GIPR activity in α cells is required for the complete metabolic response to a meal.
Abstract
Glucose-dependent insulinotropic polypeptide (GIP) communicates nutrient intake from the gut to islets, enabling optimal levels of insulin secretion via the GIP receptor (GIPR) on β cells. The GIPR is also expressed in α cells, and GIP stimulates glucagon secretion; however, the role of this action in the postprandial state is unknown. Here, we demonstrate that GIP potentiates amino acid–stimulated glucagon secretion, documenting a similar nutrient-dependent action to that described in β cells. Moreover, we demonstrate that GIP activity in α cells contributes to insulin secretion by invoking paracrine α to β cell communication. Last, specific loss of GIPR activity in α cells prevents glucagon secretion in response to a meal stimulus, limiting insulin secretion and driving glucose intolerance. Together, these data uncover an important axis by which GIPR activity in α cells is necessary to coordinate the optimal level of both glucagon and insulin secretion to maintain postprandial homeostasis.
INTRODUCTION
The incretin axis is an essential component of normal glucose tolerance that links nutrient absorption from the gut to pancreatic islet hormone secretion. The current model of the incretin system is based on two gut-derived peptides, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), that are secreted in response to nutrient intake (1, 2). Both these peptides are potent insulin secretagogues that interact with specific G protein–coupled receptors (GPCRs) on islet β cells. GLP-1 also reduces circulating glucagon, an action that combined with its insulinotropic activity has led to the development of a range of new drugs for the treatment of diabetes (3). Of the two incretins, GIP has a much more robust secretory pattern, with a clear dose-dependent response to meal size and a much wider dynamic range than GLP-1 (4, 5). Recent evidence suggests that GIP makes a greater contribution than GLP-1 to prandial insulin secretion in healthy humans (6), although its effect is muted relative to GLP-1 in persons with type 2 diabetes (T2D) (7). Another important difference between the incretins is that GIP stimulates glucagon secretion from islet α cells.
The GIP receptor (GIPR) is expressed in similar amounts in α and β cells, and previous work in this area has universally reported that GIP stimulates glucagon secretion (8). This effect has been observed across a range of experimental systems from an α cell line (9), isolated rat α cells (10), perifused mouse islets (11), perfused rat pancreas (12), and systemic infusion into humans (13). In healthy humans, GIP only stimulates glucagon secretion at fasting glucose concentrations, leading to the proposal that GIP is a bifunctional glucose stabilizer, enhancing glucagon secretion to limit hypoglycemia and stimulating insulin secretion to lower hyperglycemia (13). However, these findings are based on studies with exogenously administered GIP and have limited extension to normal physiology. In particular, GIP is stimulated by meals, and concentrations are low in the fasting state. Thus, plasma GIP is not typically secreted during periods of eu- or hypoglycemia when stimulation of glucagon would be important for counter-regulation.
In contrast to nondiabetic humans, hyperglycemic subjects with T2D respond to infusions of GIP with increased glucagon secretion during hyperglycemia (14, 15). This enhanced response of diabetic α cells to GIP has been proposed to contribute to the relative hyperglucagonemia that is thought to contribute to prandial hyperglycemia (9, 14). However, recent evidence raises questions about this presumption. We (16, 17), and others (18, 19), have recently identified important interactions between α and β cells, by which nutrient-stimulated proglucagon gene products, including glucagon, robustly stimulate insulin secretion through paracrine interactions in the islet. We, and others, have shown that α to β cell communication is required for normal nutrient-stimulated insulin secretion (16, 18, 19) and that this mechanism permits exogenous glucagon to lower, not raise, glycemia through enhanced insulin secretion (17).
In the experiments described herein, we set out to investigate the role of GIP to stimulate α cells through the lens of α to β cell communication and the prandial insulin response. In summary, we observed that (i) GIP potentiates amino acid–stimulated glucagon secretion, revealing a nutrient-dependent relationship in the α cell that mimics the well-described glucose-dependent incretin stimulation in β cells, (ii) GIP-stimulated glucagon secretion at high glucose is capable of enhancing insulin secretion beyond direct GIPR activity in β cells alone, and (iii) specific deletion of Gipr in α cells produces glucose intolerance in response to a mixed nutrient stimulus that is associated with attenuated secretion of both glucagon and insulin. These findings support an important role for GIPR activity in α cells that links prandial amino acid flux to insulin secretion and glucose homeostasis to complement well-known glucose-based mechanisms.
RESULTS
GIP potentiates alanine-stimulated glucagon secretion
The defining function of incretin hormones is potentiation of glucose-stimulated insulin secretion, facilitated by agonism of the GIPR and GLP-1 receptor (GLP-1R) (1). We looked to extend this concept to incretin action in α cells and glucagon secretion. The GIPR is expressed in α cells to a similar degree documented in β cells (20) and increases glucagon secretion in some settings (9, 12, 13). In addition, the well-established effect of certain amino acids to robustly increase glucagon secretion raised the possibility of a nutrient-incretin synchronous action on α cells. We choose to study alanine because (i) it is the most potent amino acid α cell secretagogue in isolated islets (fig. S1A) and (ii) postprandial concentrations of alanine are among the highest of the amino acids (fig. S1B).
We tested potential interactions between GIP and alanine by first determining glucagon secretion in response to these stimuli alone and in combination in isolated islets from wild-type (WT) mice at low glucose concentrations (Fig. 1). Alanine alone produced a biphasic increase in glucagon levels, similar to what we have previously reported for glutamine, glycine, and arginine (Fig. 1A) (16). GIP alone produced a modest increase in glucagon secretion (Fig. 1B), resembling the levels produced during the second-phase alanine stimulation. The increase in GIP-stimulated glucagon secretion during low-glucose conditions is similar to previous reports (13, 21). However, GIP potently potentiated glucagon secretion when given together with alanine, exhibiting a synergistic relationship that has not been previously reported (Fig. 1, C and D). Insulin secretion was not altered by either GIP alone or alanine + GIP at low glucose concentrations (fig. S1C). Thus, the actions of GIP in α cells generally mimic those in β cells, where agonism of the GIPR potentiates glucose-stimulated insulin secretion. In α cells, GIP potentiates alanine-stimulated glucagon secretion.
