant changes in QT interval corrected for heart rate (QTc), i.e. QTc of >500 ms according to Fridericia’s formula or a >60 ms change from baseline (Figure 3E); absence of QT prolongation, a highly sensitive marker of pro-arrthythmic properties, suggested low likelihood of pro-arrhythmic effects of CDR132L.19 Heart rate and systolic and diastolic blood pressure appeared not to be influenced by study treatment (Figure 3F–H), and no study treatment-related syncope was reported.
CDR132L is associated with largely consistent, predictable, linearly dose-dependent pharmacokinetics
CDR132L exposure from Days 1 and 28 administrations was consistent (Figure 4), and intra-patient variability was generally within the expected physiological norm (<30%). CDR132L was quantifiable in the plasma of all patients in the respective cohorts until 9 h after all intravenous infusions of 0.32 mg/kg, until 24 h after all infusions of 1 mg/kg, and until at least 48 h, the last measurement point, after all infusions of 3 or 10 mg/kg. No evidence of drug accumulation was seen in any CDR132L dosage group after the second (Day 28) administration, in line with short observed t½ (Supplementary material online, Table S3).
Figure 4.
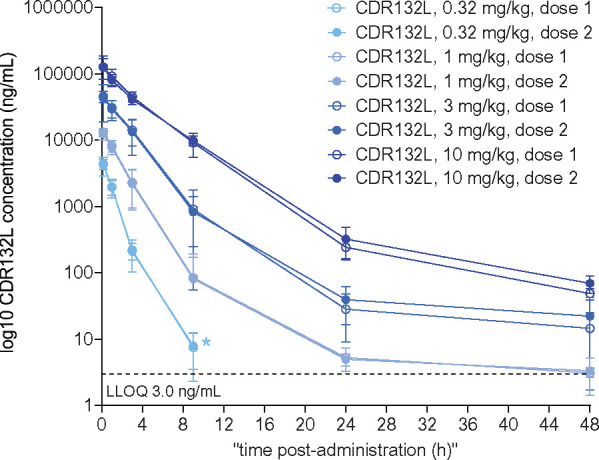
Plasma CDR132L concentrations through 48 h after CDR132L administrations. Data are mean (95% confidence interval). Measurements were taken 9 min and 1, 3, 9, 24, and 48 h after CDR132L administration. *CDR132L concentration fell below the lower limit of quantification at subsequent time points.
The relationship between dose and CDR132L concentration was almost linear with slightly increasing slope over time up to 9 h and then again decreasing (Supplementary material online, Figure S1). Statistical evaluation of dose proportionality supported the high linearity of Cmax with dose. When the power model was fitted to dose-normalized Cmax, the slope [90% confidence interval (CI)] was −0.023 (−0.068 to 0.021); inclusion of 0 in this CI suggested dose proportionality. Mean tmax was relatively consistent across doses, generally occurring ∼0.4 h after the start of the 15-min infusion (Supplementary material online, Table S3).
Statistical evaluation of dose proportionality of dose-normalized AUC0–t or AUC0–inf suggested a greater than dose-proportional increase in exposure with increasing CDR132L dose. For example, when the power model was fitted to dose-normalized AUC0–inf, the slope (90% CI), 0.264 (0.225–0.302), excluded 0, suggesting non-proportionality.
Mean CDR132L t½ in plasma was estimated at ∼4 h for doses ≥1 mg/kg. The corresponding value for the 0.32 mg/kg dose was ∼1 h (Supplementary material online, Table S3). This shorter t½ reflects the briefer post-infusion period of CDR132L quantifiability in plasma and is more likely to reflect drug distribution than elimination. For all studied dosages, CDR132L showed a small mean Vz, suggesting drug delivery largely to the vascular space and surrounding extravascular fluid. However, the Vz seemingly accounted for the short t½ in plasma, since mean CL generally remained protracted, at ∼2–5 L/h, slowing as dosage increased (Supplementary material online, Table S3). These data are in line with the results from the non-clinical pig studies that provided sufficient distribution into the heart tissue to achieve therapeutic levels.
