Abstract
Throughout the COVID-19 pandemic, governments and individuals have attempted a wide variety of strategies to limit the damage of the pandemic on human lives, population health, and economies. Contact tracing has been a commonly used strategy, and various approaches have been proposed and attempted. We summarise some methods of contact tracing and testing, considering the resources demanded by each and how features of SARS-CoV-2 transmission affect their effectiveness. We also propose an approach focusing on tracing transmission events, which can be particularly effective when superspreading events play a large role in transmission. Accounting for the best available evidence on a pathogen and for the availability of resources can make control strategies more effective, even if they are not perfect.
Standard approaches to contact tracing
Contact tracing has a long history in public health and has been used nearly universally during the COVID-19 pandemic.1, 2, 3, 4 So-called test and trace strategies, which rely on routine and symptomatic surveillance to identify cases and then trace their possible contacts, have been shown to be effective in some areas.3, 4, 5 These strategies are especially important to prevent successive waves of outbreaks when social distancing measures are relaxed and schools and businesses reopen.6, 7, 8, 9
Contact tracing can take many forms, depending on the features of its target infectious disease.10, 11, 12 In any form, however, it relies on substantial public health investment and public acceptance and participation, including the collaboration of infected individuals, who must share their potential contacts, and of the identified contacts, who must follow appropriate public health protocols.11, 12, 13 It also depends on how accurately and completely people who are infected recall their potential contacts, and on the ability of public health agencies to locate and deliver effective interventions to those contacts.14 Without these elements, contact tracing can be slow and lead to an ineffective response.15 Indeed, if cases start to increase exponentially, contact tracing can become a poor use of restricted resources.
During the COVID-19 pandemic, contact tracing has generally been used with presumptive quarantine of contacts. This approach has been used in the USA, implemented by individual states, and in the UK.1, 2, 9, 13 However, contact tracing does not need to involve only presumptive quarantine; other strategies, such as active monitoring or testing contacts and acting according to the test results, have been proposed.14, 16 There are also many different ways to identify potential contacts, including personal outreach by public health workers, automated notification through mobile phone apps, or combinations of these approaches.17
Tracing transmissions
Quarantining contacts of a known case to prevent onward transmission is not the only approach to contact tracing. The strategy of backward contact tracing has been a key part of the pandemic response in some locations. With backward contact tracing, when an index case is identified, the tracers attempt to identify the source of infection (either an infected individual or a transmission event), and then forward trace from that source to identify new infections and halt transmission by immediately isolating all contacts.18, 19 This strategy has several advantages, especially for a virus such as SARS-CoV-2, which transmits in clusters, and which many infected individuals do not transmit at all.20, 21, 22, 23 For such a pathogen, it is important to identify the source of infection when not known because there is a high probability that an infected invididual who has transmitted the virus at least once will transmit multiple times. Knowledge of the source is particularly useful in stopping outbreaks starting from individual imported cases.
We propose two processes of transmission tracing with rapid, high-sensitivity tests: one based on forward tracing, and another based on backward tracing (panel ), shown schematically in figure 1 along with other contact tracing strategies. In describing this approach, we use the phrase isolation of contacts (as opposed to quarantine), even for those who test negative, because this approach treats them as presumptive positives. Depending on the setting, other epidemiological control measures might be more appropriate for the presumptive positives and these can be used in the next step of the transmission tracing process (panel). Both of these approaches rely on the key point that identification of exposure to a common transmission event is far more informative of the infection state of other individuals than is identification of exposure to a person with a positive test result alone.
Panel. Transmission tracing.
-
•
Test all potential contacts of an index case using a rapid, high-specificity test
-
•
If any contacts test positive:
-
•Immediately isolate all contacts and do more thorough epidemiological assessments of these individuals Control measures can include more sensitive testing, active monitoring, presumptive quarantine, or self-isolation (with appropriate support measures to ensure it can be followed), and tracing of their contacts, potentially using this same strategy
-
•
-
•
If no contacts test positive:
-
•All contacts are presumptively treated as negative and self-isolation is not suggested
-
•
-
•
Similarly, if an individual tests positive and the source of infection can be identified, treat all contacts of the source as presumptive positives as described above
Figure 1.