Fig. 1. GIP potentiates alanine-stimulated glucagon secretion.
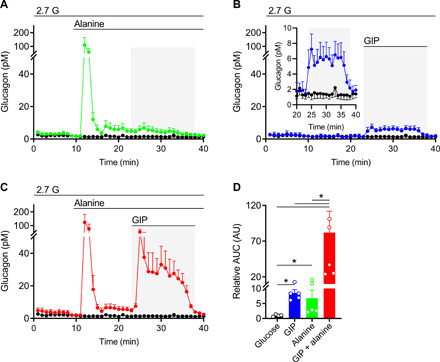
WT isolated mouse islets were perifused with alanine, GIP, or with both sequentially. (A) Glucagon secretion in response to (A) 3 mM alanine (green, n = 5), (B) 10 nM GIP (blue, n = 4; inset scaled to view GIP treated area), or (C) 3 mM alanine +10 nM GIP (red, n = 6). Control conditions, glucose (2.7 mM) alone, are shown in each panel (black, n = 4). (D) Relative area under the curve (AUC) was calculated for the stimulation period (23 to 38 min) for each condition and normalized to the glucose alone condition. *P < 0.05. Data are shown as means ± SEM.
The α cell GIPR is required for potentiation of alanine-stimulated glucagon secretion
The GIPR is a class B GPCR that is predominantly Gs coupled, suggesting that GIP agonism should increase cyclic adenosine 3′,5′-monophosphate (cAMP) production in α cells. We used a cAMP biosensor expressed exclusively in α cells and found that alanine alone led to a modest rise in cAMP levels, but alanine + GIP led to a prompt, steep rise in cAMP (Fig. 2A). Stimulating islets with GIP alone led to a similar rise in cAMP (Fig. 2A). Next, we measured changes in calcium levels specifically in α cells in response to alanine alone, GIP alone, and the combination of the two. GIP had no significant effect on increasing calcium levels in α cells (Fig. 2B), while alanine stimulation led to robust increases in calcium levels (Fig. 2B). Alanine + GIP was not additive for the level of calcium in α cells (Fig. 2B). These findings suggest that the synergy between alanine and GIP on glucagon secretion is the result of the interaction of these two independent mechanisms.
Fig. 2. The α cell GIPR is required for potentiation of alanine-stimulated glucagon secretion.
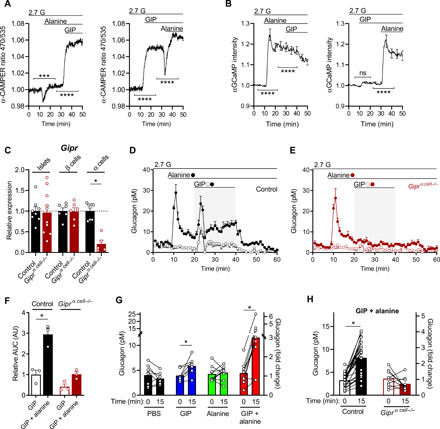
(A) cAMP levels were measured in response to alanine alone (3 mM) then alanine (3 mM) + GIP (10 nM), or GIP alone (10 nM) then alanine + GIP in isolated islets from α-CAMPER mice and reported as the emission ratio R470/535 (n = 131 and 124). ***P < 0.001, ****P < 0.0001 versus single treatment steady state. (B) Calcium levels measured in response to alanine alone (3 mM) then alanine (3 mM) + GIP (10 nM), or GIP alone (10 nM) then alanine + GIP in isolated islets from α-GCaMP mice and reported as normalized fluorescence intensity (F/F0) (n = 101 and 79). ****P < 0.0001 versus single treatment steady state. ns, not significant. (C) Gipr expression of whole-islet extracts or enriched populations of β cell populations or α cell populations in control versus Giprα cell−/− mice (n = 7 control and n = 6 Giprα cell−/−). (D and E) Glucagon secretion in islets isolated from (C) control or (D) Giprα cell−/− mice stimulated with GIP (10 nM) ± alanine (3 mM) (n = 3). (F) Relative AUC for GIP-stimulated glucagon secretion (min 20 to 40) for control or Giprα cell−/− islets. (G) Plasma glucagon levels in WT mice injected with phosphate-buffered saline (PBS) (n = 8), GIP (4 nmol/kg) (n = 8), alanine (0.325 g/kg) (n = 9), or GIP + alanine (n = 9). (H) Plasma glucagon levels in response to GIP + alanine injection in control (n = 27) versus Giprα cell−/− mice (n = 10). *P < 0.05, data are shown as means ± SEM.
To directly test the significance of GIPR agonism in α cells, we generated an α cell–specific Gipr knockout model (Giprα cell−/−) by crossing Gcg-CreERT2 (22) with Giprflox mice (23). Four weeks following tamoxifen exposure, we isolated islets, dispersed them into single cells, and sorted these into enriched populations of α and β cells, following established protocols (fig. S2, A to C) (24). The level of Gipr in enriched α cells from Giprα cell−/− was reduced by 85% relative to controls, while Gipr expression in whole islets (~10 to 20% α cells) or enriched β cells was unchanged (Fig. 2C). Expression of Gcgr and Glp1r, two class B insulinotropic GPCRs, were low to undetectable in WT and Giprα cell−/− α cells, and unchanged in whole islets or β cells (fig. S2, D and E). Insulin and glucagon content was similar in islets from control and Giprα cell−/− mice (fig. 2, F and G). Alanine produced similar levels of glucagon secretion in islets from control and Giprα cell−/− mice; however, the synergistic effect of GIP + alanine on glucagon secretion was absent in islets from Giprα cell−/− mice (Fig. 2, D to F). In vivo, the combination of GIP + alanine synergistically increased plasma glucagon levels (Fig. 2G), mimicking the results in isolated islets ex vivo. However, this synergy was lost in Giprα cell−/− mice (Fig. 2H), demonstrating the requirement of the GIPR in α cells to produce this effect.