CDR132L administration provides beneficial add-on effect on cardiac function and fibrosis
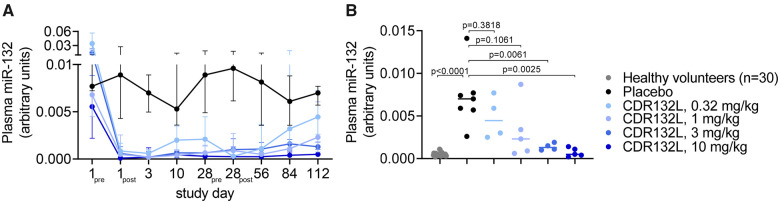
HF patients show high miR-132 expression in cardiac tissue and elevated plasma concentrations associated to NYHA class.20,21 CDR132L administration achieved rapid and, especially at higher dose levels ≥1 mg/kg, sustained and sharp reduction in plasma miR-132 over the 4-month course of the study. This observation was demonstrated by decreases from baseline in median plasma miR-132 levels (Figure 5A and Supplementary material online, Table S4). By contrast, patients receiving placebo showed relatively constant, much higher median plasma miR-132 concentrations over the same period. Circulating miR-132 levels were dose-dependently decreased in CDR132L patients when compared to concentrations in the placebo group at study endpoint (Day 112). We also assessed miR-132 in plasma samples from healthy volunteers obtained from a blood bank (n = 30) since the study was carried out in HF patients. Interestingly, patients treated with 3 or 10 mg/kg CDR132L showed sustained low plasma miR-132 levels similar to the range in healthy volunteers (median level: 0.0004) (Figure 5B and Supplementary material online, Table S4). Relative to placebo, two infusions of CDR132L, 0.32 mg/kg, reduced median miR-132 concentration by 36%, and of CDR132L, 1 mg/kg, by 67%. Two infusions of the highest studied CDR132L doses, 3 and 10 mg/kg, suppressed median plasma miR-132 levels by 81% and 93%, respectively, relative to placebo, differences that attained statistical significance (P ≤ 0.0061, Mann–Whitney U test; Figure 5B). As tissue sampling by invasive myocardial biopsy for pharmacokinetic or miR-132 quantification was not considered to be ethically justified or acceptable to the potential study participants, we developed a pharmacokinetic/pharmacodynamic (PK/PD) modelling approach to predict effective CDR132L dose levels. Therefore, PK/PD data from cardiac tissue and plasma measurements in CDR132L studies in large animal models of HF with relatively large sample sizes5,6 were combined with plasma miR-132 measurements from the present study regarding CDR132L PK and target engagement (Figures 4 and 5 and Supplementary material online, Tables S3 and S4) to estimate dose ranges likely to deliver sufficient drug to suppress excess target in cardiac tissue. Methodological details appear in the Supplementary material online. Based on this modelling approach, monthly intravenous CDR132L doses of 1–5 mg/kg or quarterly doses of 9–14 mg/kg would appear to provide sufficient cardiac miR-132 reduction (see Supplementary material online, Figure S2) and, presumably, related clinical benefits; such dosages may be appropriate for further clinical studies in HF patients.
Figure 5.
Plasma miR-132 levels in patients after CDR132L treatment. (A) Median with 25%/75% interquartile ranges of plasma miR-132 levels in patients over the study course (pre = immediately before, post = 1h after administration). (B) Individual median miR-132 levels in healthy subjects (n = 30; blood bank samples) and in patients (n = 25) at study end (Day 112). P-value: Mann–Whitney U test comparing to the placebo group.
