INTRODUCTION:
Dipeptidyl peptidase-4 (DPP-4) is a membrane-bound glycoprotein that acts as a receptor but also exists in a soluble form. It has been recognized as a mediator of inflammation and considered a biomarker in inflammatory bowel disease (IBD).
METHODS:
We evaluated a prospectively recruited cohort, consisting of 101 patients with IBD, using validated clinical indexes; 22 patients with ulcerative colitis (UC) underwent endoscopic evaluation. Fecal DPP-4 (fDPP-4) levels were analyzed and correlated with clinical scores, Mayo endoscopic score (in UC patients), serum DPP-4, C-reactive protein, and fecal calprotectin. Immunohistochemical staining for DPP-4 in intestinal biopsies was also performed.
RESULTS:
When compared with remitters, median fDPP-4 levels were higher in patients with ileal Crohn's disease (CD) (7,584 [1,464–7,816] vs 2,104 [630–2,676] ng/mL, P = 0.015) and lower in patients with UC exhibiting clinical activity (1,213 [559–1,682] vs 7,814 [2,555–7,985] ng/mL, P < 0.001). Patients with UC presenting endoscopic activity also had lower levels than remitters (939 [559–1,420] vs 7,544 [4,531–7,940] ng/mL, P = 0.006). Fecal DPP-4 discriminated clinical activity from remission with areas under the curve of 0.76 (95% confidence interval [CI] 0.58–0.94, P = 0.015) and 0.80 (95% CI 0.68–0.93, P < 0.001) in CD and UC, respectively; it allowed to differentiate endoscopic activity in patients with UC, with areas under the curve of 0.84 (95% CI 0.63–1.00, P = 0.009). Immunohistochemical analysis revealed higher DPP-4 apical expression in UC remitters, but no statistically significant differences were revealed between patients with ileal CD.
DISCUSSION:
Our results suggest that fDPP-4 can be used as a biomarker of IBD activity, particularly in UC. The expression profiles in intestinal tissue might represent a functional compartmentalization of DPP-4 expression.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses 2 main distinct forms, Crohn's disease (CD) and ulcerative colitis (UC). These conditions are characterized by repeated episodes of mucosal inflammation and result in marked alterations in intestinal structure and function (1). Although the etiology of IBD is still unknown, the most accepted hypothesis states that the disease is initiated and maintained by an inappropriate immune response to unknown environmental or microbial antigen(s) in genetically susceptible individuals (1).
Although the diagnosis of IBD is still a challenge, over the past few years, several studies managed to identify key molecules involved in IBD pathogenesis. In this context, dipeptidyl peptidase-4 (DPP-4) emerged as a potential noninvasive biomarker of IBD activity and as a monitoring tool of the response to biological treatments (2). DPP-4, also known as the cell surface antigen CD26, is a transmembrane glycoprotein expressed in numerous tissues, including intestinal brush border membrane and cells of the immune system (3,4). Its activity and expression have been studied in epithelial cells from different regions of the human small intestine and colon. Protein activity and expression levels are high in the ileum and jejunum, low in the duodenum, and not detectable in the colon (5). DPP-4 is also present in a soluble form believed to result from specific proteolytic cleavage of the membrane-bound form, through a process called shedding, mediated by various enzymes in a cell type-specific manner (6). Potent cytokines such as RANTES (regulated on activation, normal T cell expressed, and secreted) and tumor necrosis factor-α (TNF-α) are some of the substrates described for DPP-4, suggesting a complex immunomodulatory role of DPP-4 in serum (7–10).
Although published data regarding DPP-4 expression among patients with IBD are scarce, a few studies demonstrated that when compared with healthy controls, patients with IBD present lower levels of circulating/serum DPP-4 and higher membrane expression of CD26 in T lymphocytes (11–13). DPP-4/CD26 was associated to the pathogenesis of IBD because of its involvement in immune regulation, extracellular matrix formation, and tissue remodeling. Our group also demonstrated that serum DPP-4 (sDPP-4) is able to discriminate patients with IBD in remission from those with active disease (clinical and endoscopic activity), with low levels indicating mucosal inflammation. Baseline sDPP-4 levels proved to be important predictors for treatment escalation and showed to be useful to monitoring response to IBD treatment, thus avoiding unnecessary treatments and lowering the risk of adverse events (2).
In this context and considering the demonstrated data about the different expression profiles of DPP-4 in patients with IBD, the study of the correlation of fecal DPP-4 (fDPP-4) with IBD can contribute to further explain the role of this protein in this disease.
This prospective study aims to clarify the role of fDPP-4 as a biomarker of IBD activity and to correlate it with clinical parameters and other biomarkers, namely: sDPP-4, fecal calprotectin (FC), and C-reactive protein (CRP). In addition, our study intends to assess the mechanisms underlying the variations of DPP-4 expression levels in IBD, through the evaluation of the expression of DPP-4/CD26, in intestinal samples of patients with IBD.
MATERIAL AND METHODS
Patients and study design
This study included 101 patients diagnosed with CD and UC previously recruited for another study performed by our research group (2). The cohort included active and remitting patients with IBD attending routine outpatient consultations or who were admitted to the hospital because of an IBD flare. A control group of 40 healthy volunteers was recruited from phlebotomy clinics for blood and fecal samples collection purposes. These were randomly selected after screening with a standardized questionnaire to ensure no medical history of IBD. An additional 10 individuals who underwent ileocolonoscopy for non-IBD/noninflammatory diseases were included for immunohistochemical analysis. Inclusion and exclusion criteria have been previously described (2). The local Research Ethics Committee granted approval for this study. All patients signed an informed written consent.
