Abstract
The purpose of this article is to inform nurse practitioners and other healthcare professionals regarding the utilization of CardioMEMs, a wireless pulmonary artery pressure monitoring device, in reducing heart failure-related hospital readmission rates. This article will briefly explain how CardioMEMs also helps to reduce the risk of Covid-19 in patients with heart failure.
Keywords: CardioMEMS, heart failure, hemodynamic monitoring, hospital readmission, wireless pulmonary artery pressure monitoring system
Introduction
The prevalence of cardiovascular disease is increasing worldwide due to the aging of the population,1 higher prevalence of risk factors (obesity, diabetes mellitus, and hypertension),1 , 2 and advances in revascularization therapy.3. According to the National Health and Nutrition Examination Survey, cardiovascular disease is the leading cause of death in the United States.4 The prevalence of heart failure (HF) is significant. Approximately 6.5 million adults in the United States had HF in 2017.4 HF caused 1 in 8 deaths in United States in the same year.4 An acute exacerbation of the HF is also the leading cause of hospital readmission within 30 days of discharge in the United States.1 , 4 Frequent hospital readmissions due to HF is associated with increased mortality,1 , 4 poor quality of life (QOL),1 and increased health care costs.1 , 4 For example frequent readmissions potentially increase the risk for hospital acquired infections that can possibly increase the health care cost and decrease patients’ time with their family, which may contribute to poor QOL in HF patients.
CardioMEMS, a wireless implantable hemodynamic monitoring system, is currently the only US Food and Drug Administration (FDA)-approved wireless pulmonary artery pressure monitoring device that has demonstrated the capacity to substantially reduce the HF-related hospital readmission rate.5 The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association (NYHA) class III Heart Failure Patients (CHAMPION) trial showed a reduction in HF-related hospitalization by 37% during the follow-up period of 15–18 months.6 By reducing frequent hospitalizations and 30-day readmission rates, this HF-monitoring system may improve QOL and decrease morbidity, mortality, and health care cost in advanced HF patients with acute exacerbation.
What Is CardioMEMS?
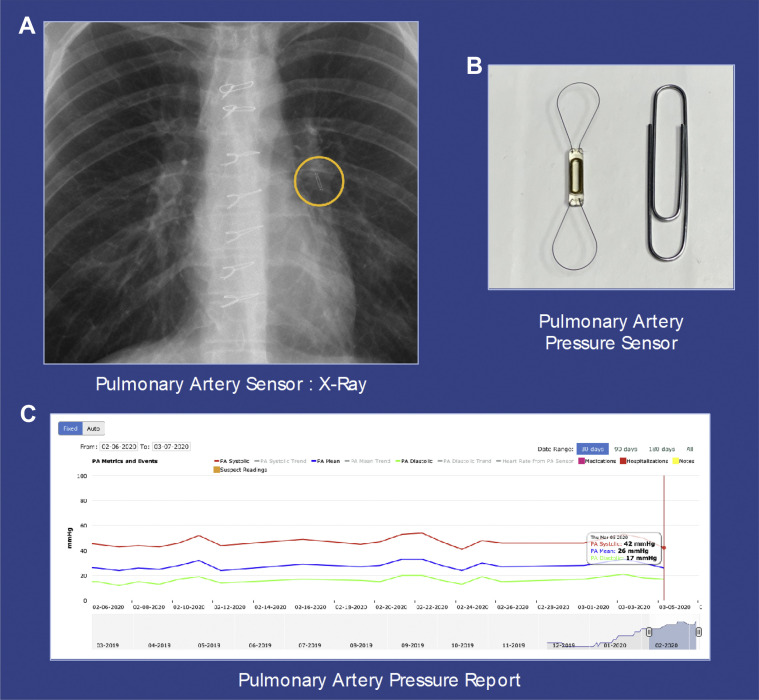
CardioMEMS is a wireless pulmonary artery pressure monitoring system that is percutaneously implanted in an inferior and later branch of the left pulmonary artery (PA). The left-sided placement allows for optimal interrogation of the device as left-sided PA branches course posteriorly. The procedure is initially involves performing a right heart catheterization using a Swan Ganz catheter via the common femoral vein. After selecting an optimal vessel, the Swan Ganz catheter is removed over a wire, and the sensor is inserted over the wire and subsequently implanted. This device allows remote hemodynamic monitoring by using a wireless system that provides 18-second measurements of systolic, diastolic, and mean PA pressures. Patients can upload readings by simply laying on a pillow-like device that interrogates the sensor. This self-prompted pillow guides a patient for proper positioning. The transmitted PA pressure readings can be viewed by a designated health care provider (Figure ). This type of remote monitoring device allows the health care provider to manage the patients’ fluid volume status by adjusting diuretics and other cardiac medications based on their pulmonary artery pressure reading that is above or below the patient’s baseline.