α cell GIPR activity contributes to insulin secretion
We (16, 17), and others (18, 19), have recently demonstrated the importance of α to β cell communication for insulin secretion. This paracrine interaction is mediated by proglucagon-derived peptides acting through the GLP-1R and GCGR to establish β cell tone through intracellular levels of cAMP, the level of glucose-stimulated insulin secretion, and, ultimately, the glycemic set point of an organism (16, 25). In our previous studies, we demonstrated that the insulinotropic actions of specific amino acids (glutamine, arginine, glycine, and alanine) require α cell input into β cells and fail to stimulate insulin secretion when α to β cell communication is prevented (16). With this in mind and because GIPR stimulation at high glucose strongly increases β cell activity, we next aimed to determine whether the synergistic effect of GIP + alanine on glucagon secretion persisted at elevated glucose concentrations and had an effect on insulin.
At stimulatory glucose concentrations (10 mM), GIP and alanine each produced a modest increase in glucagon secretion (Fig. 3A). Similar to our previous reports with other glucagonotropic amino acids (16), the effect of alanine was reduced at high glucose relative to low glucose. However, the combination of GIP + alanine continued to be synergistic for glucagon secretion at high glucose (Fig. 3A). Because both GIP and alanine levels are elevated in the period immediately following a mixed nutrient meal, we hypothesized that the synergistic actions of these α cell stimuli would enhance α to β cell communication and contribute to postprandial insulin secretion. To test the possibility that GIPR agonism in α cells indirectly stimulates insulin secretion, we first determined the maximal activation of insulin secretion by GIP in control islets. A concentration-response curve for GIP-stimulated insulin secretion at high glucose was generated and identified 0.26 nM GIP as the median effective concentration (EC50) and 1 nM GIP as the Emax (fig. S3). In our next experiments, we used 10 nM GIP alone and in combination with alanine to induce maximal GIPR signaling in β cells. Individually, both GIP and alanine robustly stimulated insulin secretion; however, there was an additive effect on insulin secretion when GIP was combined with alanine (Fig. 3B). This suggests that the maximal effect of GIP on insulin secretion mediated through direct actions on the β cell can be further augmented by GIP activity in α cells. In other words, α to β cell communication invoked by GIP + alanine adds to the direct and maximal stimulation of insulin secretion by GIP alone. Supporting evidence for this dual effect of GIP was derived from experiments using GLP-1 in the place of GIP. The GLP-1R is not expressed in α cells (20, 24), and GLP-1R agonism reduces glucagon secretion. Furthermore, combining GLP-1 + alanine failed to increase insulin secretion beyond GLP-1 stimulation alone (fig. S4), indicating that the combination of alanine and a β cell GPCR ligand per se does not produce an additive effect on insulin secretion.
Fig. 3. α Cell GIPR activity contributes to insulin secretion.
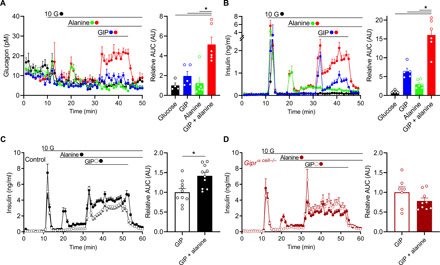
(A and B) Glucagon secretion (A) and insulin secretion (B) in WT isolated mouse islets in response alanine (3 mM, green), GIP (10 nM, blue), or GIP + alanine (red) at stimulatory glucose levels (10 mM, black). AUCs are shown relative to control during the stimulatory period (min 31 to 46). (C and D) Insulin secretion in response to GIP (open circles) or GIP + alanine (closed circles) in isolated islets from (C) control or (D) Giprα cell−/− mice, following the same protocol in (B). AUCs are relative to GIP alone conditions for each group. *P < 0.05; data are shown as means ± SEM.
To directly test the hypothesis that GIPR activity in α cells contributes to insulin secretion, we repeated the combination of GIP + alanine in islets from control and Giprα cell−/− mice. Compared to controls, islets from Giprα cell−/− mice had similar glucose- and alanine-stimulated insulin secretion, suggesting intact β cell function and normal α to β cell communication in response to alanine (Fig. 3, C and D). However, the combination of GIP + alanine only enhanced insulin secretion in the control islets, with no effect in islets from Giprα cell−/− mice (Fig. 3, C and D). Thus, specific deletion of the GIPR in α cells limited the maximal insulin secretion observed when fully recruiting both α and β cell activity with alanine and glucose, respectively.
Complete GIPR-stimulated insulin secretion requires α to β cell communication
The rise in glucose and amino acids in the postprandial state and our observations that GIP potentiates both insulin and glucagon secretion in response to these nutrients, respectively, suggest a mechanism of insulin secretion that integrates both direct and indirect actions. We have previously demonstrated that the direct actions of GIP on β cells require elevated glucose and a β cell GIPR (16). Our current work suggests that GIP also invokes an indirect mechanism for insulin secretion that requires the α cell GIPR and expands our previous work with a novel mechanism of activating α to β cell communication (16, 17). To test this directly, we used three separate models of impaired α to β cell communication that we have previously characterized in mouse and human islets. First, we inhibited α cell input to the β cell pharmacologically with the GLP-1R antagonist, exendin(9-39) (Ex9), which inhibits ~90% of α to β cell communications (16). Ex9 had no effect on the ability of GIP + glucose to stimulate insulin secretion but significantly reduced alanine- and glucose-stimulated insulin secretion, confirming the importance of α cell input in the latter condition (Fig. 4, A and B). Furthermore, Ex9 completely prevented the increase in insulin secretion in response to GIP + alanine (Fig. 4, A and B). Next, we used islets from Glp1r:Gcgrβ cell−/− mice, which have complete loss of α to β cell communication (16, 17). This model produced greatly diminished glucose- and alanine-stimulated insulin secretion, while at the same time enhancing GIP-stimulated insulin secretion (Fig. 4, C and D), in recapitulating our previous work with these mice. Preventing α to β cell communication using this orthogonal approach also impaired the additive effect of GIP + alanine on insulin secretion (Fig. 4, C and D). Thus, in two models with blockade of α to β cell communication at the level of the β cell, the effect of GIP + alanine on insulin secretion was impaired. As a final approach, we repeated these experiments with islets from Gcg−/− mice, which fail to produce any α cell proglucagon products (26) and have a proximal impairment of α to β cell communication due to lack of signal from the α cell (16). Islets from Gcg−/− mice had a reduced insulin response to glucose, alanine, and GIP + alanine (Fig. 4, E and F), a further affirmation that complete GIP-stimulated insulin secretion requires α to β cell communication.