Demographic and clinical data were collected at baseline. Disease location and clinical phenotypes were evaluated with the Montreal classification (14). Disease activity was assessed using the Harvey-Bradshaw index (HBI) (15) (clinical remission ≤4) for patients with CD and the partial Mayo Score (pMS) (16) (clinical remission ≤1) for patients with UC.
This article was designed to meet the Standards for Reporting Diagnostic Accuracy requirements (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A521).
Biomarkers assessment
Stool samples were collected and kept at 4 °C, for a maximum of 48 hours, until shipment to the laboratory. Fecal DPP-4 was extracted from stool samples, within a maximum of 7 days after collection using the Fecal Sample Preparation kit (Roche Diagnostics, Mannheim, Germany) and stored at −80 °C until quantification. These fecal samples underwent only one freeze-thaw cycle. DPP-4 levels were measured using a quantitative enzyme-linked immunosorbent assay (Sigma-Aldrich, St. Louis, MO), according to the manufacturers' instructions.
The protocols for the measurement of fecal calprotectin, sDPP-4, and serum C-reactive protein were followed as described in a previous study (2).
Endoscopic assessment
Endoscopic procedures were performed on some of the patients in the context of a previous study, with the endoscopist blinded for the biomarker results (2). The endoscopic reports (including photographs) were reviewed by an IBD physician (F.M.) to grade endoscopic activity. Because of the low number of patients with ileal CD with endoscopic evaluation, the authors used these data only for patients with UC. In these patients, endoscopic activity was assessed according to the Mayo endoscopic score, which reflects the severity of intestinal inflammation on colonoscopy as follows: 0 points, normal or inactive disease; 1 point, mild disease (erythema, decreased vascular pattern, and mild friability); 2 points, moderate disease (marked erythema, absent vascular pattern, friability, and erosions); and 3 points, severe disease (spontaneous bleeding and ulcerations). Endoscopic remission was defined as a Mayo endoscopic score of 0 (17).
Immunohistochemical assessment
Biopsies and surgical resections of small intestine and colon from 25 patients with IBD (UC: 13 colon samples; CD: 12 ileum samples) and 10 healthy volunteers (5 ileum and 5 colon samples) were managed following conventional protocols. Immunohistochemical staining for DPP-4/CD26 was carried out in formalin-fixed and paraffin-embedded tissues. These were immunostained with a monoclonal antibody specific for DPP-4/CD26 (Novus Biologicals NB 100–59021, rabbit anti-human, working dilution 1:800) using the BenchMark ULTRA automatic system (Ventana Medical Systems, Oro Valley, AZ). Reactions were revealed using the UltraView Universal DAB (Ventana Medical Systems). Immunohistochemical expression of DPP-4/CD26 was evaluated in the epithelial apical side, cytoplasm, and remaining membrane. Membranous and cytoplasmic expression of DPP-4 was semiquantitatively analyzed. The scoring system was mainly based on membranous staining: 0, no membranous staining of epithelial cells; 1+, membrane staining in up to 25% of epithelial cells; +2, membrane staining observed in 26%–50% of epithelial cells; and +3, membrane staining in >50% of epithelial cells.
Statistical analyses
Categorical variables are described as absolute and relative frequencies and analyzed using Fisher exact tests. Continuous variables are presented as median and interquartile range (for asymmetrical distributions). When testing hypothesis concerning continuous variables, the nonparametric Mann-Whitney test was used as appropriate, taking into account normality assumptions and the number of compared groups. Correlation between variables was assessed using the Spearman rank-order correlation test. Receiver operating characteristics (ROC) curves were generated by plotting sensitivity against specificity, and the areas under the curve (AUC) were calculated. The level of statistical significance was set at 0.05. Statistical Package for Social Sciences V.25.0 (IBM SPSS Statistics for MacOS, Armonk, NY) and GraphPad Prism V.8.00 for Mac OS X (GraphPad Software, La Jolla, CA) were used for the statistical analysis and plot design.
RESULTS
Cohort characterization and disease outcomes
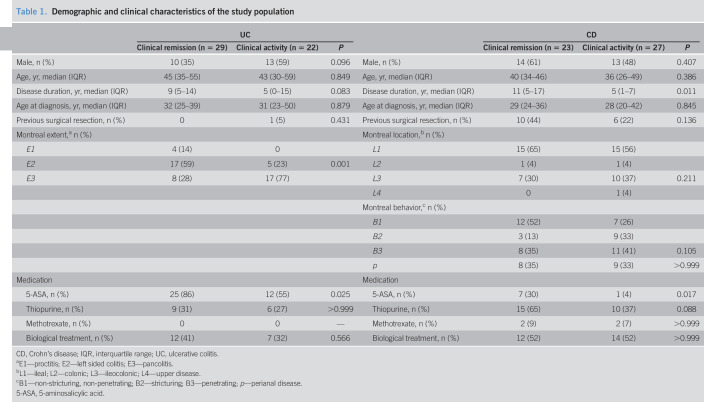
The cohort enrolled in this study included 101 patients with IBD (50 with CD and 51 with UC), and 40 healthy controls; of the former, 46% of the patients with CD and 57% of the patients with UC were in clinical remission (Table 1). Age, sex, disease duration, age at diagnosis, and need for previous surgical resection were similarly distributed among the groups, except for patients with CD in clinical remission, who had significantly longer disease duration. Montreal classification was equally balanced between patients with CD in remission and with active disease, but not between patients with UC: the E3 category was more frequent in patients with active disease (77%), whereas E1 and E2 categories were more frequent among remitters (14% and 59%, respectively). As for medication, remitters were mainly patients with CD and UC on 5-amino-salicylic acid (30% and 86%, respectively).
Table 1.