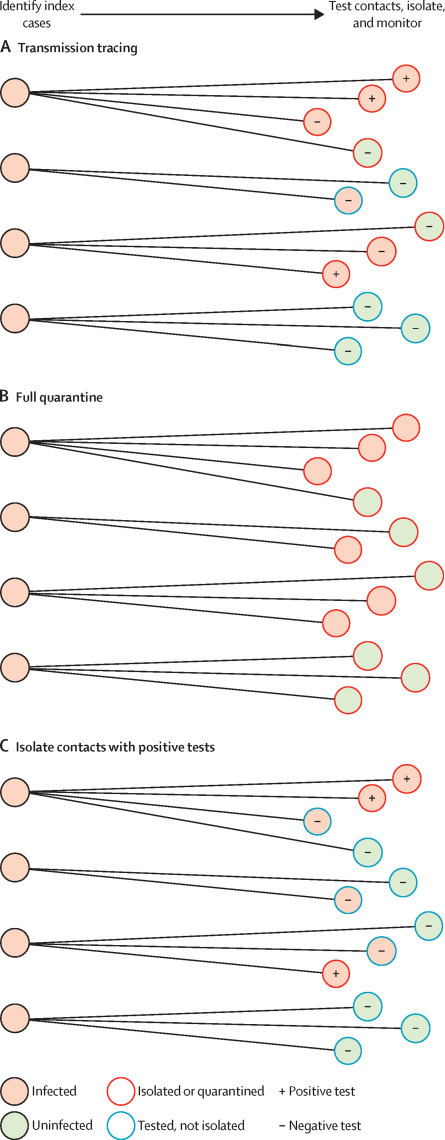
Schematic presentation of three approaches to test and trace contacts of infected individuals
Transmission tracing (A). Quarantine of all identified contacts (B). Isolation of contacts who are tested and found to be positive for the pathogen (C).
Variations in the implementation of the approach can account for variability in the timing of recall and testing of contacts. The ideal approach involves near-simultaneous testing of contacts. However, depending on the balance of the benefits of each approach, contacts can be isolated until a sufficient number of contacts have tested negative, or contacts who test negative can forgo isolation until any other contact tests positive, regardless of the rate at which tests are done. More complex risk-based approaches can also be used, as suggested for symptom monitoring in contact tracing.24
The transmission tracing approach relies on the availability of a rapid, high-specificity test. The test sensitivity, however, does not need to be high. Figure 2 shows how, even with low-sensitivity tests, transmission tracing can lead to a reduced burden on contacts by requiring fewer contacts to isolate, while still resulting in the isolation of a high proportion of infected contacts. These proportions vary as the dispersion of the number of secondary cases per index case, parameterised by k, varies; a lower value of k indicates more overdispersion. Overdispersed transmission arises when many infected individuals transmit to few (or even no) contacts, whereas some transmit to many contacts.25 More overdispersion leads to a higher rate of superspreading events, in which a large number of individuals are infected by a single source, as has been seen for COVID-19.20, 21, 22, 23 Such events illustrate how models can explain how one key feature of the pathogen's transmission affects the relative effectiveness of different test, trace, and isolate strategies. The code for this analysis is available online;26 assumptions can be altered and more complex models can be used to evaluate the effectiveness of transmission tracing in a specific setting. Mathematical details are available in appendix pp 1–2.
Figure 2.
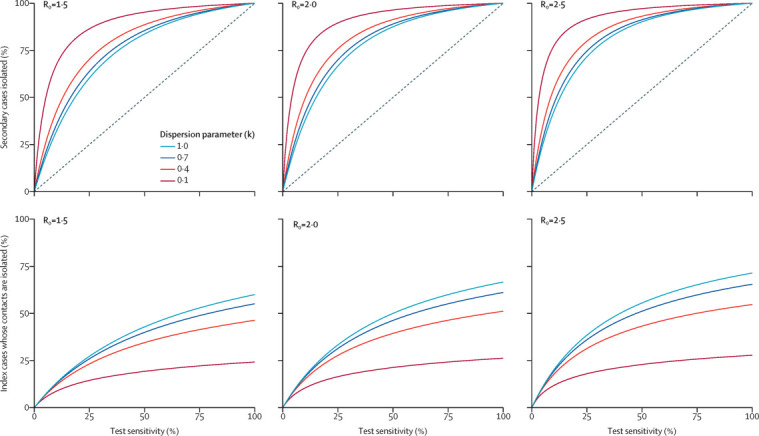
Effectiveness and isolation burden of the transmission tracing strategy in different settings
Proportion of secondary cases isolated under the transmission tracing strategy (top row) and proportion of index cases whose contacts are isolated under the transmission tracing strategy (bottom row) versus test sensitivity by R0 and k. The diagonal line in the top row indicates the proportion of secondary cases who test positive and are isolated under strategy C (figure 1).