Fig. 4. α cell GIPR activity stimulates insulin secretion through α to β cell communication.
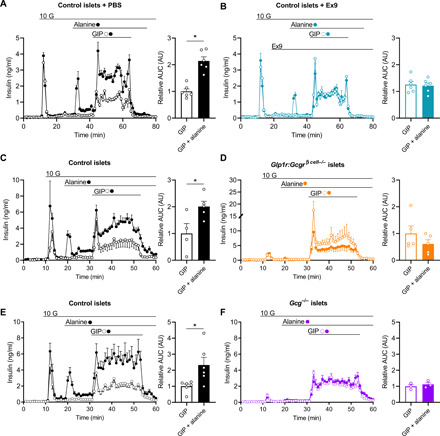
(A and B) Insulin secretion in response to GIP (open circles) or GIP + alanine (closed circles) in WT isolated mouse islets in the presence of (A) control conditions or (B) 1 μM Ex9. AUCs are shown relative to control during the stimulatory period (min 42 to 65). (C and D) Insulin secretion in response to GIP (open circles) or GIP + alanine (closed circles) in islets isolated from (C) control or (D) Glp1r:Gcgrβ cell−/− mice. AUCs are shown relative to control during the stimulatory period (min 31 to 53). (E and F) Insulin secretion in response to GIP (open circles) or GIP + alanine (closed circles) in islets isolated from (E) control or (F) Gcg−/− mice. AUCs are shown relative to control during the stimulatory period (min 31 to 53). *P < 0.05; data are shown as means ± SEM.
α cell GIP activity is required for glycemic control following a mixed nutrient stimulus
Our results in isolated islets demonstrate that GIP potentiates alanine-stimulated glucagon secretion, which, in turn, enhances insulin secretion through α to β cell communication beyond what is achieved by GIPR agonism in β cells alone. However, it is important to understand the impact of this indirect mechanism in vivo. Thus, we next set out to test the metabolic phenotype of Giprα cell−/− mice in response to a variety of nutrient challenges. In previous studies, we found that the importance of α to β cell communication is clearly apparent during the metabolic stress of a high-fat diet (HFD) (16). Therefore, we fed control and Giprα cell−/− mice a HFD for 8 weeks to induce obesity and insulin resistance. Weight gain and body composition during this period were similar between groups (fig. S5, A to D). Ambient glycemia gradually increased in the Giprα cell−/− mice relative to the control mice, producing modest hyperglycemia after 8 weeks of HFD (fig. S5E). After reaching a fat mass of >40% in both groups (fig. S5D), animals underwent intraperitoneal glucose, oral glucose, and oral mixed nutrient tolerance tests (IPGTT, OGTT, and MTT, respectively), each of which provided the same glucose (1.5 g/kg) challenge. In control mice, glucose tolerance, measured by the integrated area under the curve (iAUC), progressively improved from IPGTT to OGTT to MTT (Fig. 5, A to D), accompanied by a progressive increase in both the insulin and glucagon response to each stimuli (Fig. 5, E and F, and fig. S5, E and F). We attributed the difference in iAUC between an IPGTT and OGTT to the involvement of the incretin axis invoked by enteral nutrients (1), and the further decrease in iAUC produced by the MTT to the inclusion of α cells and enhancement of α to β cell communication through glucagon secretion (Fig. 5F). Compared to control mice, Giprα cell−/− mice had similar glycemia, insulin, and glucagon responses to both the IPGTT and OGTT (Fig. 5, A and B), whereas the Giprα cell−/− mice had significantly higher blood glucose levels during the MTT (Fig. 5, C and D). Both the OGTT and MTT produced a similar increase in plasma GIP levels, which was comparable to the Giprα cell−/− mice (fig. S5H). However, the MTT failed to produce a greater insulin response in the Giprα cell−/− mice relative to the OGTT (Fig. 5G and fig. S5F) and did not stimulate an increase in plasma glucagon levels (Fig. 5H and fig. S5G). This suggests that the glucose intolerance seen in the Giprα cell−/− mice relative to the controls during the MTT arises from an impairment in α to β cell communication due to lack of GIPR activity in α cells.
Fig. 5. GIP activity in the α cell is required for glycemic control following a mixed nutrient stimulus.
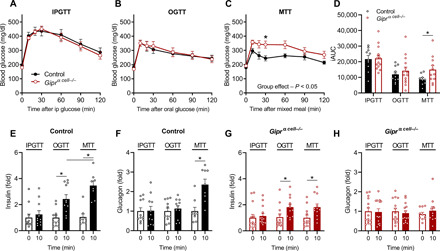
(A) Intraperitoneal (ip) glucose tolerance (n = 10, 14; control, Giprα cell−/−), (B) oral glucose tolerance (n = 11, 14; control, Giprα cell−/−), and (C) mixed nutrient glucose tolerance (n = 9, 12; control, Giprα cell−/−) in control or Giprα cell−/− mice. (D) Incremental AUCs (iAUC) are shown for each test. (E) Plasma insulin and (F) plasma glucagon at 0 and 10 min after stimuli in control mice. (G) Plasma insulin and (H) glucagon at 0 and 10 min after stimuli in Giprα cell−/− mice. *P < 0.05; data are shown as means ± SEM.
DISCUSSION
GIP was found more than 50 years ago, and over that time, its proposed physiologic role has been fairly static, i.e., an endocrine factor that matches the insulin response to the amount of carbohydrate absorbed in a meal. In the studies reported here, we expand this role and demonstrate an adjunct mechanism by which GIP links dietary protein to islet function. Our results demonstrate that one important action of the GIPR in the α cells is to integrate amino acid signals with the β cell response to other nutrients through α to β cell communication. These data extend the current incretin model of insulin secretion, demonstrating that GIP has both direct and indirect actions to potentiate insulin secretion by engaging both β and α cells (Fig. 6).
Fig. 6. GIP stimulates insulin secretion through both direct and indirect mechanisms in the islet.
