Abstract
Background:
Closed incision negative pressure therapy (ciNPT) has been shown to improve wound healing for patients at high risk for wound complications. Current devices consist of opaque interface dressings that do not allow ongoing visual evaluation of the surgical incision and utilize a negative pressure of −80 mm Hg to −125 mm Hg. The Negative Pressure Platform Wound Dressing (NP-PWD) was developed to address these aspects. This case series is the first evaluation of the NP-PWD in a clinical setting.
Methods:
Patients aged 18–85 undergoing an operation with an anticipated incision and primary closure were screened. Demographics, comorbidities, and operation performed were recorded. Following closure, the incision was measured and photographed before NP-PWD placement. The NP-PWD was removed at the first postoperative check (POC) between postoperative days (PODs) 3–5. Subjects were followed until PODs 9–14. POCs consisted of incision assessment, measurement, photography, and adverse event monitoring.
Results:
A total of 8 patients with 10 incisions were included in the study. Five patients were men. Median age was 56 years (IQR 53–74 years). All incisions were intact and without inflammation or infection at all POCs. Three adverse events, including small blisters and interruption of therapy, were noted.
Conclusions:
This case series reports that patients tolerated the NP-PWD on closed surgical incisions well and that all incisions were intact without evidence of inflammation or infection after 2 weeks of follow-up. Future controlled, clinical studies should further examine the safety and efficacy of the use of the NP-PWD.
Introduction
The effects of negative pressure wound therapy (NPWT) on wound healing have been studied extensively over the past few decades, as this therapy was found to drastically improve healing of open wounds by decreasing bacterial burden, decreasing interstitial fluid, promoting blood flow, and most importantly, encouraging formation of granulation tissue.1–3 On a cellular level, NPWT promotes pro-inflammatory gene expression, which initiates migration and differentiation of cells involved in angiogenesis and the proliferative stages of wound healing.4
More recently, NPWT has been utilized to prevent complications after wound closure, such as dehiscence, surgical site infection, hematoma, and seroma, in which case the device is utilized on closed incisions, as opposed to within the wound as for complicated, non-healing wounds.5 Patients at high risk of wound complications due to multiple comorbidities, such as hypertension, diabetes, body mass index > 35 kg/m2, and peripheral vascular disease, have been pinpointed to receive the most benefit from closed incision negative pressure therapy (ciNPT), although studies have shown mixed results.6–13 A meta-analysis examining the use of ciNPT found a 51% relative risk reduction of wound infection for wounds treated with ciNPT when compared with control.14
Although significant improvements in wound healing have been seen with use of this therapy, it should be noted that opaque foam placed on incisions prevents visibility of the wound, an important factor in assessments, especially in the days immediately following intervention. In our experience, another disadvantage of foam dressing is that it requires a high negative pressure (such as −125 mm Hg) that may cause discomfort and can lead to decreased microvascular blood flow at the wound edges.15 In a study, 63% of closed incisions following arthroplasty developed blisters at the sponge-adhesive dressing interface following ciNPT, requiring the authors to stop the study early.16
The Negative Pressure Platform Wound Dressing (NP-PWD) is a ciNPT system that does not utilize an interface dressing material. It was conceptualized and developed to mitigate limitations the authors have recognized of current devices requiring foam or gauze. The dressing consists of a transparent yet impermeable polyurethane membrane that is secured to the healthy skin surrounding the incision site by an integral self-adhesive that includes a peel-away backing for easy application. Once applied, the dressing has a connection port that attaches to a suction pump. Negative pressure is then delivered at the desired level. The membrane has a grid-like embossment that is pulled into contact with the skin or wound surface once negative pressure is applied, providing uniform negative pressure throughout the area covered by the dressing. The embossment is designed to evacuate fluid and exudate from the wound through a network of channels created by small pyramids and to evenly distribute the NPWT across the treatment area. The transparency of the membrane allows for continuous assessment of the wound without having to remove the dressing. In addition, preclinical studies in swine have shown that the impermeable membrane and lack of foam or gauze reduces the negative pressure required to maintain therapeutic effects in comparison with other NP devices (Fig. 1).17
Fig. 1.