Figure.
(A) The CardioMEMS sensor is identified on a chest radiograph and positioned in a branch of the left pulmonary artery (PA). (B) The sensor has 2 nitinol hoops that allow for contact with the PA branch. (C) PA pressure reports can be viewed by the health care provider and provide a longitudinal display of PA pressure readings.
HF and Its Classification
What Is HF?
HF is a clinical syndrome resulting from the inability of the ventricular heart muscles to pump enough blood to meet the body’s requirements. Left-sided HF is more common than the right-sided HF. 1 , 7 Structural and functional heart pathologies can result in HF. For example, hypertension, coronary artery disease, heart attack or myocardial infarction, valvular and congenital heart pathologies, and cardiomyopathy can predispose one to congestive HF.7
Major Classifications of HF
There are 3 major classifications of HF based on its pathophysiology. The first classification is based on the nature of the structural defect (Table 1 ), the second is based on the patient’s functional capacity (Table 2 ), and the third is based on the percentage of the left ventricular ejection fraction (LVEF; Table 3 ).
Table 1.
American College of Cardiology and American Heart Association Structural Classification of HF
| Classification | Description | Examples of Selected Type of HF/Conditions at This Stage |
|---|---|---|
| Stage A | At high risk for HF but without structural heart disease or symptoms of HF | Only risk factors are present, eg, hypertension, CAD, family history of cardiomyopathy, diabetes mellitus |
| Stage B | Structural heart disease but without signs or symptoms of heart failure | Previous MI, left ventricular systolic dysfunction, asymptomatic valvular disease |
| Stage C | Structural heart disease with before current symptoms of heart failure | CAD, valvular disease, hypertrophic cardiomyopathy, and dilated cardiomyopathy; peripartum cardiomyopathy; congenital heart disease; myocarditis caused by inflammation; myocardial toxicity induced by chemotherapy, radiation, illicit drugs (cocaine, methamphetamine) or alcohol |
| Stage D | Refractory heart failure requiring specialized interventions such as a heart transplant LVAD | Structural heart disease as described in Stage C with marked heart failure symptoms (shortness of breath, fatigue, decreased exercise tolerance) at rest despite maximal medical therapy |
CAD = coronary artery disease; HF = heart failure; LVAD = left ventricular assist device; MI = myocardial infection.
Table 2.
NYHA Functional Classification of Heart Failure
| NYHA functional classification | Symptoms | Correlation With ACC/AHA Stages of Structural Heart Disease |
|---|---|---|
| Class I | No symptoms or limitations during ordinary physical activities. | Stages B and C |
| Class II | Having symptoms during ordinary activities | Stage C |
| Class III | Having symptoms during less-than-ordinary activities | Stage C |
| Class IV | Having symptoms even when at rest | Stage D |
The New York Heart Association (NYHA) classifies heart failure into 4 categories based on the functional capacity of the patients.20 The functional classification of heart failure is based on the symptoms associated with patient’s functional capacity.
ACC = American College of Cardiology; AHA = American Heart Association.
Table 3.