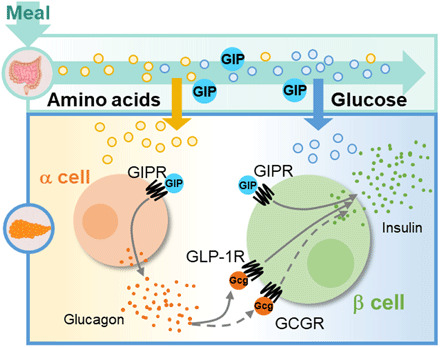
Ingested macronutrients stimulate the secretion of GIP and are sensed by islet cells. GIP directly stimulates insulin secretion through the β cell GIPR. Indirectly, GIP potentiates α cell activity to enhance α to β cell communication through the GLP-1R/GCGR. Thus, GIP indirectly stimulates insulin secretion through the α cell. The combination of both events facilitates maximal insulin secretion in response to mixed nutrient stimuli.
The classic model of the incretin axis includes the secretion of both GIP and GLP-1 from enteroendocrine cells, and the broad presumption that both peptides act in an endocrine fashion to enhance insulin secretion through GPCR actions in β cells (1). However, the hallmark work defining the incretin axis has been glucose centric, relying on oral and intravenous glucose administration (27). Moreover, the α cell has not been previously incorporated into the incretin effect even though glucagon was the first peptide proposed to serve this function (28). The work presented here builds on a number of recent studies and suggests the current incretin model needs to be reconsidered (4). For one thing, there is ongoing debate as to whether gut-derived GLP-1 enhances insulin secretion through endocrine actions or whether the primary role of the GLP-1R is to mediate α cell signals. Mice with β cell deletion of the Glp1r have normal oral glucose tolerance but impaired intraperitoneal glucose tolerance (29). Moreover, systemic antagonism of the GLP-1R only causes glucose intolerance when Gcg is expressed in the pancreas, whereas this effect is absent when Gcg is made only in the gut (26). Last, specific deletion of Gcg in the gut does not impair the insulin response to oral or intraperitoneal glucose tolerance (30). Together, these observations suggest that endocrine actions of gut-derived GLP-1 are not the primary stimulus to β cell GLP-1R. Contemporaneous with this idea is emerging evidence demonstrating important paracrine interactions between α and β cells, which support a model whereby the ligands mediating physiologic β cell GLP-1R signaling originate from the α cell, not the gut (31). One important corollary of a revised view of GLP-1 action is that GIP is the sole incretin, e.g., the enteral hormone that communicates information about meal constituents to the islet. In support of this, recent reports show that GIPR antagonism was more effective at raising glycemia and impairing insulin secretion compared to Ex9 in healthy humans (6). Other investigators, using exogenous peptide infusion in healthy people, have also concluded that GIP is the predominant incretin hormone (32).
A number of recent reports have described the importance of α to β cell communication facilitated by proglucagon peptides (16–19). Our in vivo work in this area demonstrated that amino acids are potent α cell activators with the potential to initiate α to β cell communication (17). However, even alanine, the amino acid with the greatest effect on glucagon secretion, is only modestly effective when given alone in isolated islets and to mice (Figs. 1A and 2F (33)). The secretory profile of alanine-induced glucagon secretion has features that are comparable to glucose-stimulated insulin release (Figs. 3 and 5E). In particular, during physiologic β cell secretion, e.g., meals, glucose is a permissive factor that enables GPCR potentiation of insulin secretion. Estimates from experiments in isolated islets indicate that GPCR activity accounts for >80% of insulin secretion (16). The findings presented here suggest that amino acids such as alanine serve a similar function, acting as a permissive factor to activate α cells and allow potentiation by a GPCR ligand. GIP potentiated alanine-stimulated glucagon secretion by 5- and 10-fold at high and low glucose, respectively (Figs. 1D and 3A). Thus, similar to β cells, GPCR input in α cells accounts for a significant portion of glucagon secretion.
The GIPR is expressed in both α and β cells, allowing incretin communication of nutrient absorption in the gut to both cell types, with the magnitude of the signal dependent on the amounts of meal protein and carbohydrate constituents. The efficient activation of β cells through both direct and indirect actions is also tied to the nutrient content of the meal. A glucose-only stimulus enables GIPR activity primarily in β cells, producing sufficient insulin to allow for glucose disposal. The addition of an amino acid component augments GIPR activity in both α and β cells, with the increased glucagon secretion further enhancing insulin secretion through α to β cell communication (Fig. 4). The combination of insulin and glucagon in the postprandial state mediates the efficient metabolism and storage of both glucose and amino acids. In mouse models, impairment of either insulin or glucagon signaling causes substantial increases in glycemia (34) or amino acid concentrations (35, 36), respectively. The loss of α cell GIPR impairs glucose metabolism in our mouse model only in response to a mixed nutrient stimulus, demonstrating that without increased circulating amino acids to prompt α to β cell communication, there is insufficient insulin secreted for normal control of glycemia. Thus, the metabolic actions of GIP are essential for glucose tolerance in the context of mixed nutrient meals (Fig. 6).
In summary, we provide strong evidence for an additional component of the incretin axis mediated by GIP action in α cells. First, we show an amino acid–dependent action of α cell GIPR for glucagon secretion that strongly resembles the glucose-dependent actions of incretin peptides in β cells (Figs. 1 and 2). Second, we demonstrate that GIP-stimulated glucagon secretion produces substantially more insulin secretion than what is achieved by GIPR activity in β cells alone, by engaging α to β cell communication (Figs. 3 and 4). Last, we provide in vivo evidence that preventing GIPR activity in α cells produces glucose intolerance when mice are challenged with a mixed nutrient stimulus that includes a complete diet composition (Fig. 5). Future studies are needed to extend this work from mouse models to humans. Together, we propose an expanded redefinition of the incretin effect in islets, whereby GIP produces robust, nutrient-dependent increases in both insulin and glucagon as a means of efficiently coordinating postprandial metabolism and nutrient sensing (Fig. 6).