Depiction of the Negative Pressure Platform Wound Dressing (NP-PWD). Here the embossed surface created by small pyramids can be visualized as well as the adhesive rim that surrounds the dressing. The access port is connected to the pump to provide negative pressure. The NP-PWD fully assembled includes the dressing, tubing, and pump.
Prior studies have shown that the NP-PWD has promoted wound healing by increasing granulation tissue and neo collagen formation at negative pressures of −80 mm Hg and −50 mm Hg in the preclinical setting. The round NP-PWD dressing utilized in these studies were found to reduce tissue necrosis, inflammation, and bacterial burden in porcine full-thickness wounds.17 The NP-PWD was also compared with the 3 commonly used ciNPT devices that utilize foam or gauze: the V.A.C.VIA, the PREVENA (3M, Saint Paul, Minn.), and the PICO (Smith and Nephew, London, UK). The study by Nuutila et al demonstrated that, in a porcine model, the NP-PWD performed equally, in terms of reduction in wound area, re-epithelialization, and vascularity, when compared with these conventional devices. The wound area reduction in the NP-PWD treated wounds was 41.4 ± 5.3%, which was significantly more than the PICO and VIA treated wounds (P = 0.0403 and P = 0.014, respectively). There was no statistically significant difference in re-epithelialization or amount of bleeding between groups. The current case series was designed as the first evaluation of the NP-PWD in patients.
Materials and Methods
Subject Enrollment
This study was performed under the IntegReview IRB with protocol number NP-PWD-01. Potential subjects were screened over 5 months at a single center as part of this prospective case series study. Inclusion criteria included any patient 18–85 years of age scheduled to undergo a surgical intervention for which an incision would be made and primarily closed at the time of operation. Exclusion criteria included patients with active infection, use of immunosuppressive agents, radiation or chemotherapy within the past 30 days, pregnancy, or inability to give informed consent. Comorbidities screened for included hypertension, diabetes, coronary artery disease, history of smoking, and active malignancy, among others. All patients enrolled in the study gave written informed consent before enrollment.
Negative Pressure Platform Wound Dressing
Negative Pressure Platform Wound Dressings (NP-PWDs; Applied Tissue Technologies LLC, Hingham, Mass.) were used with the InVia Motion NPWT pump (Medela, Baar, Switzerland) at −80 mm Hg continuous pressure in the study. A negative pressure tube was connected to each PWD and to the negative pressure pump. Oblong NP-PWD with wound openings measuring 1” × 3” (Part no. AT1073-01) and 3” × 5” (Part no. AT1074-01) were utilized in this study (Fig. 1).
Study Design
Immediately following incision closure in the operating room, the length of the incision was measured and photographs obtained. The NP-PWD (Applied Tissue Technologies LLC, Hingham, Mass.) was applied using sterile techniques. Excess hair adjacent to the wound was removed with a razor before application. The backing on the underside of the NP-PWD was then removed and the dressing was gradually applied, so that the center of the NP-PWD was directly over the center of the wound and the adhesive was in contact with the intact skin. The InVia motion pump, collection canister, and tubing were then assembled, and the pump was securely connected to the NP-PWD. The pump was then powered on and negative pressure was set to −80 mm Hg. The NP-PWD was removed at the first postoperative check (POC) between postoperative days (PODs) 3–5. Patients were required to undergo an additional POC between PODs 9–14.
Incision Assessment
POCs consisted of incision assessment, measurement, photography, and adverse event screening. Assessments specifically addressed erythema, drainage, itching, and pain at the incision site. At each POC, 2 members of the study team indicated presence or absence of the above physical examination findings. The same study members assessed the incisions at each time point to maintain consistency. Evaluation of erythema and drainage were made qualitatively by members of the study team, and assessment of itching and pain were made based on patient reports. Incision measurements were recorded in centimeters at each POC by members of the study team. A digital photograph of each wound was obtained at each POC as well. All AEs were recorded and assessed for relation to the NP-PWD.