Classification of HF based on LVEF
| Type of HF Based on LVEF | Percentage of LVEF | Type of HF/Major Cause |
|---|---|---|
| HF with reduced EF | LVEF ≤ 40% | Systolic HF& concomitant diastolic HF due to cardiomyopathy related to CAD, Other causes include: Hypertrophic cardiomyopathy and dilated cardiomyopathy; peripartum cardiomyopathy; congenital heart disease; myocarditis caused by inflammation; myocardial toxicity induced by chemotherapy, radiation, illicit drugs (cocaine, methamphetamine) or alcohol |
| HF with preserved EF | LVEF ≥50% | Diastolic HF; chronic hypertension is the major cause |
| HF with mid-range ejection fraction | LVEF (41–49%) | Diagnosis should be based on echocardiograms and left ventricular catheterization; comparisons should be made to assess declining of the EF% |
HF is also classified based on the percent LVEF (LVEF%).
EF = ejection fraction; HF = heart failure; LVEF = left ventricular ejection fraction.
The American College of Cardiology and American Heart Association classifies HF into 4 stages (Table 1) based on its structural defects on the tissue of the heart, or the heart valves (eg, cardiomyopathy, aortic insufficiency, mitral regurgitation).8
Pathophysiology of Clinical Progression
Etiology
The most common causes of HF are coronary artery disease, hypertension, valvular heart disease, obesity, and diabetes mellitus.1 , 2 , 7 , 9 Other pathologic etiologies include inherited cardiomyopathies (hypertrophic cardiomyopathy and dilated cardiomyopathy); peripartum cardiomyopathy; congenital heart disease; myocarditis caused by inflammation; and myocardial toxicity induced by chemotherapy, radiation, illicit drugs (cocaine, methamphetamine), or alcohol.7 , 10
Clinical Congestion
Acute exacerbations of chronic HF commonly result from increased left ventricular diastolic pressure. Common clinical symptoms manifesting chronic HF include but are not limited to the symptoms of shortness of breath, fatigue, edema of feet, ankles, and legs, orthopnea, and jugular vein distention. These symptoms are referred to as clinical congestion. Clinical congestion represents the hemodynamic abnormalities resulting from elevated filling pressures or diastolic pressure in the left ventricle. Clinical congestion may occur several days or even weeks before the clinical manifestations.11
Venous Congestion and Neurohormonal Activation
Venous congestion and neurohormonal activation are central to the underlying pathophysiology of acute HF.12 The impaired cardiac output from myocardial dysfunction leads to sympathetic activation and release of neurohormones such as angiotensin II, endothelin, and arginine vasopressin. Upregulation of the renin–angiotensin–aldosterone system and sympathetic activation can promote maladaptive remodeling, arrhythmias, sodium retention, endothelial dysfunction, apoptosis, and fibrosis. Negative remodeling may alter the shape and size of the left ventricle and cause secondary (functional) mitral regurgitation.12
Cardiorenal Syndrome and Hepatic Congestion
Hemodynamic congestion and impaired renal perfusion are central to cardiorenal syndrome, which is associated with renal dysfunction and poor outcomes in HF. Left ventricular dysfunction and elevated left ventricular end-diastolic pressures may lead to elevated pulmonary artery pressures and eventually right HF if left untreated or as the disease progresses. Hepatic congestion, ascites, gastrointestinal mucosal edema, and peripheral edema are hallmarks of advanced right HF.13
Intermittent Hypoxia
Medical conditions such as sleep apnea and chronic obstructive pulmonary disease could potentially trigger intermittent hypoxia, which may activate episodic sympathetic hyperactivity. The activation of sympathetic hyperactivity could result in systemic hypertension, subendocardial ischemia, and rapid elevations in filling pressure causing imbalances in circulating blood volume. Elevation in the filling pressure results in HF symptoms.12
Regardless of the underlying pathophysiology of the classification or type of HF, the patient experiences the fluid volume overload. The fluid volume overload underpins signs and symptoms as well as functional capacity of the patient. Patient goals are to treat the underlying cause and manage patients’ signs and symptoms. HF is a complex physiological process, and often the underlying cause is not reversible.13
Negative Impact of Frequent Hospital Readmissions in the HF Patient
Hospital readmissions have a direct negative impact on patient outcomes and QOL. It also increases patient and family burden and health care costs.
