Abstract
Purpose:
Numerous randomized trials have demonstrated noninferiority of single- versus multiple-fraction palliative radiation therapy (RT) in the management of uncomplicated bone metastases; yet there is neither a clear definition of what constitutes a complicated lesion, nor substantial data regarding the prevalence of such complicating features in clinical practice. Thus, we identify a range of evidence-based operational definitions of complicated symptomatic bone metastases and characterize the frequency of such complicating features at a high-volume, tertiary care center.
Methods and Materials:
A retrospective review of patients seen in consultation for symptomatic bone metastases between March 1, 2007, and July 31, 2013, at Johns Hopkins Hospital identified patient and disease characteristics. Descriptive statistics characterized the frequency of the following complicating features: prior RT, prior surgery, neuraxis compromise, pathologic fracture, and soft tissue component at the symptomatic site. A range of definitions for complicated bone metastases was evaluated based on combinations of these features. Uni- and multivariable logistic regressions evaluated the odds of complicated bone metastases as a function of site of primary cancer and of the symptomatic target lesion.
Results:
A total of 686 symptomatic bone metastases in 401 patients were evaluated. Percent of target sites complicated by prior RT was 4.4%, prior surgery was 8.9%, pathologic fracture was 20.6%, neuraxis compromise was 52.0% among spine and medial pelvis sites, and soft tissue component was 38.6%. More than 96 possible definitions of complicated bone metastases were identified. The presence of such complicated lesions ranged from 2.3% to 67.3%, depending on the operational definition used. Odds of a complicated lesion were significantly higher for spine sites and select nonbreast histologies.
Conclusions:
In this retrospective study, we found complicated symptomatic bone metastases may be present in up to two-thirds of patients. Literature review also demonstrates no clear standard definition of complicated bone metastases, potentially explaining underutilization of single-fraction palliative RT in this setting.
Introduction
In the seminal systematic review of randomized trials of radiation therapy (RT) in the management of uncomplicated symptomatic bone metastases, Chow et al found no significant difference in pain control across studies comparing single- versus multiple-fraction RT.1 These data have resulted in consensus recommendations from the American Society of Radiation Oncology (ASTRO),2 the American College of Radiology (ACR),3–5 and the National Comprehensive Cancer Network (NCCN),6–8 supporting use of single-fraction RT in a range of clinical scenarios.
Yet, the definition of uncomplicated bone metastases for which single-fraction RT may be most appropriate remains ill-defined. Recently, Cheon et al, sought to clarify this definition by reviewing inclusion and exclusion criteria for 25 trials included in the aforementioned systematic review.9 The authors concluded that a conservative definition of “uncomplicated metastases” supported across studies is the “presence of painful bone metastases unassociated with impending or existing pathologic fracture or existing spinal cord or cauda equina compression.”9
Table 1 summarizes clinical features that would result in exclusion from the trials reviewed by Cheon et al, plus 4 additional randomized trials published subsequent to their analysis10,11 and included in ASTRO’s most recent systematic review.2 After combining separate studies that reported the same patient population in the same row and excluding one study reported as abstract alone,12 this review results in 23 unique sets of exclusion criteria.
Table 1.