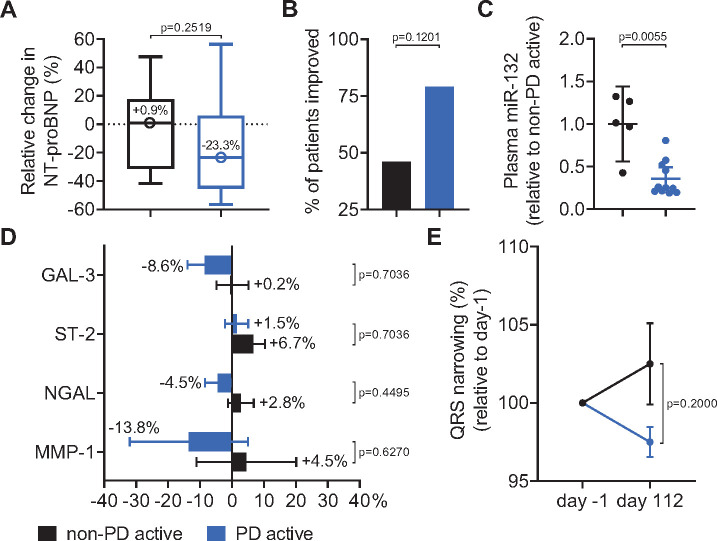
Based on the PK/PD modelling approach described above, we categorized all patients into 'pharmacodynamically active' (PD active) and ‘non-pharmacodynamically active’ (non-PD active) study treatment groups following the above described target engagement data. Pharmacodynamic activity was classified based on plasma miR-132 reduction in large animal studies and in the present Phase 1b trial on LVEF changes and other pharmacodynamic variables in the animal studies. From this we pooled patients receiving 1, 3, or 10 mg/kg (PD active: n = 14) and compared with the pooled placebo and the lowest dose of 0.32 mg/kg groups (non-PD active: n = 13).
NT-proBNP levels showed a median relative decrease in the PD active group with 23.3% from baseline to end of study (Day 112), whereas the non-PD active group showed in contrast a median relative increase of 0.9% (P = 0.2519, Fisher’s exact test; odds ratio: 2.9167; Figure 6A and Supplementary material online, Table S5). Both groups were additionally compared regarding a combined endpoint consisting of patients with a >10% relative decrease in NT-proBNP levels and/or a >2% absolute increase in LVEF from baseline to Day 112. The combined endpoint was met by 11/14 (79%) in the active group vs. 6/13 (46%) in the non-active group (P = 0.1201, Fisher’s exact test; odds ratio: 4.2778; Figure 6B and Supplementary material online, Table S6). These efficacy data are supported by markedly lower end-of-study plasma miR-132 concentrations in the active group relative to the non-active group (Figure 6C).
Figure 6.
Changes in exploratory pharmacodynamic variables, before first study treatment vs. end of study (Day 112). ‘Non-PD active’: patients receiving placebo or the 0.32 mg/kg CDR132L, ‘PD active’: patients receiving 1, 3, or 10 mg/kg CDR132L. (A) Median (circles) and 25%/75% interquartile ranges (bars) for relative changes in NT-proBNP from baseline to Day 112. (B) Percentages of patients with a >2% absolute increase in left ventricular ejection fraction and a >10% reduction in NT-proBNP, or both. (C) Corresponding mean ± SEM end-of-study plasma miR-132 levels. (D) Mean ± SEM changes in galectin-3, suppression of tumourgenicity-2, lipocalin-2, and matrix metallopeptidase-1. (E) Mean ± SEM changes in QRS narrowing normalized to pre-dosing measurements. P-values: Fisher's exact test (A, B, D, and E) or Mann–Whitney U test (C) comparing to ‘non-PD active’. Gal-3, galectin-3; MMP-1, matrix metallopeptidase-1; NGAL, lipocalin-2; ST-2, suppression of tumourgenicity-2.
Additional biomarkers related to HF and cardiac fibrosis including GAL-3, ST-2, NGAL, and MMP-1 were measured in this study (odds ratios for these parameters: >1; Figure 6D and Supplementary material online, Tables S7–S10). From baseline until end of study, the active group had modest relative decreases in median concentrations of 4/5 of these biomarkers and NT-proBNP, suggesting amelioration of disease. The non-active group displayed a diametrically opposite pattern of changes in these biomarkers. Likely due at least partly to the small subgroup size, none of the changes for either group was statistically significant.