Poor Patient Outcomes
During any hospital admission, there are chances of hospital-acquired illnesses and injuries, such as infections, falls, and depression, for example.13, 14, 15 In 2012, HF involved 23.4% of all hospitalizations with a discharge diagnosis of urinary tract infection, pneumonia, or sepsis.13 These problems result in increased length of stay and cause further complications in HF patients. For example, among patients aged 65 years and older, 61.55% of 4,754 fall cases resulted in a femur fracture.14 Similarly, it is estimated that 35–60% of acute HF patients are depressed. Depression is one of the factors most influencing QOL, length of hospital stays, and posthospital discharge mortality.15
Poor QOL and Increased Mortality
Adding to the burden of poor patient outcomes, hospital readmissions takes patients’ and caregivers’ time away from their family, work, and other essential matters in their lives, affecting one’s QOL. Hospital readmissions are also linked to an increased death rate in HF patients. Contemporary research indicates that there is a greater than 75% increase in 5-year mortality after the first hospitalization for HF.16
Economic Burden
HF 30-day readmission represents a profound economic burden. The cost of 30-day readmissions was estimated at $30.7 billion in 2012 and is predicted to be $69.7 billion by 2030.17 The mean estimated cost of each HF readmission within 30 days of hospital discharge was $11,552 in 2014, with a total estimated cost greater than $11 billion. Patients, hospitals, and insurance companies must bear the financial burden. Medicare incurs one of the largest burdens of HF hospital readmission expenditures.18
Benefits of CardioMEMS in HF Patients
Reduces Hospital Readmissions
This wireless system correlates well with a Swan-Ganz catheter and echocardiographic PA pressure measurements.19 Elevations in PA pressures can occur days or sometimes weeks before the onset of clinical HF symptoms.20 This system provides the opportunity for timely intervention to mitigate an acute HF decompensation and hospital admission. This system has been effective in HF with both reduced and preserved ejection fraction in preventing hospital readmissions. 21
Another reason for frequent hospital readmission in HF patients is that a predischarge clinical assessment of congestion is not performed routinely and systematically.10 With the help of CardioMEMS, we can easily monitor pulmonary artery pressure readings every day, and upward or downward trends can be monitored. This type of monitoring device helps clinicians ensure that the patient’s volume status is stable before discharge, which will help prevent immediate rebound to the hypervolemic state after discharge. This wireless system also has a low rate of system- and device-related complications. Most of the procedures are done in an outpatient setting,22 and patients typically go home the day of the procedure.
Minimizes Right Heart Catheterization
This PA pressure monitoring system also reduces the number of right heart catheterizations in patients with HF. The current gold standard for assessing hemodynamic congestion in acute HF is right heart catheterization to measure pulmonary artery wedge pressure and right arterial pressure. Although the initial implant is done via right heart catheterization, this device minimizes further invasive procedures in patients with acute exacerbation of HF. The diuretics and other HF therapy can be easily adjusted on the basis of the patient’s readings from this wireless monitoring system.
Reduces the Risk of COVID-19
An added benefit of reducing early hospital readmissions in HF patients is decreasing or minimizing the risk of exposure to coronavirus disease 2019 (COVID-19) in vulnerable populations. CardioMEMS also helps prevent the spread of COVID-19 by reducing the number of hospital readmissions and clinic visits for patients with HF. COVID-19 is primarily transmitted in 3 ways: contact, droplet, and airborne transmission.23 The Centers for Disease Control and Prevention (CDC) recommends social distancing along with other measures such as wearing a mask in public, maintaining hand hygiene, surface cleaning and disinfection, ventilation, and avoidance of crowded indoor spaces. Limiting close face-to-face contact with others is the best way to reduce the spread of COVID-19. Social distancing is especially important for people who are at higher risk for severe illness from COVID-19. Patients with cardiovascular disease including HF are considered a high-risk population for transmission of COVID-19.23 Therefore, by reducing the number of clinic visits and rehospitalizations, CardioMEMS will help to prevent the spread of COVID-19 and decrease risk factors for this vulnerable population.