Testing and isolating
In addition to contact tracing, routine surveillance testing, with isolation of individuals who test positive, has been proposed to control the COVID-19 pandemic without large-scale stay-at-home orders.27 Routine surveillance testing can be done independently or in combination with some type of contact tracing.27 There is a range of possibilities, depending on the setting and the available testing infrastructure, and which we group into three large categories: standard assays at moderate frequencies, less-sensitive tests at higher frequencies, and regular pooled or group testing.
Modelling studies have shown that frequency of testing is more important than high sensitivity of tests for outbreak control.28, 29, 30 The turnaround time from testing to results and isolation of infected individuals is also a crucial factor.28 These factors depend heavily on the tests available and their cost, as well as the ability to ensure compliance with regular testing.31, 32 In all cases, by identifying currently asymptomatic infections, regular testing reduced case counts compared with symptomatic testing alone.29
The efficiency of mass testing can also be improved with group testing, where samples from multiple individuals are combined and the pool is tested for the presence of virus. A positive result leads to follow up of the individuals who contributed to that sample. This method allows for quicker and less expensive mass testing, but can delay the time to confirmation of an individual's infection status.33, 34, 35 Group testing can be particularly effective in settings where superspreading events are probable, if the groups are likely to include multiple individuals with the same exposures to infection.
Comparing approaches
Various modelling and simulation studies have assessed these strategies individually in different settings, and we refer interested readers to these studies for more details.6, 7, 8, 9, 10, 11, 14, 16, 17, 18, 24, 27, 28, 29, 30, 33, 36, 37, 38, 39, 40, 41, 42 We summarise selected strategies for control, noting some of their advantages and disadvantages (table ). In particular, we consider the effect of overdispersed transmission and the presence of superspreading events in this comparison. These features lead to transmission tracing being more effective than traditional contact tracing at controlling transmission, with a lower burden on uninfected individuals, and lead to pooled testing being more efficient if the pool groups are probable transmission clusters. Backward contact tracing can also be more valuable, as the index cases are more likely to have been infected in superspreading events.18 Most other methods have increased variability due to overdispersed transmission, as any undetected transmissions are potentially more consequential for onward spread.
Table.
Comparison of testing and tracing strategies
| Description | Tests needed | Effect of overdispersed transmission and superspreading events | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| Contact tracing | Surveillance to identify index cases, contact tracing, and quarantine of contacts | Only for surveillance | More variability and dependence on contact recall | High control if contacts identified and quarantined quickly | High burden on contacts and public health agencies |
| Contact tracing with active monitoring | Surveillance to identify index cases, contact tracing, and active monitoring of contacts | Only for surveillance | More variability and dependence on contact recall | Low burden on contacts | More chance of onward transmission before identification of infected contacts |
| Contact tracing with testing of contacts | Surveillance to identify index cases, contact tracing, testing of contacts, and isolation of positives | For surveillance; high-sensitivity test needed for contacts | More variability and dependence on contact recall | Low burden on contacts | High-sensitivity tests need to be rapidly deployed to contacts; high chance of onward transmission otherwise |
| Transmission tracing | Surveillance to identify index cases, contact tracing, testing of contacts, and isolation of contacts of an index case with at least one positive contact | For surveillance; high-specificity test needed for contacts | Less burden on uninfected contacts, more possibilities of control of infected contacts | Moderate burden on contacts; can be done with rapid, low-sensitivity tests | High burden on public health agency; tests need to be rapidly deployed to contacts |
| Backward contact tracing | Surveillance to identify cases, tracing of their infector, tracing of contacts, and quarantine of contacts | For surveillance, potentially to identify infector | More possibilities of control of infected contacts | Moderate burden on contacts | High burden on public health agency and need to identify infector with directionality |
| Regular testing | Test full population at moderate frequency and contact tracing in between tests | Large need for high-sensitivity tests | More variability, more spreading can occur between tests | High control potential if combined with other measures | Requires high-sensitivity tests and rapid results; most useful in a closed population |
| High-frequency testing | Test full population at high frequency | Very strong need for rapid-result tests | More variability, more spreading can occur between tests | High control potential; low-sensitivity tests can be used | Requires adherence to testing regimen and availability of rapid tests |
| Pooled testing | Group known individuals for testing, pooled testing capability | Pooled tests | If groups represent probable clusters, fewer positive groups and more positive individuals per positive group | Low burden on testing facilities | Requires groups that are likely to be infection clusters |
Because contact tracing and testing efforts also depend on the speed of testing and tracing, cost to public health resources, burden on individuals, and acceptability of interventions to individuals, we consider these factors as well.14, 36 Many of these approaches depend on the willingness and ability of individuals to identify and recall potential contacts, so contact tracing or testing programmes with less severe interventions or less likelihood of quarantine or isolation for contacts might lead to higher reporting of contacts or higher uptake of testing, and those that can occur more rapidly (eg, by use of a point-of-care test) might reduce recall bias by reducing the time delay.13 For example, transmission tracing can have a reduced efficacy when contacts are missed because it results in a lower probability of isolating recalled contacts who were infected. However, this reduced efficacy can be offset, in some settings, by the higher acceptability of the approach. The factors that make a contact tracing or testing programme effective are not independent from the programme's features, but are closely related. These features can be adjusted to improve acceptability of the programme, even at some cost to its absolute efficacy, in ways that improve real-world effectiveness.