Demographic and clinical characteristics of the study population
| UC | CD | ||||||
| Clinical remission (n = 29) | Clinical activity (n = 22) | P | Clinical remission (n = 23) | Clinical activity (n = 27) | P | ||
| Male, n (%) | 10 (35) | 13 (59) | 0.096 | Male, n (%) | 14 (61) | 13 (48) | 0.407 |
| Age, yr, median (IQR) | 45 (35–55) | 43 (30–59) | 0.849 | Age, yr, median (IQR) | 40 (34–46) | 36 (26–49) | 0.386 |
| Disease duration, yr, median (IQR) | 9 (5–14) | 5 (0–15) | 0.083 | Disease duration, yr, median (IQR) | 11 (5–17) | 5 (1–7) | 0.011 |
| Age at diagnosis, yr, median (IQR) | 32 (25–39) | 31 (23–50) | 0.879 | Age at diagnosis, yr, median (IQR) | 29 (24–36) | 28 (20–42) | 0.845 |
| Previous surgical resection, n (%) | 0 | 1 (5) | 0.431 | Previous surgical resection, n (%) | 10 (44) | 6 (22) | 0.136 |
| Montreal extent,a n (%) | Montreal location,b n (%) | ||||||
| E1 | 4 (14) | 0 | L1 | 15 (65) | 15 (56) | ||
| E2 | 17 (59) | 5 (23) | 0.001 | L2 | 1 (4) | 1 (4) | |
| E3 | 8 (28) | 17 (77) | L3 | 7 (30) | 10 (37) | 0.211 | |
| L4 | 0 | 1 (4) | |||||
| Montreal behavior,c n (%) | |||||||
| B1 | 12 (52) | 7 (26) | |||||
| B2 | 3 (13) | 9 (33) | |||||
| B3 | 8 (35) | 11 (41) | 0.105 | ||||
| p | 8 (35) | 9 (33) | >0.999 | ||||
| Medication | Medication | ||||||
| 5-ASA, n (%) | 25 (86) | 12 (55) | 0.025 | 5-ASA, n (%) | 7 (30) | 1 (4) | 0.017 |
| Thiopurine, n (%) | 9 (31) | 6 (27) | >0.999 | Thiopurine, n (%) | 15 (65) | 10 (37) | 0.088 |
| Methotrexate, n (%) | 0 | 0 | — | Methotrexate, n (%) | 2 (9) | 2 (7) | >0.999 |
| Biological treatment, n (%) | 12 (41) | 7 (32) | 0.566 | Biological treatment, n (%) | 12 (52) | 14 (52) | >0.999 |
CD, Crohn's disease; IQR, interquartile range; UC, ulcerative colitis.
E1—proctitis; E2—left sided colitis; E3—pancolitis.
L1—ileal; L2—colonic; L3—ileocolonic; L4—upper disease.
B1—non-stricturing, non-penetrating; B2—stricturing; B3—penetrating; p—perianal disease.
5-ASA, 5-aminosalicylic acid.
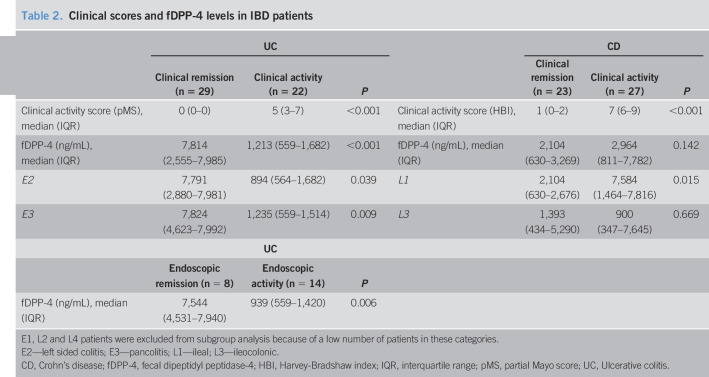
The main outcomes of the study are described in Table 2. Twenty-two patients with UC underwent colonoscopy, and according to the Mayo endoscopic score, 8 patients (36%) were in endoscopic remission and 14 (64%) had endoscopic activity.
Table 2.
Clinical scores and fDPP-4 levels in IBD patients
| UC | CD | ||||||
| Clinical remission (n = 29) | Clinical activity (n = 22) | P | Clinical remission (n = 23) | Clinical activity (n = 27) | P | ||
| Clinical activity score (pMS), median (IQR) | 0 (0–0) | 5 (3–7) | <0.001 | Clinical activity score (HBI), median (IQR) | 1 (0–2) | 7 (6–9) | <0.001 |
| fDPP-4 (ng/mL), median (IQR) | 7,814 (2,555–7,985) | 1,213 (559–1,682) | <0.001 | fDPP-4 (ng/mL), median (IQR) | 2,104 (630–3,269) | 2,964 (811–7,782) | 0.142 |
| E2 | 7,791 (2,880–7,981) | 894 (564–1,682) | 0.039 | L1 | 2,104 (630–2,676) | 7,584 (1,464–7,816) | 0.015 |
| E3 | 7,824 (4,623–7,992) | 1,235 (559–1,514) | 0.009 | L3 | 1,393 (434–5,290) | 900 (347–7,645) | 0.669 |
| UC | |||||||
| Endoscopic remission (n = 8) | Endoscopic activity (n = 14) | P | |||||
| fDPP-4 (ng/mL), median (IQR) | 7,544 (4,531–7,940) | 939 (559–1,420) | 0.006 | ||||
E1, L2 and L4 patients were excluded from subgroup analysis because of a low number of patients in these categories.
E2—left sided colitis; E3—pancolitis; L1—ileal; L3—ileocolonic.