MATERIALS AND METHODS
Animals
All animals were maintained and used in accordance with protocols approved by the Duke University Institutional Animal Care and Use Program. Mice were housed under a 12-hour light/dark cycle and provided free access to a food and water. Mice with LoxP sites in the Gipr allele (23) were crossed with Gcg-CreERT2 mice (22) to generate α cell–specific Gipr deletion (Giprα cell−/−). Control mice were homogeneous for the LoxP sites in the Gipr allele but did not express the Glucagon-CreERT2. Mice lacking β cell glucagon receptor (Gcgr) and glucagon-like peptide receptor (Glp1r) were produced as previously described (Gcgr:Glp1rβ cell−/−) (16). Inactivation of the genes was induced at 6 weeks of age by oral gavage of tamoxifen (prepared at 50 mg/ml in corn oil); each mouse was given 100 μl consecutively for 5 days. For in vivo studies, experiments were performed in 10- to 22-week-old group-housed male mice. For ex vivo studies, experiments were performed in islets isolated from 10- to 18-week-old male and female mice. Animals were fed either standard chow diet or HFD (45% kcal from fat; Research Diets, D12451i) for 8 weeks prior to tolerance testing. Littermates of the same sex were randomly assigned to experimental groups.
Islet isolation
Primary islets were isolated from mice according to previously published methods (16). Briefly, the pancreas was inflated through the pancreatic duct with collagenase V (0.7 mg/ml) in Hanks’ balanced salt solution. The pancreas was then excised and digested for 12 min at 37°C. Digestion was quenched with cold RPMI [2 mM l-glutamine, 11.1 mM glucose, 0.25% bovine serum albumin (BSA), penicillin (100 U/ml), and streptomycin (100 μg/ml)]. Islets were separated using a Histopaque gradient. Islets recovered overnight in RPMI containing 10% fetal bovine serum (FBS) prior to all experiments.
Islet perifusion
After incubation, equal numbers of islets (75 to 100 islets) were handpicked and placed into chambers containing 2.7 mM glucose Krebs-Ringer-phosphate-HEPES (KRPH) buffer [140 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 1 mM NaH2PO4, 1 mM MgSO4, 2 mM NaHCO3, 5 mM Hepes, and 0.1% FA-free BSA (pH 7.4)] with 100 μl of Bio-Gel P-4 Media (Bio-Rad). Islets were equilibrated for 48 min and then perifused in intervals based on the experimental conditions. All treatments were prepared in KRPH buffer +1% BSA. Islet proteins were extracted in acid ethanol to assess total insulin and glucagon levels. Insulin and glucagon secretion were assayed with Insulin AlphaLISA (PerkinElmer) and Lumit Glucagon Immunoassay Kit (Promega, CS3037A02), respectively, and measured using the EnVision plate reader (PerkinElmer). Concentrations for treatments in perifusion were as follows: alanine (3 mM), glucose (2.7 or 10 mM), GLP-1 (10 nM), GIP (10 nM), and Ex9 (1 μM).
Peptides
Exendin 9 (Ex9), mouse GIP (mGIP) (Phoenix Pharmaceuticals), mouse [D-Ala2] GIP (in vivo, Phoenix Pharmaceuticals), and GLP-1 (Bachem) were reconstituted in phosphate-buffered saline (PBS), aliquoted, and stored at −80°C.
Islet dispersion
We followed procedures previously described (24). After overnight recovery, 70 to 100 islets were collected from each mouse and rinsed once in PBS before incubation with Accutase (Sigma-Aldrich, A6964) for 12 to 15 min at 37°C with intermittent vortexing. Digestion was stopped with addition of cold RPMI, and dispersed islet cells were centrifuged for 3 min, 350g, at 4°C. RPMI was aspirated, and islets were washed with sorting buffer [RPMI 1640 without phenol red (11835030), 11.1 mM glucose, 1% FBS, 1% penicillin/streptomycin, 20 mM Hepes, and deoxyribonuclease (10 U/ml)].
Flow cytometry
Dispersed islet cells were sorted into enriched populations as previously described in (24). Following Accutase dispersion, islet cells were filtered through a 30-μm mesh and held on ice before fluorescence-activated cell sorting (FACS) using a Beckman-Coulter MoFlo Astrios. Forward and side scatter (SSC) were used to separate single cells from debris and doublets. Islet cells were separated by autofluorescence and SSC into α, β, and δ cell populations into TRIzol. Cell percentages reported in this manuscript are calculated from FACS sorts (i.e., absolute number of cells sorted to TRIzol).
RNA extraction, DNA synthesis, and reverse transcription polymerase chain reaction
Whole islets and sorted cells were collected into TRIzol for RNA extraction, and cDNA was synthesized from 100 ng of RNA (Thermo Fisher Scientific, catalog #4368814) as previously described (24). Quantitative polymerase chain reaction (qPCR) was run using TaqMan reagents and primers (table). Data were analyzed by calculating ΔΔCT, and each gene of interest was normalized to cyclophilin A. qPCR primers used are shown in table S1. Data are shown as fold change relative to whole-islet lysates in control animals.
α cell cAMP and Ca2+ imaging
α-CAMPER mice were generated by crossing CAMPER mice (Jax 032205), a Cre-dependent cAMP indicator strain (37), with Glucagon-CreERT2 mice (Jax 030346) that express tamoxifen-inducible CreERT2 in islet α cells (22). α-GCaMP6s mice were generated by crossing GCaMP6s mice (Jax 028866), a Cre-dependent Ca2+ indicator strain (38), with Glucagon-CreERT2 mice (Jax 030346) that express tamoxifen-inducible CreERT2 in islet α cells (22). Isolated islets were incubated overnight in islet media (Invitrogen RPMI1640, 10% FBS, and penicillin/streptomycin) containing 1 μM 4-hydroxytamoxifen (Sigma-Aldrich, H7904) and imaged the following day. Islets were preincubated in islet media containing 2.7 mM glucose for 45 min at 37°C and then placed in an RC-41LP imaging chamber inserted in a QE-1 chamber holder (Warner Instruments) mounted on a Nikon Ti2 microscope equipped with a 10×/0.5NA SuperFluor objective (Nikon Instruments). The chamber was perfused with a standard bath solution containing 135 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 20 Hepes, and 2.7 glucose (pH 7.35). Alanine (3 mM) and 10 nM GIP (Thermo Fisher Scientific NC9048828) were added as indicated. The flow rate was maintained at 0.25 ml/min (Fluigent MCFS-EZ), and temperature was maintained at 33°C using solution and chamber heaters (Warner Instruments). LED excitation (CAMPER: Semrock 430/21, 5% output; GCaMP6s: Chroma ET500/20, 20% output) from an AURA (CAMPER) or SOLA (GCaMP6s) LED Light Engine (Lumencor) was used in combination with a Semrock dichroic (CAMPER: FF459/526/596-Di01; GCaMP6s: FF444/521/608-Di01) and emission filters (CAMPER: Semrock FF02-470/30 and FF01-542/27; GCaMP6s: Chroma ET535/30 m), with cAMP levels reported as the emission ratio R470/542, and Ca2+ levels reported as normalized intensity (F/F0). Fluorescence emission for CAMPER was collected with a Prime 95B back-illuminated sCMOS camera (Photometrics) with 2 × 2 binning, and for GCaMP6s with a Hamamatsu Flash4.0v2 sCMOS camera (Hamamatsu), every 6 s. A single region of interest was used to quantify the average response of each islet using Nikon Elements.