Other features of the specific pathogen and its transmission, as well as the local setting, should also be considered when deciding on a strategy or mix of strategies. The structure of contacts in a location is particularly important, as societies with more interaction across age or risk groups might require different methods.11, 37, 38 The variation in viral load between individuals, and the consequence of this variation on the likelihood of transmission, is also important. If viral load is highly correlated with infectiousness, tests that identify only high viral loads will be more effective at halting transmission.28, 31, 43 As more is learned about SARS-CoV-2 and COVID-19, the appropriate control measures should be regularly reconsidered.
The path forward
Many of the strategies described here can be implemented by use of current technologies, techniques, and tests. Some, however, can be improved by the approval and use of new tests. High-frequency testing is ideally suited for the use of rapid, inexpensive tests, even if they are of lower sensitivity than current assays.28, 31 The short turnaround time of these tests is especially important for mass testing and isolation, and is also useful for contact tracing because it enables tracers to talk to index cases earlier, when contact recall might be higher. Rapid tests would also be valuable for transmission tracing and backward contact tracing because they are most likely to identify superspreading events, where multiple individuals have been infected. Although rapid tests with lower sensitivity can allow some individual cases to go undetected, such tests would detect most of the major transmission events, providing a good opportunity for appropriate isolation and quarantine measures to halt onward transmission. This approach might provide the best use of scarce public health resources.44 Rapid tests are even more effective if they are more sensitive to high viral loads, as might be true for antigen tests, and high viral loads contribute to the likelihood of superspreading events and onward transmission of high viral loads.45, 46 Tests that can contribute to important public health efforts, and not just those that are most clinically useful for diagnosis, should be approved.
We have discussed how rapid tests that are highly specific, but not necessarily very sensitive, could be added to control strategies making use of contact tracing. However, it must be recognised that contact tracing is only expected to be useful for control when the prevalence of a pathogen in a given population is low. If the number of cases is growing exponentially, the capacity of contact tracing to make a difference can be rapidly outpaced. Situational awareness can be maintained by rapid self-administered tests of the type we discuss here, but it could be enhanced by other surveillance methods, including environmental surveillance, for example of wastewater. Wastewater surveillance has a long history in detecting outbreaks of viruses such as polio, and has been applied to SARS-CoV-2 in several different settings.47, 48, 49 None of these approaches are expected to solve the problem on their own, or allow a community to return to normal. However, they might help communities to manage the pandemic far more comfortably and with less risk of surges in infections, which add pressure on health-care facilities.
There will always be uncertainty as to the value of any control method in stopping an infectious disease. However, with modelling and understanding of the advantages and disadvantages of various methods, it is possible to estimate which methods will be most effective in specific conditions. As new tests, with different sensitivities and specificities, are approved, or new contact tracing apps are rolled out, the ability to use and combine different strategies will continue to expand. Many methods have been proposed and we consider a selection of test, trace, and isolate methods here. Different populations and locations should appropriately weigh the balance between resource availability, the burden on individuals, and the risks of future surges of disease. These considerations should include and account for the best evidence on the transmission of the pathogen in estimating the efficacy of various strategies. Waiting for the perfect test or the perfect control method must not prevent the implementation of good control measures as soon as possible.