Previous HF large animal data suggested positive effects on the shortening of prolonged action potential.5 Indeed, QRS complex narrowing is an electrocardiographic variable predicting beneficial treatment effects in patients with HF.22 HF patients with the initial abnormal durations of >120 ms within the non-PD active group showed a further widening of the QRS complex between baseline and study endpoint, while the PD active group showed a substantial narrowing leading to an intergroup difference of 5% (Figure 6E and Supplementary material online, Table S11). When placebo patients and patients receiving CDR132L were compared, the effect on QRS narrowing by CDR132L administration was statistically significant (Supplementary material online, Figure S3, P = 0.0333, Mann–Whitney U test).
Discussion
This is the first study report of an ASO inhibiting a miRNA in HF patients. The FiH study demonstrated a selective, dose-dependent, and potent reduction in miR-132 expression by the selective inhibitor CDR132L. The mechanism of action of CDR132L is to prevent and revert pathological cardiac remodelling as described earlier.3,5,6 A summary of the mode of action is provided in the Take home figure. Since novel generation ASOs are widely considered as safe treatment due to the high target selectivity and a well-established PK,23 we performed the FiH study already in the target indication HF as advised in meetings with regulators [Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM), MHRA, FDA]. Involving healthy volunteers would not add new and relevant information to what is already known from other ASOs of the same chemical class.24,25
Take home figure.
This first-in-human study confirmed that the novel treatment concept with an antisense oligonucleotide directed against miR-132 is safe and well-tolerated and provided indicative beneficial pharmacodynamic effects for the treatment of heart failure.
Although this study was designed primarily to assess the safety and pharmacokinetics of four ascending dosages of CDR132L, exploratory analysis of pre-planned pharmacodynamic endpoints revealed positive and encouraging effects demonstrating the translation of findings from various animal HF models into HF patients.5,6
Overall, intravenous administration of escalating dose levels from 0.32 up to 10 mg/kg of CDR132L demonstrated a consistent safety profile in the target HF population as already seen in preclinical animal studies.5,6
Thrombocytopenia was defined in the study protocol as potential AESI since some ASOs have been associated with effects on platelet number and function.15–18 Thorough analysis of platelets and other coagulation parameters did not show any abnormalities and remained well within normal ranges in all treatment groups. The safety of CDR132L is confirming of what is known from that of other locked nucleic acid ASOs,24,25 given the apparent inability of ASO chemistries like CDR132L’s to cross the intact blood–brain barrier,26 and given suggestions in preclinical studies of possible beneficial effects of miR-132 suppression in the diseased liver27 or kidney.28
One main outcome of this study was that CDR132L reliably demonstrated target engagement with, and potent reduction in its molecular target, miR-132, in plasma. The results showed a dose-dependent, statistically significant reduction in plasma miR-132 as already seen in preclinical animal studies.5,6
Since taking cardiac biopsies were seen unethical in this Phase 1b study, no data were gleaned on tissue PK or direct target engagement in human cardiac tissue. However, extensive pig studies5,6 found strong correlations between plasma and cardiac tissue concentrations of miR-132. In addition, linear inverse correlation was seen between CDR132L and miR-132 concentrations in the porcine heart and a positive correlation between miR-132 and LVEF improvement post-treatment could be established. These findings were supported by an observational sub-study21 of a 953-patient subset of the GISSI Heart Failure Study, where a correlation between plasma miR-132 levels and HF severity could be demonstrated. A reduction in plasma miR-132 in the present study indeed reflects CDR132L activity against its molecular target in the diseased human heart. One might speculate that, in turn, increasing levels of activity against miR-132 excess in that tissue would correlate with the clinical improvement of HF. The target engagement data demonstrate the successful translation of preclinical (pig) target engagement by CDR132L treatment into HF patients. It also indicates that the assessment of circulating miR-132 is suitable to be used as target engagement monitoring biomarker in the context of further CDR132L clinical development.
Results from our PK/PD modelling approach provide the opportunity to select a suitable CDR132L administration scheme and mode of administration with a high level of freedom to meet the needs for later clinical development. Our modelling approach allowed subgrouping of patients (n = 14, doses 1–10 mg/kg) receiving CDR132L associated with relatively high and prolonged plasma miR-132 reduction to be compared with those patients with absent or sub-optimal plasma miR-132 reduction (n = 13, placebo and 0.32 mg/kg).