Clinical Trials: Past, Present, and Future
The CHAMPION Trial
The first major trial that showed the effectiveness of this wireless hemodynamic monitoring system was the CHAMPION trial. A total of 270 control patients and 280 treatment group patients underwent CardioMEMS implantation. Over the 15-month follow-up period, the HF-related hospital admission rate was decreased by 37% in the treatment group. The result also indicated a lower risk of death and HF-related hospitalization.20 Following the completion of the randomized access period in the CHAMPION trial, a longitudinal analysis was done on all study participants for 13 months. The result of this longitudinal analysis also reinforced the result of the CHAMPION trial.21 Another randomized clinical trial done on 249 Medicare eligible patients showed a 49% reduction in total HF hospitalizations and a 58% reduction in all-cause 30-day readmissions.21
Post Approval Study
The Post Approval Study (PAS) was a prospective, multicenter, open-label trial conducted in 150 sites in the United States.22 This trial had 297 patients with HF NYHA class III symptoms and at least 1 HF hospitalization within a year was included in the study. Once the device was approved by the FDA, there were more studies done in the commercial settings, which also showed a reduction in HF-related readmission.24 In a large retrospective cohort study using US Medicare claim data on 1,114 patients who had CardioMEMS device implanted for 18 months, there was a greater reduction of HF hospitalization over 6 months compared with the initial CHAMPION trial.
Hemodynamic-GUIDEd Management of HF (GUIDE-HF)
GUIDE-HF is currently being conducted to study the impact of the wireless PA pressure monitoring device in improving survival and QOL in patients with HF. Unlike previous trials, this study will also include NYHA class II, III, and IV patients. This study will enroll in 3,600 HF patients at 140 hospitals across North America. Study participants are required to have an elevated BNP or at least 1 hospitalization within 1 year. The randomized arm has completed enrolling ACC stage C, NYHA class II–IV patients, and the single-arm will have ACC stage C, NYHA class III–IV patients. 24
International Trials
MEMS-HF (CardioMEMS European Monitoring Study for Heart Failure) trial is a prospective, nonrandomized, open-label, multicenter trial to study the safety and feasibility of using the device in a real-world setting in Germany, the Netherlands, and Ireland. This study will include 230 patients who have had NYHA class III symptoms for at least 30 days. The duration of this study will be 36 months with 24 months of the enrollment period and 12 months of follow-up.25
Previous Experience With Other Hemodynamic Monitoring Methods
There has been substantial research done in both invasive and noninvasive methods of ambulatory HF monitoring to reduce morbidity, mortality, and hospital readmission rate in patients with HF.
Noninvasive Monitoring Systems
Telemonitoring and Tele-HF (Telemonitoring to Improve Heart Failure Outcomes) were both noninvasive systems that primarily monitored the weight and symptoms of patients and the clinician’s adjusted medications daily.26 In the telemonitoring system, a commercial system called Tel-Assurance was used. The intervention group was told to make daily toll-free calls to the Tel-Assurance system. During each call, the participants heard a series of automated questions and entered the response using a telephone keypad. The responses were then downloaded to the secured Internet site and were reviewed every weekday by the site coordinators.25 Telemonitoring helped to reduce the mortality rate but was unable to reduce 6 months of hospital readmission.26 , 27
Tele-HF was designed to compare an automated daily symptom and self-reported monitoring intervention with usual care in reducing all-cause hospital readmissions and mortality among the patients who were recently hospitalized with decompensated HF.28 Tele-HF did not demonstrate a significant difference in reducing both mortality and readmission rate.26 , 27 TIM-HF trial was a more sophisticated system that automatically transmitted 3-lead electrocardiographic data, blood pressure, and daily weights. However, the result showed no difference in mortality or hospitalization rate.26 Other noninvasive monitoring systems were evaluated in the Prevent-HF (Prevention of Heart Failure Events with Impedance Cardiography Testing) and PREDICT (Prospective Evaluation of Cardiac Decompensation in Patients with Heart Failure by Impedance Cardiography Test) trials but failed to meet primary endpoints.28 Likewise, the VeriCor System used to provide an indirect measurement of LV filling pressure. The VeriCor system also has had insufficient data supporting its use.26