In vivo glucagon secretion
Mice were fasted 5 hours before injection of [D-Ala2] GIP (4 nmol/kg body weight) and/or alanine (0.325 g/kg body weight). Solutions were made in PBS. Tail vein blood was collected in EDTA-coated capillary tubes before injections and 15 min after injections, and plasma separated by centrifugation at 21,000g for 15 min.
Glucose and meal tolerance tests
Animals were fasted for 5 hours before given glucose or mixed meal by oral gavage, or glucose by intraperitoneal injection. Glucose was administered at 1.5 g/kg body weight, and Ensure (liquid, Abbott Laboratories) was administered at 10 ml/kg body weight. Blood glucose was measured from the tail vein with a Contour Blue glucometer (Bayer). Blood was collected from the tail vein in EDTA-coated capillary tubes and plasma separated by centrifugation at 21,000g for 15 min.
Plasma analysis
Insulin and glucagon plasma levels were measured by enzyme-linked immunosorbent assay (ELISA) (Mercodia). Total GIP was measured by ELISA (Millipore Sigma).
Statistics
All data are presented as means ± SEM. Where appropriate, data were normalized to body weight. The Student’s t test or two-way analysis of variance (ANOVA) (with Bonferroni post hoc tests as appropriate) was performed using GraphPad Prism 8.3 software (La Jolla, CA, USA) to detect statistical differences. P < 0.05 was considered statistically significant. Statistical details of experiments can be found in figure legends.
Acknowledgments
We thank L. Martinek of the Duke Cancer Institute Flow Cytometry Shared Resource for assistance with FACS experiments. Funding: K.E., S.M.G., and M.E.C. received support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (K.E., T32 DK007012; S.M.G., F32 DK121420; M.E.C., F32 DK116542). J.E.C. is supported by a career development award from the American Diabetes Association (1-18-JDF-017) and by funding from the NIDDK, NIH (DK123075 and DK125353), and is a Borden Scholar. D.A.D. is supported by the NIDDK, NIH (DK101991). Author contributions: K.E., S.M.G., M.E.C., E.R.K., E.J., B.S., A.C., J.L.B., S.E.E., and B.M.C. performed the experiments. K.W.S. and D.J.N. provided key conceptual insights. K.E., M.J.M., D.A.D., and J.E.C. designed and directed the study. K.E., D.A.D., and J.E.C. wrote and edited the manuscript. All authors reviewed and approved the final manuscript for submission. Competing interests: K.W.S. is an employee of Eli Lilly and Company. The Campbell Laboratory receives funding from E. Lilly for basic science research. All other authors confirm that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/11/eabf1948/DC1
REFERENCES AND NOTES
- 1.Campbell J. E., Drucker D. J., Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Holst J. J., The incretin system in healthy humans: The role of GIP and GLP-1. Metabolism 96, 46–55 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Drucker D. J., Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 19, 277–289 (2020). [DOI] [PubMed] [Google Scholar]
- 4.D’Alessio D., Is GLP-1 a hormone: Whether and when? J Diabetes Investig 7 ( Suppl. 1), 50–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilsboll T., Krarup T., Sonne J., Madsbad S., Vølund A., Juul A. G., Holst J. J., Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 88, 2706–2713 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Gasbjerg L. S., Helsted M. M., Hartmann B., Jensen M. H., Gabe M. B. N., Sparre-Ulrich A. H., Veedfald S., Stensen S., Lanng A. R., Bergmann N. C., Christensen M. B., Vilsbøll T., Holst J. J., Rosenkilde M. M., Knop F. K., Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68, 906–917 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Nauck M. A., Heimesaat M. M., Orskov C., Holst J. J., Ebert R., Creutzfeldt W., Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 91, 301–307 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El K., Campbell J. E., The role of GIP in α-cells and glucagon secretion. Peptides 125, 170213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia C. W., Carlson O. D., Kim W., Shin Y. K., Charles C. P., Kim H. S., Melvin D. L., Egan J. M., Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 58, 1342–1349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moens K., Heimberg H., Flamez D., Huypens P., Quartier E., Ling Z., Pipeleers D., Gremlich S., Thorens B., Schuit F., Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes 45, 257–261 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Opara E. C., Go V. L., Influence of gastric inhibitory polypeptide (GIP) and glucose on the regulation of glucagon secretion by pancreatic alpha cells. Regul. Pept. 32, 65–73 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Sparre-Ulrich A. H., Gabe M. N., Gasbjerg L. S., Christiansen C. B., Svendsen B., Hartmann B., Holst J. J., Rosenkilde M. M., GIP(3–30)NH 2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochem. Pharmacol. 131, 78–88 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Christensen M., Vedtofte L., Holst J. J., Vilsboll T., Knop F. K., Glucose-dependent insulinotropic polypeptide: A bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes 60, 3103–3109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia C. W., Odetunde J. O., Kim W., Carlson O. D., Ferrucci L., Egan J. M., GIP contributes to islet trihormonal abnormalities in type 2 diabetes. J. Clin. Endocrinol. Metab. 