Results
No difficulties directly related to patients’ willingness to participate in the study were identified. All patients enrolled in the study maintained the dressing for the duration intended. All patients enrolled in the study were present at both of their scheduled POCs, and no patients were lost to follow-up.
There was no difficulty applying the dressing. Because no adjustments to size or shape need to be made to the dressing before application, those applying the dressing felt very comfortable doing so after just 1–2 applications.
A total of 8 non-consecutive patients with 10 incisions were included in the study. Five patients were men. Three patients were women. Half of the patients enrolled were Hispanic (4, 50%), and half were White (4, 50%). The median age was 56 years (IQR 53–74 years), and median BMI was 28.4 (IQR 25.2–35) (Table 1).
Table 1.
Overview of Demographic Data
| Demographics | |
|---|---|
| Total number enrolled, n | 8 |
| Age (y), median (IQR) | 56 (53.5, 74) |
| Gender, n (%) | |
| Men | 5 (62.5) |
| Women | 3 (37.5) |
| Ethnicity, n (%) | |
| White | 4 (50) |
| Hispanic | 4 (50) |
| BMI, median (IQR) | 28.4 (25.2, 35) |
BMI, Body mass index; IQR, Interquartile range.
Comorbidities
All patients had at least 1 comorbidity recorded. The most common comorbidities present were hypertension (n = 6), active malignancy (n = 4), and type 2 diabetes mellitus (n = 2). All comorbidities are listed in Table 2. Operations ranged from full-thickness skin graft donor site obtained from the groin (n = 1), panniculectomy (n = 2), coronary artery bypass graft (CABG, n = 2), open mitral valve repair (n = 1), hemicolectomy (n = 1), mastectomy (n = 2), and pulmonary resection (n = 1).
Table 2.
All Comorbidities
| Comorbidities | n (%) |
|---|---|
| Hypertension | 6 (75) |
| Active malignancy | 4 (50) |
| Type 2 diabetes mellitus | 2 (25) |
| Neuropathy | 2 (25) |
| Coronary artery disease | 2 (25) |
| GERD | 2 (25) |
| Asthma | 1 (12.5) |
| Chronic kidney disease | 1 (12.5) |
| Smoking | 1 (12.5) |
| Hyperlipidemia | 1 (12.5) |
| Atrial fibrillation | 1 (12.5) |
| Mitral valve stenosis | 1 (12.5) |
GERD, Gastroesophageal reflux disease.
Incision Length and Dressing Duration
Median initial incision length was 20 cm (IQR 7.4 cm–20 cm). Incision length remained the same throughout the duration of the treatment. For the majority of incisions, the dressing remained in place for 3 days (60%). All dressings were removed by POD 5 (Table 3).
Table 3.
Incision Length and NP-PWD Duration
| Incision Number | Operation | Incision Length (cm) | Duration (d) |
|---|---|---|---|
| 1 | FTSG donor site | 8 | 4 |
| 2 | Panniculectomy | 20 | 3 |
| 3 | Panniculectomy | 20 | 3 |
| 4 | CABG | 20 | 3 |
| 5 | CABG | 20 | 3 |
| 6 | Open mitral valve repair | 23 | 4 |
| 7 | Hemicolectomy | 20 | 3 |
| 8 | Mastectomy | 7 | 5 |
| 9 | Mastectomy | 7 | 5 |
| 10 | Pulmonary resection | 7.5 | 3 |
CABG, coronary artery bypass graft; FTSG: full-thickness skin graft.