CD, Crohn's disease; fDPP-4, fecal dipeptidyl peptidase-4; HBI, Harvey-Bradshaw index; IQR, interquartile range; pMS, partial Mayo score; UC, Ulcerative colitis.
Fecal DPP-4 levels were lower in patients with UC with clinical activity comparing with remitters (1,213 [559–1,682] vs 7,814 [2,555–7,985] ng/mL, P < 0.001) and healthy controls (1,213 [559–1,682] vs 2,842 [1,570–5,712] ng/mL, P = 0.019). However, remitters presented higher levels of fDPP-4 than healthy controls (7,814 [2,555–7,985] vs 2,842 [1,570–5,712] ng/mL, P = 0.006). Patients with UC exhibiting endoscopic activity also had lower levels than remitters (939 [559–1,420] vs 7,544 [4,531–7,940] ng/mL, P = 0.006) and healthy controls (939 [559–1,420] vs 2,842 [1,570–5,712] ng/mL, P = 0.004). CD patients with active ileal disease showed higher fDPP-4 than those in remission (7,584 [1,464–7,816] vs 2,104 [630–2,676] ng/mL, P = 0.015). No statistically significant differences were found among ileocolonic patients with CD (clinical remission: 1,393 [434–5,290] ng/mL vs clinical activity: 900 [347–7,645] ng/mL, P = 0.669), between patients with active ileal CD and healthy controls (7,584 [1,464–7,816] vs 2,842 [1,570–5,712] ng/mL, P = 0.086), and between patients with ileal CD in clinical remission and healthy controls (2,104 [630–2,676] ng/mL vs 2,842 [1,570–5,712] ng/mL, P = 0.061) (Table 2 and Figure 1).
Figure 1.
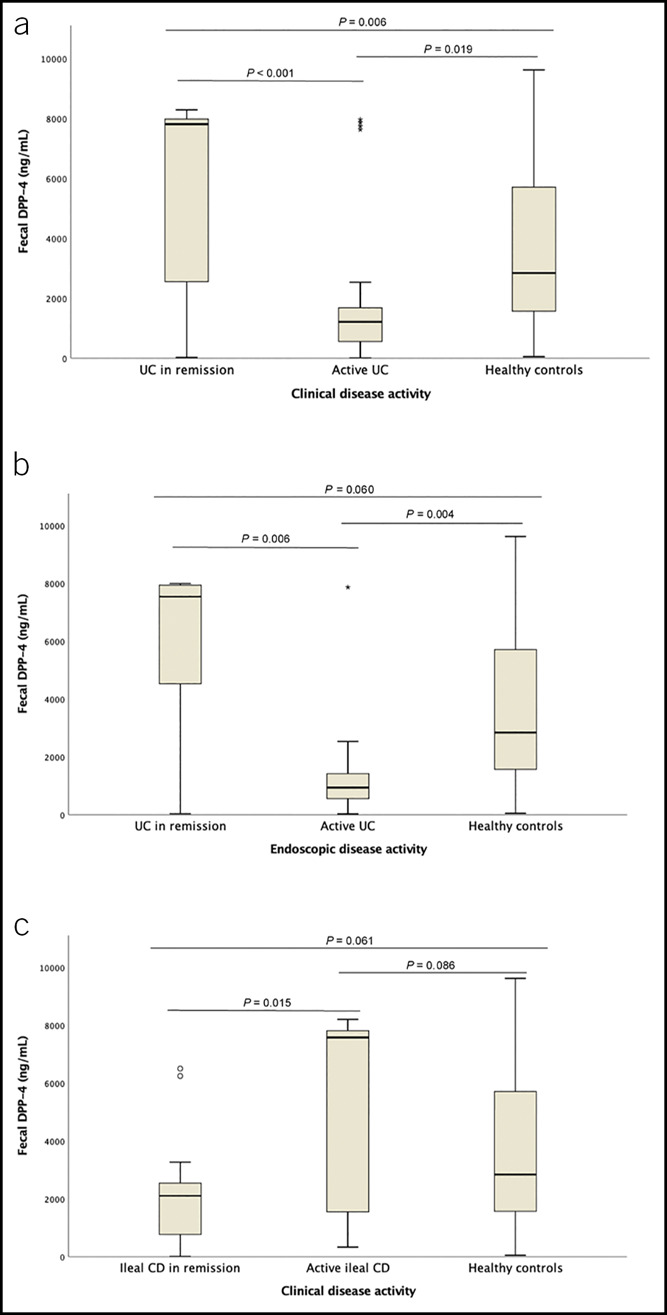
Comparison of median fecal DPP-4 levels in healthy controls and patients with UC regarding clinical activity (a) and endoscopic activity (b) and in healthy controls and patients with ileal CD regarding clinical activity (c). CD, Crohn's disease; DPP-4, dipeptidyl peptidase-4; UC, ulcerative colitis.