Interestingly, although small patient numbers, the study results for CDR132L are already suggesting some clinical benefit in chronic HF patients on the top of standard of care. This included an improvement in HF severity, reflected in a clinically meaningful median reduction in NT-proBNP and narrowing of the QRS complex. Even if the human data are to be interpreted with caution due to the small patient numbers, the pharmacodynamic findings are to be viewed as encouraging especially as they confirm the efficacy results seen in large animal studies.5,6
Data availability statement
The data underlying this article are available in the article and in the Supplementary material online.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank all patients and study staff who made this research possible. We gratefully acknowledge the contributions of RPL regarding Richard Francis-Sakai, Jessica Partleton, and Graham Blakey (PK-consultant), Axolabs regarding Alina Kern, Prolytic regarding Dorothee Krone and Lisa Hentschel, and excellent technical support at Cardior regarding Christin Albrecht. Robert J. Marlowe, Spencer-Fontayne Corporation, Jersey City, NJ, USA, helped to edit this manuscript.
Funding
The study was supported by Cardior Pharmaceuticals GmbH. The funder, who was also the sponsor according to Directive 2001/20/EC of the European Parliament and of the Council Article 2 (e), participated in designing the study, collecting, analysing, interpreting data, and writing this report.
Conflict of interest: S.B. and T.T. are co-founders and shareholders, W.H., S.R., and H.W. are consultants, and S.B., C.G., J.P., L.R., and J.V. are fulltime staff members of that company. T.T. filed and licenced patents about miR-132 to Cardior Pharmaceuticals GmbH through Hannover Medical School. T.T. has personal fees from Novo Nordisk, as well as other from Sanofi-Genzyme, Takeda, and Amicus Therapeutics. J.T. and U.L. are fulltime employees of Richmond Pharmacology Ltd where the study was performed. A.A.L receives personal fees from Cardior, Atalanta, Civi Therapeutics, Deep Genomics, ProQR, and Stoke Therapeutics. J.B. reports personal fees from Abbott, grants and personal fees from Abiomed, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from BMS, personal fees from Boehringer Ingelheim, grants and personal fees from CVRx, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Servier, grants and personal fees from Vifor, grants and personal fees from Zoll, and personal fees from Cardior, outside the submitted work. S.D.S. reports personal fees from Cardior, during the conduct of the study, grants and personal fees from Alnylam, Amgen, AstraZeneca, Bellerophon, BMS, Celladon, Gilead, and GSK, grants from Ionis, Lone Star Heart, and Mesoblast, grants and personal fees from MyoKardia, grants from NIH/NHLBI, grants and personal fees from Novartis, grants from Sanofi Pasteur, grants and personal fees from Theracos, personal fees from Akros, grants and personal fees from Bayer, personal fees from Corvia, personal fees from Ironwood, personal fees from Merck, personal fees from Roche, personal fees from Takeda, personal fees from Quantum Genomics, personal fees from AoBiome, personal fees from Janssen, personal fees from Cardiac Dimensions, grants from Eidos, grants and personal fees from Cytokinetics, personal fees from Tenaya, personal fees from Daichi-Sankyo, personal fees from Arena, personal fees from Dinaqor, and personal fees from Cardurion, outside the submitted work.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 2.Rossignol P, Hernandez AF, Solomon SD, Zannad F.. Heart failure drug treatment. Lancet 2019;393:1034–1044. [DOI] [PubMed] [Google Scholar]
- 3.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T.. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012;3:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PBL, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP.. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 2013;14:11190–11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyöngyösi M, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Weber N, Zlabinger K, Hašimbegović E, Winkler J, Fiedler J, Dangwal S, Fischer M, Roche J. D L, Wojciechowski D, Kraft T, Garamvölgyi R, Neitzel S, Chatterjee S, Yin X, Bär C, Mayr M, Xiao K, Thum T.. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat Commun 2020;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Zlabinger K, Hašimbegović E, Winkler J, Garamvölgyi R, Neitzel S, Gyöngyösi M, Thum T.. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur J Heart 2021;42:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu D, Thum T.. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 2019;16:661–674. [DOI] [PubMed] [Google Scholar]
- 8.Lavenniah A, Luu TDA, Li YP, Lim TB, Jiang J, Ackers-Johnson M, Foo RS-Y.. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Mol Ther 2020;28:1506–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail England 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 10.NICE chronic heart failure guideline. NICE guideline. Guidelines. https://www.guidelines.co.uk/cardiovascular/nice-chronic-heart-failure-guideline/454369.article (22 October 2020).