Incision Assessment
At the first POC, all incisions were observed to have serosanguinous (SS) drainage. Less than half of the incisions were erythematous (40%) and only 2 were reported to be painful (20%). By the second POC, all SS drainage had resolved. Three incisions were found to be erythematous (30%), 1 was reported to be itchy (10%), and none were painful (Table 4, Figs. 2–6). No wound breakdown or infection was observed. There were 3 adverse events: small blisters were noted under the adhesive of 2 incisions, and for 1 patient, tubing was disconnected upon transfer from the ICU, resulting in interruption of therapy.
Table 4.
Postoperative Check Assessments
| Incision Assessment | ||
|---|---|---|
| POD 3–6 | POD 9–14 | |
| Erythema, n (%) | 4 (40) | 3 (30) |
| Drainage, n (%) | 10 (100) | 0 (0) |
| Itching, n (%) | 0 (0) | 1 (10) |
| Pain, n (%) | 2 (20) | 0 (0) |
Fig. 2.
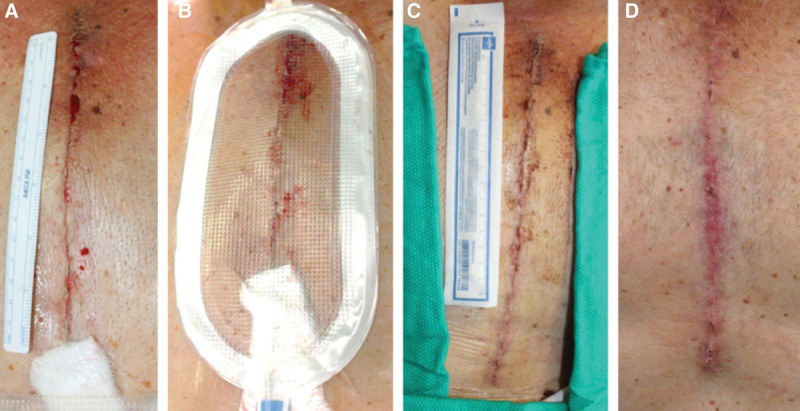
Median sternotomy. A, Closed incision before dressing application, Day 0. B, Following uneventful study dressing application and maintenance of –80 mm Hg pressure, Day 0. C, Immediately following dressing removal, Day 4. D, Incision appearance at Day 13. Of note, the cardiac surgeon added a gauze sponge to decrease pressure to the skin, where the closed suction Jackson-Pratt drain was traveling superficially.
Fig. 6.
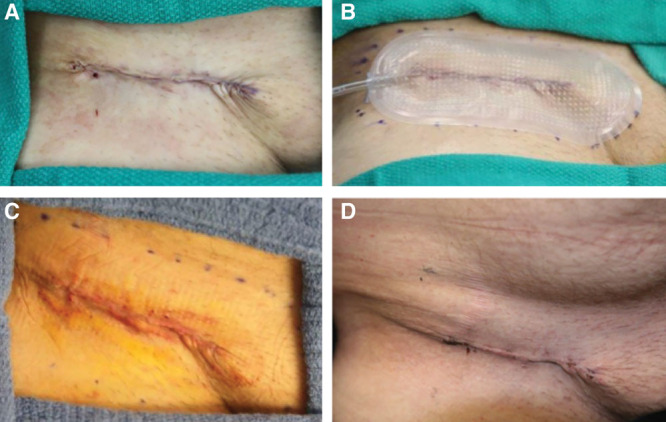
Full-thickness skin graft donor site (groin). A, Closed incision before dressing application, Day 0. B, Following uneventful study dressing application and maintenance of –80 mm Hg pressure, Day 0. C, Immediately following dressing removal, Day 4. D, Incision appearance at Day 13.
Fig. 3.