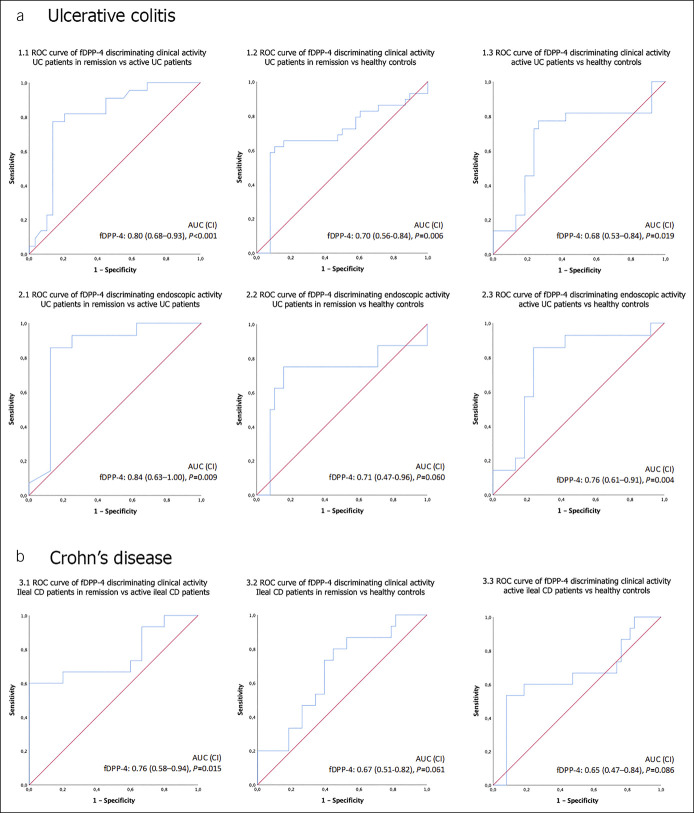
ROC curves confirmed that in UC (Figure 2a), fDPP-4 is able to discriminate between (i) healthy controls and patients in clinical remission and with clinical activity, with an AUC of 0.70 (95% confidence interval [CI] 0.56–0.84, P = 0.006) and 0.68 (95% CI 0.53–0.84, P = 0.019), respectively (Figure 2, 1.2 and 1.3); (ii) healthy controls and patients with endoscopic activity, with an AUC of 0.76 (95% CI 0.61–0.91, P = 0.004) (Figure 2, 2.3); (iii) patients with clinical activity and remitters, with an AUC of 0.80 (95% CI 0.68–0.93, P < 0.001) (Figure 2, 1.1); and (iv) patients with endoscopic activity and those in endoscopic remission, with an AUC of 0.84 (95% CI 0.63–1.00, P = 0.009) (Figure 2, 2.1). ROC curves also showed that in patients with ileal CD (Figure 2b), fDPP-4 can discriminate remitters from patients with clinical activity, with an AUC of 0.76 (95% CI 0.58–0.94, P = 0.015) (Figure 2, 3.1).
Figure 2.
Receiver operating characteristics (ROC) curve analysis of fecal DPP-4 in predicting clinical and endoscopic activity in patients with UC (a) and clinical activity in patients with ileal CD (b). 1.1—patients with UC in clinical remission vs patients with UC with clinical activity; 1.2—healthy controls vs patients with UC in clinical remission; 1.3—healthy controls vs patients with UC with clinical activity; 2.1—patients with UC in endoscopic remission vs patients with UC with endoscopic activity; 2.2—healthy controls vs patients with UC in endoscopic remission; 2.3—healthy controls vs patients with UC with endoscopic activity; 3.1—patients with ileal CD in clinical remission vs patients with ileal CD with clinical activity; 3.2—healthy controls vs patients with ileal CD in clinical remission; and 3.3—healthy controls vs patients with ileal CD with clinical activity. AUC, areas under the curve; CD, Crohn's disease; CI, confidence interval; DPP-4, dipeptidyl peptidase-4; fDPP-4, fecal DPP-4; UC, ulcerative colitis.
In patients with UC, fDPP-4 correlated positively with sDPP-4 (r = 0.52, P < 0.001) and CD26 intestinal immunostaining (r = 0.54, P = 0.049) and inversely correlated with other biomarkers such as FC (r = −0.43, P = 0.001) and serum CRP (r = −0.32, P = 0.022), as well as with pMS (r = −0.51, P < 0.001) and Mayo endoscopic score (r = −0.57, P = 0.005). In this group of patients, sDPP-4 correlated directly with CD26 intestinal expression (r = 0.72, P = 0.006). Patients with CD, with ileal involvement, presented a direct correlation between fDPP-4 and CRP (r = 0.43, P = 0.018) and between sDPP-4 and CD26 intestinal expression (r = 0.64, P = 0.025) (Table 3).
Table 3.
Correlation coefficients (R Spearman) between DPP-4 and other biomarkers, clinical indexes (HBI and pMS), Mayo endoscopic score, and immunohistochemical expression in IBD patients
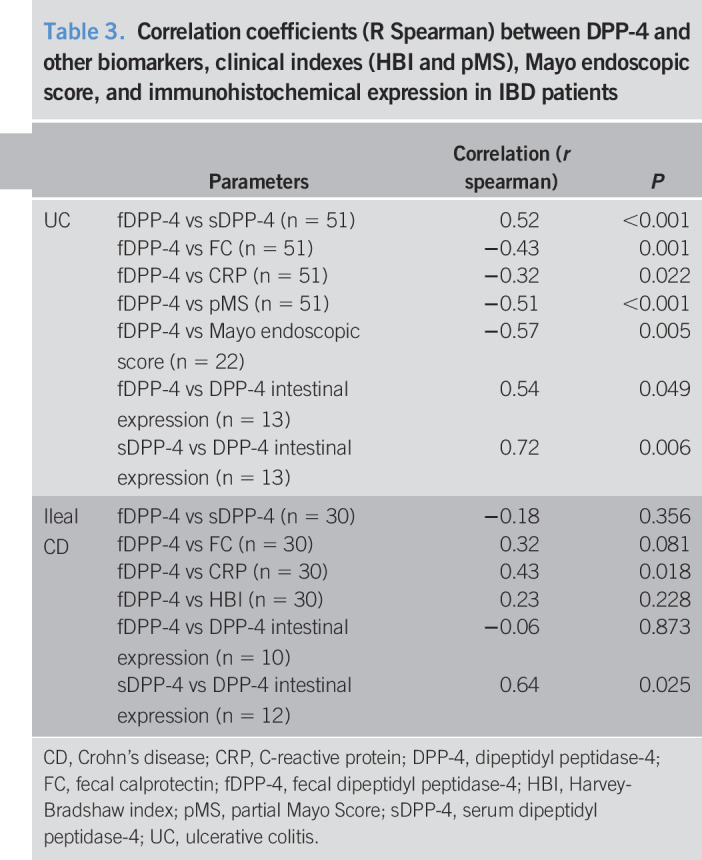
| Parameters | Correlation (r spearman) | P | |
| UC | fDPP-4 vs sDPP-4 (n = 51) | 0.52 | <0.001 |
| fDPP-4 vs FC (n = 51) | −0.43 | 0.001 | |
| fDPP-4 vs CRP (n = 51) | −0.32 | 0.022 | |
| fDPP-4 vs pMS (n = 51) | −0.51 | <0.001 | |
| fDPP-4 vs Mayo endoscopic score (n = 22) | −0.57 | 0.005 | |
| fDPP-4 vs DPP-4 intestinal expression (n = 13) | 0.54 | 0.049 | |
| sDPP-4 vs DPP-4 intestinal expression (n = 13) | 0.72 | 0.006 | |
| Ileal CD | fDPP-4 vs sDPP-4 (n = 30) | −0.18 | 0.356 |
| fDPP-4 vs FC (n = 30) | 0.32 | 0.081 | |
| fDPP-4 vs CRP (n = 30) | 0.43 | 0.018 | |
| fDPP-4 vs HBI (n = 30) | 0.23 | 0.228 | |
| fDPP-4 vs DPP-4 intestinal expression (n = 10) | −0.06 | 0.873 | |