- 11.ICH E6 (R2) Good clinical practice. European Medicines Agency. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice (22 October 2020).
- 12.Revised guideline on first-in-human clinical trials. European Medicines Agency. https://www.ema.europa.eu/en/news/revised-guideline-first-human-clinical-trials (22 October 2020).
- 13.FDA. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; 2005. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (22 October 2020).
- 14.Smith BP, Vandenhende FR, DeSante KA, Farid NA, Welch PA, Callaghan JT, Forgue ST.. Confidence interval criteria for assessment of dose proportionality. Pharm Res 2000;17:1278–1283. [DOI] [PubMed] [Google Scholar]
- 15.Chi X, Gatti P, Papoian T.. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today 2017;22:823–833. [DOI] [PubMed] [Google Scholar]
- 16.Sewing S, Roth AB, Winter M, Dieckmann A, Bertinetti-Lapatki C, Tessier Y, McGinnis C, Huber S, Koller E, Ploix C, Reed JC, Singer T, Rothfuss A.. Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. PLoS One 2017;12:e0187574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazier KS. Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol 2015;43:78–89. [DOI] [PubMed] [Google Scholar]
- 18.Stein CA, Castanotto D.. FDA-approved oligonucleotide therapies in 2017. Mol Ther 2017;25:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICH E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. European Medicines Agency. https://www.ema.europa.eu/en/ich-e14-clinical-evaluation-qtqtc-interval-prolongation-proarrhythmic-potential-non-antiarrhythmic (22 October 2020).
- 20.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, Laake L. V, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J.. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 2007;116:258–267. [DOI] [PubMed] [Google Scholar]
- 21.Masson S, Batkai S, Beermann J, Bär C, Pfanne A, Thum S, Magnoli M, Balconi G, Nicolosi GL, Tavazzi L, Latini R, Thum T.. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail 2018;20:78–85. [DOI] [PubMed] [Google Scholar]
- 22.McMurray JJV. Clinical practice. Systolic heart failure. N Engl J Med 2010;362:228–238. [DOI] [PubMed] [Google Scholar]
- 23.Levin AA, Yu RZ, Geary RS, Basic principles of the pharmacokinetics of antisense oligonucleotide drugs. In Crooke ST, ed. Antisense Drug Technology. 2nd ed.; Taylor & Francis Inc, 2008. [Google Scholar]
- 24.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng M-J, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, Tribble DL, Flaim JD, Crooke ST.. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:998–1006. [DOI] [PubMed] [Google Scholar]
- 25.Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, Meer A. V D, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR.. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685–1694. [DOI] [PubMed] [Google Scholar]
- 26.Salta E, Strooper BD.. microRNA-132: a key noncoding RNA operating in the cellular phase of Alzheimer’s disease. FASEB J 2017;31:424–433. [DOI] [PubMed] [Google Scholar]
- 27.Hanin G, Yayon N, Tzur Y, Haviv R, Bennett ER, Udi S, Krishnamoorthy YR, Kotsiliti E, Zangen R, Efron B, Tam J, Pappo O, Shteyer E, Pikarsky E, Heikenwalder M, Greenberg DS, Soreq H.. miRNA-132 induces hepatic steatosis and hyperlipidaemia by synergistic multitarget suppression. Gut 2018;67:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijkerk R, Bruin R. D, Solingen C. V, Gils J. V, Duijs JMGJ, Veer E. V D, Rabelink TJ, Humphreys BD, Zonneveld A. V.. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int 2016;89:1268–1280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in the Supplementary material online.