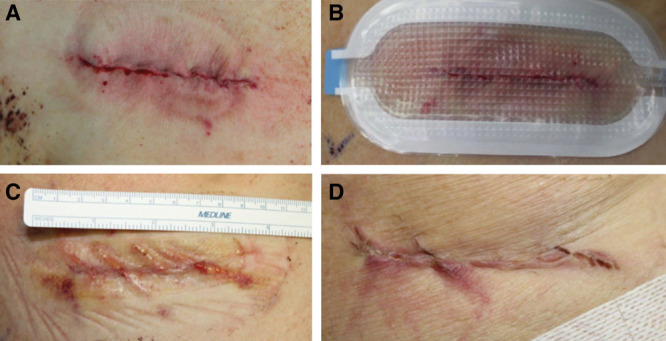
Thoracotomy. A, Closed incision before dressing application, Day 0. B, Following uneventful study dressing application and maintenance of –80 mm Hg pressure, Day 0. C, Immediately following dressing removal, Day 3. D, Incision appearance at Day 9.
Fig. 4.
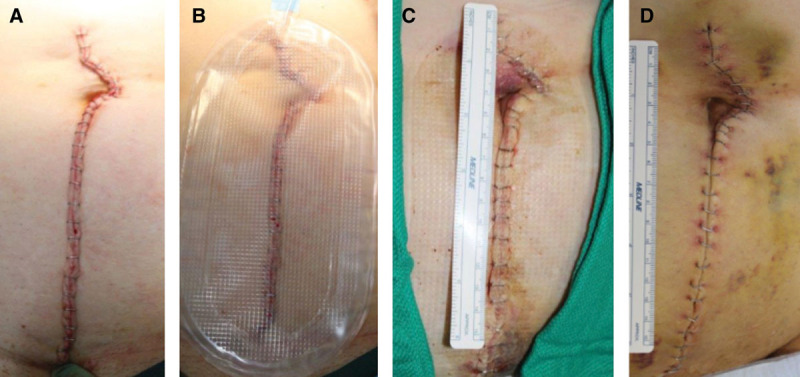
Laparotomy. A, Closed incision before dressing application, Day 0. B, Following uneventful study dressing application and maintenance of –80 mm Hg pressure, Day 0. C, Immediately following dressing removal, Day 3. D, Incision appearance at Day 14.
Fig. 5.
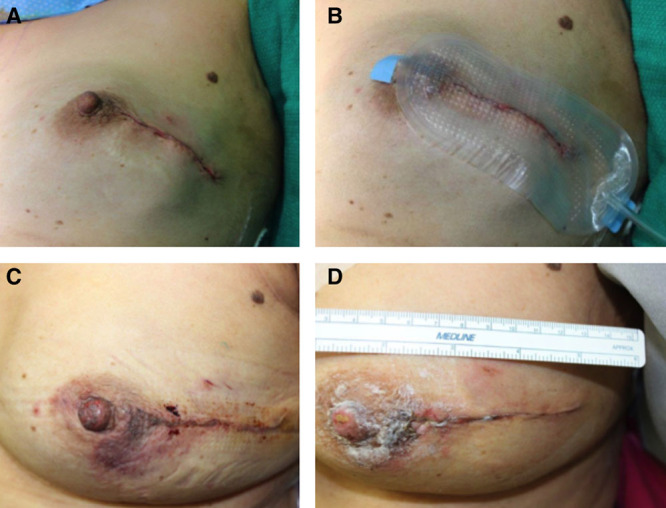
Breast reconstruction. A, Closed incision before dressing application, Day 0. B, Following uneventful study dressing application and maintenance of –80 mm Hg pressure, Day 0. C, Immediately following dressing removal, Day 5. D, Incision appearance at Day 14.
Discussion
This study was the first to examine the use of the NP-PWD in the clinical setting. In this study, the study dressing was applied onto a variety of closed surgical incisions following operations to different areas of the body, including the breast, chest, abdomen, and groin. No wound infection occurred during this study. Of note, the patients included in this study had significant comorbidities, including diabetes and active malignancies, that are known to impair wound healing.5,6 The dressing was easy to apply by the clinical staff, patients did not report any intolerable side effects, and incisions healed well without signs of infection or dehiscence. In the case of the blisters that developed under the adhesive of 2 incisions, this was felt to more likely be due to incision location (panniculectomy) and patient factors, as opposed to the device itself.