| sDPP-4 vs DPP-4 intestinal expression (n = 12) | 0.64 | 0.025 |
CD, Crohn's disease; CRP, C-reactive protein; DPP-4, dipeptidyl peptidase-4; FC, fecal calprotectin; fDPP-4, fecal dipeptidyl peptidase-4; HBI, Harvey-Bradshaw index; pMS, partial Mayo Score; sDPP-4, serum dipeptidyl peptidase-4; UC, ulcerative colitis.
DPP-4/CD26 immunohistochemical evaluation
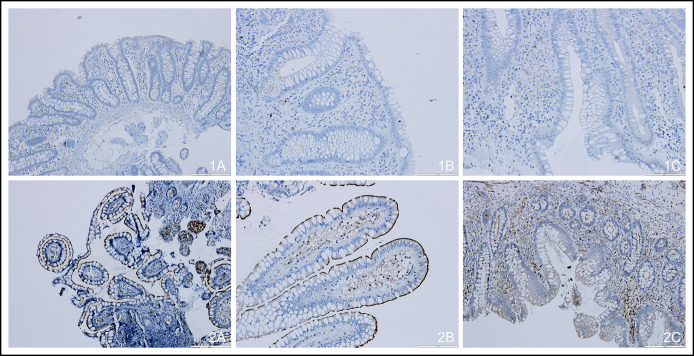
Figure 3 shows an example of the immunohistochemical expression of DPP-4/CD26 in healthy volunteers and in patients with IBD. Normal colon mucosa (Figure 3, 1A) did not express DPP-4/CD26, whereas the ileum (Figure 3, 2A) displayed positive luminal membrane reinforcement (P = 0.002). Sixty percent of the patients with UC in clinical remission (n = 5) presented some degree of apical DPP-4/CD26 expression (Figure 3, 1B) compared with none of those with clinical activity (n = 8) (P = 0.012) (Figure 3, 1C). All patients with CD with ileal disease in clinical remission (n = 5) showed high apical DPP-4/CD26 expression, in contrast to 29% of the patients with CD with clinical activity (n = 7) (Figure 3, 2B and 2C); nevertheless, these differences did not reach statistical significance (P = 0.190). Although cytoplasm and basolateral membrane were always negative for DPP-4/CD26 expression, lymphocytes of lamina propria were positive for this marker in all cases, as expected (see Supplementary Table 2, Supplementary Digital Content, http://links.lww.com/CTG/A521).
Figure 3.
Immunohistochemical expression of DPP-4/CD26 in normal colon mucosa (1A, original magnification ×40), UC in clinical remission (1B, original magnification ×100), UC with clinical activity (1C, original magnification ×100), normal ileum mucosa (2A, original magnification ×40), ileal CD in clinical remission (2B, original magnification ×100), and ileal CD with clinical activity (2C, original magnification ×40). CD, Crohn's disease; DPP-4, dipeptidyl peptidase-4; UC, ulcerative colitis.
DISCUSSION
In this prospective study, we explored, for the first time, the potential of fDPP-4 as a biomarker of IBD activity. To further understand the role of DPP-4 in IBD, we evaluated the expression of DPP-4/CD26 in biopsies and surgical resections of small intestine and colon from patients with IBD and healthy volunteers.
Our study evidenced that fDPP-4 levels were lower in patients with UC with clinical and endoscopic activity compared with those in remission. Conversely, ileal patients with CD with clinical activity presented higher fDPP-4 levels than remitters. ROC curves confirmed that fDPP-4 is a biomarker able to discriminate clinical activity in patients with IBD (UC and CD) and endoscopic activity in patients with UC. It correlated well with other biomarkers such as sDPP-4, FC, and serum CRP and with clinical and endoscopic activity scores in patients with UC. These results resemble, in some way, those obtained in a previous study of our group in which we proved that sDPP-4 was able to discriminate IBD remitters from patients with active disease (clinical and endoscopic activity), with low levels indicating mucosal inflammation (2). In fact, in that study, lower levels of sDPP-4 were associated with disease activity, in both patients with CD and UC, and sDPP-4 was inversely correlated with both disease activity scores (HBI and pMS) and endoscopic activity groups. Experimental and clinical studies on other diseases such as systemic lupus erythematosus (18), rheumatoid arthritis (19), and colorectal cancer (20) also reported changes in sDPP-4 activity.