Data sharing
All data is available in the manuscript or supplementary materials and replication code is available at https://github.com/leekshaffer/transmission-tracing.
Acknowledgments
Acknowledgments
LK-S is supported by the Morris-Singer Fund for the Center for Communicable Disease Dynamics and the US Centers for Disease Control and Prevention award U01IP001121. MB is partly supported by the David and Lucile Packard Foundation and the NIH NIGMS award R35GM133700. WPH is supported by the National Institutes of Health Models of Infectious Disease Agent Study programme through NIGMS award U54GM088558. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, analysis, decision to publish, or preparation of the manuscript.
Contributors
All authors produced the study concept and methods, did the analysis, and wrote, reviewed, and edited the paper. All authors had full access to the manuscript and results and accept responsibility of the submission for publication.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.US Centers for Disease Control and Prevention Case investigation and contact tracing: part of a multipronged approach to fight the COVID-19 pandemic. https://www.cdc.gov/coronavirus/2019-ncov/php/principles-contact-tracing.html
- 2.UK National Health Service If you're told to self-isolate by NHS Test and Trace or the NHS COVID-19 app. https://www.nhs.uk/conditions/coronavirus-covid-19/testing-and-tracing/nhs-test-and-trace-if-youve-been-in-contact-with-a-person-who-has-coronavirus/
- 3.Salathé M, Althaus CL, Neher R, et al. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20225. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrook R. Contact tracing, testing, and control of COVID-19—learning from Taiwan. JAMA Intern Med. 2020;180:1163–1164. doi: 10.1001/jamainternmed.2020.2072. [DOI] [PubMed] [Google Scholar]
- 5.Sun K, Viboud C. Impact of contact tracing on SARS-CoV-2 transmission. Lancet Infect Dis. 2020;20:876–877. doi: 10.1016/S1473-3099(20)30357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleta A, Martín-Corral D, Pastore Y, Piontti A, et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat Hum Behav. 2020;4:964–971. doi: 10.1038/s41562-020-0931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano G, Blanchini F, Bruno R, et al. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med. 2020;26:855–860. doi: 10.1038/s41591-020-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo JR, Cook AR, Park M, et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect Dis. 2020;20:678–688. doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panovska-Griffiths J, Kerr CC, Stuart RM, et al. Determining the optimal strategy for reopening schools, the impact of test and trace interventions, and the risk of occurrence of a second COVID-19 epidemic wave in the UK: a modelling study. Lancet Child Adolesc Health. 2020;4:817–827. doi: 10.1016/S2352-4642(20)30250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clémençon S, Tran VC, de Arazoza H. A stochastic SIR model with contact-tracing: large population limits and statistical inference. J Biol Dyn. 2008;2:392–414. doi: 10.1080/17513750801993266. [DOI] [PubMed] [Google Scholar]
- 11.Eames KTD. Contact tracing strategies in heterogeneous populations. Epidemiol Infect. 2007;135:443–454. doi: 10.1017/S0950268806006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson KC, Altare C, Wesseh CS, et al. Contact tracing performance during the Ebola epidemic in Liberia, 2014–2015. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lash RR, Donovan CV, Fleischauer AT, et al. COVID-19 contact tracing in two counties—North Carolina, June–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1360–1363. doi: 10.15585/mmwr.mm6938e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretzschmar ME, Rozhnova G, Bootsma MCJ, van Boven M, van de Wijgert JHHM, Bonten MJM. Impact of delays on effectiveness of contact tracing strategies for COVID-19: a modelling study. Lancet Public Health. 2020;5:e452–e459. doi: 10.1016/S2468-2667(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vize R. Too slow and fundamentally flawed: why test and trace is a weak and inequitable defence against COVID-19. BMJ. 2020;369 doi: 10.1136/bmj.m2246. [DOI] [PubMed] [Google Scholar]
- 16.Peak CM, Kahn R, Grad YH, et al. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect Dis. 2020;20:1025–1033. doi: 10.1016/S1473-3099(20)30361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo A, Leclerc QJ, Knight GM, et al. Implication of backward contact tracing in the presence of overdispersed transmission in COVID-19 outbreaks. Wellcome Open Res. 2020;5:239. doi: 10.12688/wellcomeopenres.16344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshikawa E, Fukumoto M, Iguchi A, Suzuki A. National Institute of Infectious Diseases guide on active epidemiological investigation for public health nurses in response to COVID-19 in Japan. Feb 6, 2020. https://www.niid.go.jp/niid/en/2019-ncov-e/2484-idsc/9472-2019-ncov-02-en.html