This study adds to the current body of literature demonstrating the benefits of NPWT on the healing of closed incisions. Although literature on ciNPT is mixed in terms of the benefits and ideal patient population, overall data are in support of ciNPT.8–10,13 Stannard et al studied the use of ciNPT on both draining hematomas and surgical incisions with high risk of wound healing complications and found that in both cases, drainage was significantly decreased, but no differences were seen in infection rate or wound dehiscence.18 Other studies showed that ciNPT decreases healing time, wound dehiscence, infection, and overall complication rate.5,19–21 A meta-analysis examining 11 studies that compared ciNPT with control found a 51% relative risk reduction of wound infection in the ciNPT group as 15% of wounds treated with ciNPT developed infection when compared with 28% in the control group.14
The NP-PWD is a novel ciNPT that provides an easy-to-apply, transparent, foam-less dressing that can be placed in the OR and removed within days after incision creation, although the device has its own shortcomings.22 First, the dressing cannot be altered in size and thus can only be applied to incisions or wounds that are less than or equal to the size of the dressing or multiple NP-PWDs that must be placed (many sizes and shapes are available from the manufacturer). In cases where multiple NP-PWDs are placed on 1 incision, the adhesive portion of the dressing could possibly overlap the surgical incision. Additionally, carrying the suction pump could be cumbersome to patients, especially in addition to other necessary postoperative closed suction drains. Although not all of these shortcomings are necessarily unique to this dressing, they are still factors to take into consideration when determining the utility of the NP-PWD in each specific clinical scenario.
The strengths of this study include that the study design proved to be reasonable in terms of both patient and provider participation, and no significant concerns were identified regarding the dressing within this case series, such as poor wound healing, infection, or device intolerance by enrolled patients. Incisions healed well over the course of the study, with the majority being free of drainage, erythema, itching, and pain by 14 days postoperatively. Limitations include that, as for all single-institution studies, the results are only representative of the population at 1 institution and so further multi-institutional studies must be performed to generalize the results. The small number of patients included in the study and the majority of data collected being qualitative serve as limitations as well. Selection bias was present during this study because only patients with planned surgical interventions were included. We recognize that measurement bias and lack of control are inherent limitations of case series that were present within this study as well. Overall, this case series provides preliminary data based on which additional, more rigorous studies evaluating the use of NP-PWD on closed incisions to improve wound healing can be developed.
CONCLUSIONS
This study was the first evaluation of the NP-PWD in the clinical setting. The dressing proved to be easy to apply and tolerable for patients. Blisters were noted underneath the adhesive of 2 incisions, and 1 patient experienced unintentional interrupted therapy. In summary, this case series reports that patients tolerated the NP-PWD on closed surgical incisions well and that all incisions were intact without evidence of inflammation or infection after 2 weeks of follow-up. Future controlled, clinical studies should further examine the safety and efficacy of the use of the NP-PWD.
Footnotes
Published online 11 March 2021.
Disclosure: Dr. Eriksson is the founder and chief medical officer of Applied Tissue Technologies LLC. He has not participated in the evaluation or recording of the results or the formulation of the conclusions or summary. All the other authors have no financial interest to declare in relation to the content of this article. Funding was received as a Small Business Innovation Research award from the Department of Defense (to Dr. Eriksson) for this study.