Our study also aimed to contribute toward a deeper knowledge on the mechanisms underlying the changes in DPP-4 levels in patients with IBD and on the origin of fDPP-4. Because free DPP-4 is originated from the membrane bounded form, the study of the shedding process is of utmost importance to clarify these aspects (21). Although DPP-4 shedding is poorly understood, recent studies attempted to unravel the molecular basis of this phenomenon. Röhrborn et al. (6) showed that DPP-4 shedding is based on a complex interplay between different matrix metalloproteases in a cell type-specific manner: MMP1, MMP2, and MMP14 are involved in DPP-4 shedding from human vascular smooth muscle cells and MMP9 from adipocytes. Nargis et al. verified that in patients with type 2 diabetes mellitus, circulatory Th17 cells express increased amounts of kallikrein-related peptidase 5 that consequently cleaves DPP-4 from the surface of Th17 cells, shedding them into circulation (22).
Although the knowledge on the tissues and cell types involved in the generation of the soluble pool of DPP-4 is limited, bone marrow derived cells, endothelium, adipocytes, enterocytes, and hepatocytes have been identified as important sources (23). Despite these advances, the origin and regulation of the circulating DPP-4 in various inflammatory diseases, including IBD, has not yet been clarified.
In this context, our immunohistochemical assays, implemented to evaluate the location and expression of DPP-4/CD26, lead to improved knowledge on the origin of serum and fecal DPP-4. The results obtained with healthy volunteers confirmed those from previous studies: ileal mucosa, unlike colon mucosa, expresses DPP-4/CD26 (4,5). We evidenced that DPP-4/CD26 is expressed in the lymphocytes of lamina propria in normal mucosa and in the mucosa of patients with IBD. With these results, we hypothesize that fDPP-4 is originated mainly by shedding from small intestine enterocytes because epithelial cells from normal colon mucosa did not express it. However, in patients with IBD DPP-4/CD26 was expressed in colon mucosa which also contributed to the pool of fDPP-4. UC and CD patients showed distinct immunohistochemical patterns. Patients with UC in remission presented higher immunohistochemical expression of DPP-4/CD26 and higher levels of both fDPP-4 and sDPP-4. These results suggest that this receptor/enzyme can be important to achieve and maintain remission in these patients, with a protective role against relapses. On the contrary, patients with CD with active ileitis presented lower sDPP-4 but higher levels of fDPP-4, without differences in the intestinal expression of DPP-4/CD26. We believe that this phenomenon can be associated to a loss of the receptor/enzyme, from the epithelium to the intestinal lumen. These observations might be associated with increased levels and activity of the MMPs responsible for the shedding of biologically active proteins from their membrane-anchored proforms. In fact, several animal model experiments and analysis of IBD patient biopsies demonstrated that the expression and activity of the MMPs is upregulated in this disease, especially MMP1, MMP3, MMP9, and MMP26 (24). Therefore, the different patterns of fecal and serum DPP-4 expression might be reflecting such activity in patients with IBD.
Through its enzymatic function, DPP-4 can modulate the activity of several mediators of immunity and inflammation. Therefore, we hypothesize that DPP-4 may be implicated in IBD pathogenesis being responsible for activation of proinflammatory or inactivation of anti-inflammatory mediators. Low levels of sDPP-4, as evidenced in patients with active IBD, are believed to be part of a counter-regulatory mechanism that could prevent the degradation of anti-inflammatory peptides, promoting mucosal healing (13).
This study has a few limitations: (i) patients were recruited from tertiary-level hospitals, and therefore, our findings may not be applicable to the overall IBD community; (ii) only patients with UC underwent endoscopic assessment; and (iii) the endoscopic evaluations and immunohistochemical assays were performed in a small population, and thus, we anticipate that larger prospective studies must be carried out to validate these results.
In conclusion, our results suggest that fDPP-4 can be used as a noninvasive biomarker of IBD activity, particularly in patients with UC. We believe that the expression profiles of DPP-4, previously described in other processes, are unlikely to represent simple up/downregulations induced by inflammation. In fact, the DPP-4 variations herein evidenced are governed by more complex mechanisms, suggesting the existence of a functional compartmentalization of DPP-4 expression.
CONFLICTS OF INTEREST
Guarantor of the article: Fernando Magro, MD, PhD.
Specific author contributions: P.P.-L.: study concept and design, acquisition of data, recruitment of patients and collection of samples, analysis and interpretation of data, DPP-4 assays, drafting of the manuscript, study supervision, and critical revision of the manuscript. F.M.: DPP-4 assays and analysis and interpretation of data. J.A.: DPP-4 and fecal calprotectin assays and analysis and interpretation of data. R.P.-L.: recruitment of patients and collection of samples, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. C.R.: DPP-4 and fecal calprotectin assays. D.M.: histological analysis. G.M.: critical revision of the manuscript. C.C.D.: analysis and interpretation of data and statistical analysis. F.C.: histological analysis and critical revision of the manuscript. F.M.: study concept and design, acquisition of data, analysis and interpretation of data, study supervision, and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Financial support: This study was supported by the Portuguese IBD Study Group (GEDII—Grupo de Estudo da Doença Inflamatória Intestinal) and received a research grant from Janssen-Cilag Pharmaceuticals.
Potential competing interests: F.M. served as speaker and received honoraria from Merck Sharp & Dohme, AbbVie, Vifor, Falk, Laboratórios Vitoria, Ferring, Hospira, and Biogen. All other authors have nothing to declare.