Invasive Monitoring Systems
Other implantable hemodynamic monitoring systems also have not been effective in reducing HF-related admissions. Chronicle is an implantable hemodynamic monitoring device that is like a single lead pacemaker that is placed in the right ventricular outflow tract that helps to transmit data to a subcutaneously placed device and can transmit a real-time report on cardiac hemodynamics.26 , 29 However, the COMPASS (Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure) trial did not show any benefit over optimal medical management.26 , 29 Similarly, Optivol is another implantable hemodynamic monitoring system that detects increased left ventricular filling pressures via a reduction in impedance within a right ventricular defibrillator coil of an ICD device, which occurs due to increases in the intrathoracic fluid.26 , 30 There were 2 clinical trials related to Optivol. The first was FAST (Fluid Accumulation Status Trial), which had promising results with a significant change in patient’s weight. The primary purpose of this trial was to assess the sensitivity and unexplained detection rate of Optivol associated with changes in intrathoracic impedance and with changes in daily weight and to compare the intrathoracic impedance and the weight.29 However, it was a limited study.26 , 30 The second trial was DOT-HF (Diagnostic Outcome Trial in Heart Failure), which was terminated early due to increased ambulatory visits and hospitalizations without clinical benefit.26
Conclusion
HF is highly prevalent worldwide and is associated with significant morbidity and mortality. HF is also one of the major causes of frequent hospital readmissions, which are costly and can potentially lead to poor patient outcomes. Clinical trials to date have supported the benefit of implantable wireless hemodynamic monitoring systems in reducing HF hospitalizations in HF patients with both preserved and reduced ejection fraction. CardioMEMS, a wireless pulmonary artery pressure monitoring system, offers a promising method to monitor, manage, and decrease HF-related early and frequent readmissions in HF patients.
Biographies
Rosha Joshi, MS, FNP-BC, is an adjunct faculty member at Prairie View A&M University, College of Nursing, Houston, TX, and a PhD student at Texas Woman’s University College of Nursing, Houston TX. She can be contacted at rosharani@gmail.com.
Ajith Nair, MD, is an assistant professor in the Department of Medicine, Division of Cardiology, Baylor College of Medicine, Houston, TX.
Footnotes
In compliance with standard ethical guidelines, the authors report no relationships with business or industry that would pose a conflict of interest.
References
- 1.Savarese G., Lund L.H. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakumar P., Maung-U K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Catalina C.O., Adina B., Smarandita B.E.D., Angela D., Dan G., Silvia M. Cardiovascular lipid risk factors and rate of cardiovascular events after myocardial revascularization. Int J Cardiovasc Sci. 2017;30 doi: 10.5935/2359-4802.20170015. [DOI] [Google Scholar]
- 4.Benjamin E.J., Muntner P., Alonso A., et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention What is heart failure? https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure Accessed February 20, 2020.
- 6.Abbott.com. Accessed February 20, 2020. https://www.cardiovascular.abbott/content/dam/bss/divisionalsites/cv/pdf/guides/cardiomems_hf_system_clinical_.pdf
- 7.Abraham W.T., Adamson P.B., Bourge R.C., et al. Wireless pulmonary artery hemodynamic monitoring in chronic heart failure: a randomized controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 8.Penn Medicine Heart failure classification—stages of heart failure and their treatments. https://www.pennmedicine.org/updates/blogs/heart-and-vascular-blog/2014/september/heart-failure-classification--stages-of-heart-failure-and-their-treatments Accessed November 5, 2020.
- 9.American Heart Association. https://www.heart.org/en/health-topics/heart-failure/causes-and-risks-for-heart-failure/causes-of-heart-failure Accessed February 20, 2020.