References
- 1.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997; 38:553–562 [DOI] [PubMed] [Google Scholar]
- 2.Venturi ML, Attinger CE, Mesbahi AN, et al. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) Device: a review. Am J Clin Dermatol. 2005; 6:185–194 [DOI] [PubMed] [Google Scholar]
- 3.Suh H, Lee A, Park E, et al. Negative pressure wound therapy on closed surgical wounds with dead space. Ann Plast Surg. 2016; 76:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Zhang L, Li T, et al. Negative-pressure wound therapy enhances local inflammatory responses in acute infected soft-tissue wound. Cell Biochem Biophys. 2014; 70:539–547 [DOI] [PubMed] [Google Scholar]
- 5.Stannard JP, Volgas DA, McGwin G, III, et al. Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma. 2012; 26:37–42 [DOI] [PubMed] [Google Scholar]
- 6.Masden D, Goldstein J, Endara M, et al. Negative pressure wound therapy for at-risk surgical closure in patients with mulltiple comorbidities. Ann Surg. 2012; 255:1043–1047 [DOI] [PubMed] [Google Scholar]
- 7.Manoharan V, Grant AL, Harris AC, et al. Closed incision negative pressure wound therapy vs conventional dry dressings after primary knee arthroplasty: a randomized controlled study. J Arthroplasty. 2016; 31:2487–2494 [DOI] [PubMed] [Google Scholar]
- 8.Keeney JA, Cook JL, Clawson SW, et al. Incisional negative pressure wound therapy devices improve short-term wound complications, but not long-term infection rate following hip and knee arthroplasty. J Arthroplasty. 2019; 34:723–728 [DOI] [PubMed] [Google Scholar]
- 9.Sandy-Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post-surgical wound complications: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2015; 13:253–303 [DOI] [PubMed] [Google Scholar]
- 10.Gabriel A, Sigalove S, Sigalove N, et al. The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes. Plast Reconstr Surg Glob Open. 2018; 6:e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler AL, Barry MK. Closed incision negative pressure therapy: results of recent trials and recommendations for clinical practice. Surgeon. 2020; 18:241–250 [DOI] [PubMed] [Google Scholar]
- 12.Karlakki S, Brem M, Giannini S, et al. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery. Bone Joint Res. 2013; 2:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon J, Staley C, McCullough M, et al. A randomized clinical trial evaluating negative pressure therapy to decrease vascular groin incision complications. J Vasc Surg. 2018; 68:1744–1752 [DOI] [PubMed] [Google Scholar]
- 14.Tran BNN, Johnson AR, Shen C, et al. Closed-incision negative-pressure therapy efficacy in abdominal wall reconstruction in high-risk patients: a meta-analysis. J Surg Res. 2019; 241:63–71 [DOI] [PubMed] [Google Scholar]
- 15.Borgquist O, Ingemansson R, Malmsjö M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage. 2010; 56:60–67 [PubMed] [Google Scholar]
- 16.Howell R, Hadley S, Strauss E, et al. Blister formation with negative pressure dressings after total knee arthroplasty. Current Orthopaedic Practice. 2011; 22:176–179 [Google Scholar]
- 17.Nuutila K, Yang L, Broomhead M, et al. Novel negative pressure wound therapy device without foam or gauze is effective at −50 mmHg. Wound Repair Regen. 2019; 27:162–169 [DOI] [PubMed] [Google Scholar]
- 18.Stannard JP, Robinson JT, Anderson ER, et al. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. 2006; 60:1301–1306 [DOI] [PubMed] [Google Scholar]
- 19.Atkins BZ, Wooten MK, Kistler J, et al. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov. 2009; 16:140–146 [DOI] [PubMed] [Google Scholar]
- 20.Abatangelo S, Saporiti E, Giatsidis G. Closed Incision Negative-Pressure Therapy (ciNPT) reduces minor local complications in post-bariatric abdominoplasty body contouring: a retrospective case-control series. Obes Surg. 2018; 28:2096–2104 [DOI] [PubMed] [Google Scholar]
- 21.Patel AA, Wilcox K, Bhinder J, et al. Low complication rates using closed-incision negative-pressure therapy for panniculectomies: a single-surgeon, retrospective, uncontrolled case series. Plast Reconstr Surg. 2020; 146:390–397 [DOI] [PubMed] [Google Scholar]
- 22.Nuutila K, Yang L, Broomhead M, et al. PWD: treatment platform for both prolonged field care and definitive treatment of burn-injured warfighters. Mil Med. 2019; 184:e373–e380 [DOI] [PubMed] [Google Scholar]