Data sharing and data accessibility: All data relevant to the study are included in the article. Further information will be shared on reasonable request to the corresponding author.
Study Highlights.
WHAT IS KNOWN
✓ Dipeptidyl peptidase-4 (DPP-4) exhibit altered expression profiles in patients with inflammatory bowel disease (IBD).
✓ Serum DPP-4 is a biomarker of IBD activity and biological treatment response and predictor of treatment escalation.
✓ There are no published data about fecal DPP-4 or immunohistochemical assays of DPP-4/CD26 in patients with IBD.
WHAT IS NEW HERE
✓ Fecal DPP-4 levels were higher in ileal Crohn's disease and lower in patients with UC exhibiting clinical activity.
✓ Patients with ulcerative colitis (UC) with endoscopic activity had lower fecal DPP-4 levels than remitters.
✓ Immunohistochemical analysis revealed aberrant DPP-4 apical expression in patients with UC in remission.
TRANSLATION IMPACT
✓ Fecal DPP-4 can be used as a noninvasive biomarker of IBD activity, particularly in patients with UC.
✓ Therapeutic decisions may be implemented early enough for patients at higher risk of disease progression.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Paula Pinto, PharmD, PhD (PMA—Pharmaceutical Medicine Academy), for providing medical writing and editorial assistance.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A521
Contributor Information
Pedro Pinto-Lopes, Email: pedro.lopes.md@gmail.com.
Francisco Melo, Email: fjnmelo.w@gmail.com.
Joana Afonso, Email: joanavafonso@gmail.com.
Rui Pinto-Lopes, Email: plopes.rui@gmail.com.
Cátia Rocha, Email: catia.s.l.rocha@gmail.com.
Daniel Melo, Email: drpmdanny@gmail.com.
Guilherme Macedo, Email: guilhermemacedo59@gmail.com.
Cláudia Camila Dias, Email: camila@med.up.pt.
Fátima Carneiro, Email: fcarneiro@ipatimup.pt.
REFERENCES
- 1.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15(1):79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto-Lopes P, Afonso J, Pinto-Lopes R, et al. Serum dipeptidyl peptidase 4: A predictor of disease activity and prognosis in inflammatory bowel disease. Inflamm Bowel Dis 2020;26(11):1707–19. [DOI] [PubMed] [Google Scholar]
- 3.Gorrell MD. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin Sci (Lond) 2005;108(4):277–92. [DOI] [PubMed] [Google Scholar]
- 4.Bauvois B, De Meester I, Dumont J, et al. Constitutive expression of CD26/dipeptidyl peptidase IV on peripheral blood B lymphocytes of patients with B chronic lymphocytic leukaemia. Br J Cancer 1999;79(7–8):1042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darmoul D, Voisin T, Couvineau A, et al. Regional expression of epithelial dipeptidyl peptidase IV in the human intestines. Biochem Biophys Res Commun 1994;203:1224–9. [DOI] [PubMed] [Google Scholar]
- 6.Röhrborn D, Eckel J, Sell H. Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett 2014;588(21):3870–7. [DOI] [PubMed] [Google Scholar]
- 7.Oravecz T, Pall M, Roderiquez G, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidvl peptidase IV (CD26)-mediated cleavage. J Exp Med 1997;186(11):1865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proost P, De Meester I, Schols D, et al. Amino-terminal truncation of chemokines by CD26/dipeptidylpeptidase IV. Conversion of rantes into a potent inhibitor of monocyte chemotaxix and HIV-1-infection. J Biol Chem 1998;273(13):7222–7. [DOI] [PubMed] [Google Scholar]
- 9.Lambeir AM, Proost P, Durinx C, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem 2001;276(32):29839–45. [DOI] [PubMed] [Google Scholar]
- 10.Bauvois B, Sanceau J, Wietzerbin J. Human U937 cell surface peptidase activities: Characterization and degradative effect on tumor necrosis factor-alpha. Eur J Immunol 1992;22(4):923–30. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Q, Boushey RP, Cino M, et al. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol 2000;278:R1057–63. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt M, Rose M, Rüter J, et al. Dipeptidyl peptidase IV (DP IV, CD26) in patients with inflammatory bowel disease. Scand J Gastroenterol 2001;36(10):1067–72. [DOI] [PubMed] [Google Scholar]
- 13.Moran GW, O'Neill C, Padfield P, et al. Dipeptidyl peptidase-4 expression is reduced in Crohn's disease. Regul Pept 2012;177:40–5. [DOI] [PubMed] [Google Scholar]
- 14.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;315(8167):514. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: A randomized study. N Engl J Med 1987;317:1625–9. [DOI] [PubMed] [Google Scholar]
- 17.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat- to-target. Am J Gastroenterol 2015;110:1324–38. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H, Hosono O, Mimori T, et al. Reduction of serum soluble CD26/dipeptidyl peptidase IV enzyme activity and its correlation with disease activity in systemic lupus erythematosus. J Rheumatol 2002;29:1858–66. [PubMed] [Google Scholar]
- 19.Busso N, Wagtmann N, Herling C, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol 2005;166:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larrinaga G, Perez I, Sanz B, et al. Dipeptidyl-peptidase IV activity is correlated with colorectal cancer prognosis. PLoS One 2015;10(3):e0119436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol 2008;29(6):295–301. [DOI] [PubMed] [Google Scholar]
- 22.Nargis T, Kumar K, Ghosh AR, et al. KLK5 induces shedding of DPP4 from circulatory Th17 cells in type 2 diabetes. Mol Metab 2017;6(11):1529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nargis T, Chakrabarti P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life 2018;70(2):112–9. [DOI] [PubMed] [Google Scholar]
- 24.O'Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediators Inflamm 2015;2015:964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.