99, 2477–2485 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund A., Vilsboll T., Bagger J. I., Holst J. J., Knop F. K., The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 300, E1038–E1046 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Capozzi M. E., Svendsen B., Encisco S. E., Lewandowski S. L., Martin M. D., Lin H., Jaffe J. L., Coch R. W., Haldeman J. M., MacDonald P. E., Merrins M. J., D’Alessio D. A., Campbell J. E., β cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4, e126742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capozzi M. E., Wait J. B., Koech J., Gordon A. N., Coch R. W., Svendsen B., Finan B., D’Alessio D. A., Campbell J. E., Glucagon lowers glycemia when beta-cells are active. JCI Insight 5, e129954 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svendsen B., Larsen O., Gabe M. B. N., Christiansen C. B., Rosenkilde M. M., Drucker D. J., Holst J. J., Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 25, 1127–1134.e2 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Zhu L., Dattaroy D., Pham J., Wang L., Barella L. F., Cui Y., Wilkins K. J., Roth B. L., Hochgeschwender U., Matschinsky F. M., Kaestner K. H., Doliba N. M., Wess J., Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 5, e127994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGruccio M. R., Mawla A. M., Donaldson C. J., Noguchi G. M., Vaughan J., Cowing-Zitron C., van der Meulen T., Huising M. O., Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 5, 449–458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier J. J., Gallwitz B., Siepmann N., Holst J. J., Deacon C. F., Schmidt W. E., Nauck M. A., Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia 46, 798–801 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Ackermann A. M., Zhang J., Heller A., Briker A., Kaestner K. H., High-fidelity glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting. Mol. Metab. 6, 236–244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell J. E., Ussher J. R., Mulvihill E. E., Kolic J., Baggio L. L., Cao X., Liu Y., Lamont B. J., Morii T., Streutker C. J., Tamarina N., Philipson L. H., Wrana J. L., MacDonald P. E., Drucker D. J., TCF1 links GIPR signaling to the control of beta cell function and survival. Nat. Med. 22, 84–90 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Gray S. M., Xin Y., Ross E. C., Chazotte B. M., Capozzi M. E., el K., Svendsen B., Ravn P., Sloop K. W., Tong J., Gromada J., Campbell J. E., D’Alessio D. A., Discordance between GLP-1R gene and protein expression in mouse pancreatic islet cells. J. Biol. Chem. 295, 11529–11541 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Diaz R., Molano R. D., Weitz J. R., Abdulreda M. H., Berman D. M., Leibiger B., Leibiger I. B., Kenyon N. S., Ricordi C., Pileggi A., Caicedo A., Berggren P.-O., Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab. 27, 549–558.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers A. P., Sorrell J. E., Haller A., Roelofs K., Hutch C. R., Kim K.-S., Gutierrez-Aguilar R., Li B., Drucker D. J., D’Alessio D. A., Seeley R. J., Sandoval D. A., The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 25, 927–934.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauck M. A., Bartels E., Orskov C., Ebert R., Creutzfeldt W., Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 76, 912–917 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Samols E., Marri G., Marks V., Promotion of insulin secretion by glucagon. Lancet 2, 415–416 (1965). [DOI] [PubMed] [Google Scholar]
- 29.Smith E. P., An Z., Wagner C., Lewis A. G., Cohen E. B., Li B., Mahbod P., Sandoval D., Perez-Tilve D., Tamarina N., Philipson L. H., Stoffers D. A., Seeley R. J., D’Alessio D. A., The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 19, 1050–1057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y., Koehler J. A., Baggio L. L., Powers A. C., Sandoval D. A., Drucker D. J., Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 30, 976–986.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El K., Capozzi M. E., Campbell J. E., Repositioning the alpha cell in postprandial metabolism. Endocrinology 161, bqaa169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nauck M. A., Meier J. J., The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 4, 525–536 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Galsgaard K. D., Jepsen S. L., Kjeldsen S. A. S., Pedersen J., Wewer Albrechtsen N. J., Holst J. J., Alanine, arginine, cysteine, and proline, but not glutamine, are substrates for, and acute mediators of, the liver-α-cell axis in female mice. Am. J. Physiol. Endocrinol. Metab. 318, E920–E929 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Vikram A., Jena G., S961, an insulin receptor antagonist causes hyperinsulinemia, insulin-resistance and depletion of energy stores in rats. Biochem. Biophys. Res. Commun. 398, 260–265 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Dean E. D., Li M., Prasad N., Wisniewski S. N., Deylen A. V., Spaeth J., Maddison L., Botros A., Sedgeman L. R., Bozadjieva N., Ilkayeva O., Coldren A., Poffenberger G., Shostak A., Semich M. C., Aamodt K. I., Phillips N., Yan H., Bernal-Mizrachi E., Corbin J. D., Vickers K. C., Levy S. E., Dai C., Newgard C., Gu W., Stein R., Chen W., Powers A. C., Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 25, 1362–1373.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longuet C., Robledo A. M., Dean E. D., Dai C., Ali S., McGuinness I., de Chavez V., Vuguin P. M., Charron M. J., Powers A. C., Drucker D. J., Liver-specific disruption of the murine glucagon receptor produces α-cell hyperplasia: Evidence for a circulating α-cell growth factor. Diabetes 62, 1196–1205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntean B. S., Zucca S., MacMullen C. M., Dao M. T., Johnston C., Iwamoto H., Blakely R. D., Davis R. L., Martemyanov K. A., Interrogating the spatiotemporal landscape of neuromodulatory GPCR signaling by real-time imaging of cAMP in intact neurons and circuits. Cell Rep. 22, 255–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madisen L., Garner A. R., Shimaoka D., Chuong A. S., Klapoetke N. C., Li L., van der Bourg A., Niino Y., Egolf L., Monetti C., Gu H., Mills M., Cheng A., Tasic B., Nguyen T. N., Sunkin S. M., Benucci A., Nagy A., Miyawaki A., Helmchen F., Empson R. M., Knöpfel T., Boyden E. S., Reid R. C., Carandini M., Zeng H., Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/11/eabf1948/DC1