- 10.Gheorghiade M., Follath F., Ponikowski P., et al. Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 11.Mentz R.J., Christopher M., O’Connor Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2015;13(1) doi: 10.1038/nrcardio.2015.134. [DOI] [PubMed] [Google Scholar]
- 12.Mamic P., Heidenreich P.A., Hedlin H., Tennakoon L., Staudenmayer K.L. Hospitalized patients with heart failure and common bacterial infections: a nationwide analysis of concomitant clostridium difficile infection rates and in-hospital mortality. J Card Fail. 2016;22:891–900. doi: 10.1016/j.cardfail.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Tu J.V., Nardi L., Fang J., Liu J., Khalid L., Johansen H. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180(13):E118–E125. doi: 10.1503/cmaj.081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieminen M.S., Dickstein K., Fonseca C., et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalizations. Int J Cardiol. 2015;191:256–264. doi: 10.1016/j.ijcard.2015.04.235. [DOI] [PubMed] [Google Scholar]
- 15.Mcmanus D.D., Piacentine S.M., Lessard D., et al. Thirty-year (1975 to 2005) trends in the incidence rates, clinical features, treatment practices, and short-term outcomes of patients <55 years of age hospitalized with an initial acute myocardial infarction. Am J Cardiol. 2011;108:477–482. doi: 10.1016/j.amjcard.2011.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dordunoo D., Thomas S.A., Friedmann E., Russell S.D., Newhouse R.P., Akintade B. Inpatient unit heart failure discharge volume predicts all-cause 30-day hospital readmission. J Cardiovasc Nurs. 2017;32(3):218–225. doi: 10.1097/JCN.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 17.Jackson S.L., Tong X., King R.J., Loustalot F., Hong Y., Ritchey M.D. National burden of heart failure events in the united states, 2006 to 2014. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2010;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yandrapalli S., Raza A., Tariq S., Aronow W.S. Ambulatory pulmonary artery pressure monitoring in advanced heart failure patients. World J Cardiol. 2017;9:21–26. doi: 10.4330/wjc.v9.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New York Heart Association Classes of heart failure. https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure Accessed November 5, 2020.
- 21.Adamson P.B., Abraham W.T., Stevenson L.W., et al. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002600. [DOI] [PubMed] [Google Scholar]
- 22.Shavelle D.M., Abraham W., Bourge R., Jermyn R., Costanzo M.R., Stevenson L. High procedural, and device-related success with the CardioMEMS HF system for heart failure: observations from the CardioMEMS post-approval study. J Am Coll Cardiol. 2017;69(suppl 11):804. doi: 10.1016/S0735-1097(17)34193-1. [DOI] [Google Scholar]
- 23.Centers for Disease Control and Prevention COVID-19. People with certain medical conditions. December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html#heart-conditions [PubMed]
- 24.Desai A.S., Bhimaraj A., Bharmi R., et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “real-world” clinical practice. J Am Coll Cardiol. 2017;69:2357–2365. doi: 10.1016/j.jacc.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Houston B.A., Kalathiya R.J., Kim D.A., Zakaria S. Volume overload in heart failure. Mayo Clin Proc. 2015;90:1247–1261. doi: 10.1016/j.mayocp.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry SI M.D., Mattera JA M.P.H., Curtis JP M.D., et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. doi:https://doi.org.ezp.twu.edu/10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhry S.I., Barton B., Mattera J., Spertus J., Krumholz H.M. Randomized trial of telemonitoring to improve heart failure outcomes (tele-HF): study design. J Card Fail. 2007;13:709–714. doi: 10.1016/j.cardfail.2007.06.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourge R.C., Abraham W.T., Magalski A., et al. COMPASS-HF (Chronicle® offers management to patients with advanced signs and symptoms of heart failure) study design. J Card Fail. 2004;10:S84. doi: 10.1016/j.cardfail.2004.06.242. [DOI] [Google Scholar]
- 29.Brachmann J., Böhm M., Rybak K., et al. Fluid status monitoring with a wireless network to reduce cardiovascular-related hospitalizations and mortality in heart failure: rationale and design of the OptiLink HF study (optimization of heart failure management using OptiVol fluid status monitoring and CareLink) Eur J Heart Fail. 2011;13:796–804. doi: 10.1093/eurjhf/hfr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham W.T., Compton S., Haas G., et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST) Congestive heart failure (Greenwich, Conn) 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220. [DOI] [PubMed] [Google Scholar]