- 20.Adam DC, Wu P, Wong JY, et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 21.Hodcroft EB. Preliminary case report on the SARS-CoV-2 cluster in the UK, France, and Spain. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20212. [DOI] [PubMed] [Google Scholar]
- 22.Endo A, Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Tawfiq JA, Rodriguez-Morales AJ. Super-spreading events and contribution to transmission of MERS, SARS, and SARS-CoV-2 (COVID-19) J Hosp Infect. 2020;105:111–112. doi: 10.1016/j.jhin.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrault A, Charpignon M, Gruber J, Tambe M, Majumder MS. Designing efficient contact tracing through risk-based quarantining. medRxiv. 2020 doi: 10.1101/2020.11.16.20227389. published online Nov 19. (preprint). [DOI] [Google Scholar]
- 25.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy-Shaffer L. Code to replicate analysis in transmission tracing manuscript. Dec 18, 2020. https://github.com/leekshaffer/transmission-tracing
- 27.Kucharski AJ, Klepac P, Conlan AJK, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7 doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paltiel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu S, Lo NC. Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1383. published online Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mina MJ, Parker R, Larremore DB. Rethinking COVID-19 test sensitivity—a strategy for containment. N Engl J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 32.Vogels CBF, Watkins AE, Harden CA, et al. SalivaDirect: a simplified and flexible platform to enhance SARS-CoV-2 testing capacity. Med (N Y) 2020;2:1–18. doi: 10.1016/j.medj.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleary B, Hay JA, Blumenstiel B, et al. Using viral load and epidemic dynamics to optimize pooled testing in resource-constrained settings. Sci Transl Med. 2021 doi: 10.1126/scitranslmed.abf1568. published online Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS-CoV-2 testing resources. Am J Clin Pathol. 2020;153:715–718. doi: 10.1093/ajcp/aqaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yelin I, Aharony N, Tamar ES, et al. Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clin Infect Dis. 2020;71:2073–2078. doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwok KO, Tang A, Wei VWI, Park WH, Yeoh EK, Riley S. Epidemic models of contact tracing: systematic review of transmission studies of severe acute respiratory syndrome and Middle East respiratory syndrome. Comput Struct Biotechnol J. 2019;17:186–194. doi: 10.1016/j.csbj.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eames KTD, Keeling MJ. Contact tracing and disease control. Proc Biol Sci. 2003;270:2565–2571. doi: 10.1098/rspb.2003.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.House T, Keeling MJ. The impact of contact tracing in clustered populations. PLOS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woolhouse MEJ, Dye C, Etard JF, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grassly NC, Pons-Salort M, Parker EPK, White PJ, Ferguson NM, Imperial College COVID-19 Response Team Comparison of molecular testing strategies for COVID-19 control: a mathematical modelling study. Lancet Infect Dis. 2020;20:1381–1389. doi: 10.1016/S1473-3099(20)30630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keeling MJ, Hollingsworth TD, Read JM. Efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19) J Epidemiol Community Health. 2020;74:861–866. doi: 10.1136/jech-2020-214051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hellewell J, Abbott S, Gimma A, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaafar R, Aherfi S, Wurtz N, et al. Correlation between 3790 qPCR positives samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1491. published online Sept 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberg CR. Editorial: making the best use of test kits for COVID-19. Am J Epidemiol. 2020;189:363–364. doi: 10.1093/aje/kwaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beldomenico PM. Do superspreaders generate new superspreaders? A hypothesis to explain the propagation pattern of COVID-19. Int J Infect Dis. 2020;96:461–463. doi: 10.1016/j.ijid.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young S, Taylor SN, Cammarata CL, et al. Clinical evaluation of BD Veritor SARS-CoV-2 point-of-care test performance compared to PCR-based testing and versus the Sofia 2 SARS Antigen point-of-care test. J Clin Microbiol. 2020;59:e02338–e02340. doi: 10.1128/JCM.02338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asghar H, Diop OM, Weldegebriel G, et al. Environmental surveillance for polioviruses in the Global Polio Eradication Initiative. J Infect Dis. 2014;210(suppl 1):S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Reilly KM, Allen DJ, Fine P, Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. Lancet Microbe. 2020;1:e189–e190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar M, Patel AK, Shah AV, et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available in the manuscript or supplementary materials and replication code is available at https://github.com/leekshaffer/transmission-tracing.