Summary of features used as exclusion criteria by randomized studies of singe- versus multiple-fraction radiation therapy for symptomatic bone metastases*
| Study N = 23† | Prior therapy |
Fracture |
Nervous system compromise |
|||||
|---|---|---|---|---|---|---|---|---|
| RT | Surgery | Long bone | Vertebra | NOS | Impending | CNS | PNS | |
| Altundag13 | ✓ | ✓ | ✓ | ✓ (Symptoms of SCC) |
||||
| Amouzegar Hashemi14 | ✓ | ✓ | SCC | |||||
| Badzio15 | ✓ | ✓ | ||||||
| BPTWP16 | ✓ | ✓ | ||||||
| Cole17 | ✓ | ✓ | ✓ (SCC syndrome) |
✓ (PN compression syndrome) |
||||
| El-Shenshawy18 | ✓ | ✓ (Except vertebral compression fracture) |
✓ (Suspicion of SCC) |
|||||
| Foro19 | ✓ | ✓ | ✓ (Medulla compression) |
|||||
| Foro Arnalot20 | ✓ | ✓ | ✓ (If Mirel’s criteria >9) |
✓ (Clinical or radiologic evidence of SCC) |
||||
| Gaze21 | ✓ | ✓ | ✓ (If collapse above L2) |
✓ | ✓ | ✓ (SCC) | ||
| Gutiérrez Bayard et al10 | ✓ | ✓ (If lesion requires fixation) |
✓ (SCC) |
|||||
| Prior therapy | Fracture | Nervous system compromise | ||||||
| Study | RT | Surgery | Long bone | Vertebra | NOS | Impending | CNS | PNS |
| Hamouda22 | ✓ | ✓ | ||||||
| Hartsell,23 Howell24 |
✓ | ✓ | ✓ (Clinical or radiologic evidence of SCC or effacement) |
✓ (Clinical or radiologic evidence of CEC or effacement) |
||||
| Kaasa,25 Sande26 |
✓ | ✓ (If lesion requires fixation) |
✓ (SCC) |
|||||
| Kagei27 | ✓ (Except if vertebral compress-ion fracture) |
|||||||
| Koswig28 | ✓ | |||||||
| Majumder et al11 | ✓ | ✓ | ✓ | ✓ (SCC) |
||||
| Nielsen29 | ✓ | ✓ (Except if vertebral compress-ion fracture) |
✓ (Suspicion of SCC) |
|||||
| Ozsaran30 | ✓ | ✓ | ||||||
| Price31 | ✓ | ✓ | ||||||
| Roos32 | ✓ | ✓ | ✓ (Clinical or radiologic evidence of SCC) |
✓ (Clinical or radiologic evidence of CEC) |
||||
| Safwat33 | ✓ | ✓ (Clinical or radiologic evidence of SCC) |
✓ (Clinical or radiologic evidence of CEC) |
|||||
| Sarkar34 | ✓ | ✓ | ||||||
| Steenland,35 Van der Linden,36 Meeuse37 |
✓ | ✓ (If lesion requires fixation) |
✓ (SCC) |
|||||
| Total studies with exclusion criterion, N (%) | 18 (78%) | 3 (13%) | 3 (13%) | 1 (4%) | 17 (30%) | 4 (17%) | 15 (65%) | 4 (17%) |
Abbreviations: CEC = cauda equina compression; CNS = central nervous system; NOS = not otherwise specified; PNS = peripheral nervous system; RT = radiation therapy; SCC = spinal cord compression.
The checkmarks (✓) indicate presence of exclusion criteria, with qualifying details in parenthesis if present.
Twenty-nine studies considered, with published trials containing the same study population and exclusion criteria grouped in 1 row, resulting in 23 unique sets of exclusion criteria considered.
Notably, there are limitations to the exclusion criteria rendered across studies. Although 18 out of 23 trials excluded patients on the basis of existing or impending pathologic fracture, the studies lack details regarding clinical or radiologic features that constitute fracture. Similarly, 15 of the 23 trials excluded cases because of neuraxis compromise, but there is little description of what comprises spinal cord or peripheral nerve compression across trials.
It is additionally noted that consensus recommendations for fractionation vary on the basis of features not contained with the conservative definition of complicated metastases by Cheon et al. Table 2 provides a summary of key differences across guidelines in the setting of prior RT, prior surgery, existing or impending pathologic fracture, presence of soft tissue component, location of the treatment site, and presence of neuraxis compromise. Moreover, there is little data describing the prevalence of these potentially complicating features across possible definitions despite their propensity to dictate treatment decisions.
Table 2.
Summary of key variations in consensus recommendations for radiotherapy on the basis of possible complicating features of symptomatic bone metastasis
| Clinical feature | ASTRO2 | ACR3–5 | NCCN6,7 |
|---|---|---|---|
| Prior radiation therapy | Trial result available for 1-5 fractions EBRT | Consider 1-6 fractions EBRT | Consider SBRT for spine sites |
| Prior surgery | - | Consider multiple fraction radiation therapy | Consider SBRT for spine sites if oligometastatic or radioresistant |
| Pathologic or impending fracture | - | Consider multiple fraction radiation therapy | - |
| Soft tissue component | - | - | Consider multiple fraction radiation therapy for NSCLC metastases with soft tissue component |
| Uncomplicated spine and other critical sites | Single-fraction radiation therapy most appropriate when limited life expectancy | Consider multiple fraction radiation therapy unless limited life expectancy | Consider multiple fraction radiation therapy for estimated survival >6 mo |
| Spinal cord or cauda equina compression | - | Consider multiple fraction radiation therapy unless limited life expectancy | Consider SBRT for spine sites if oligometastatic or radioresistant |
Abbreviations: ACR = American College of Radiology; ASTRO = American Society for Radiation Oncology; EBRT = external beam radiation therapy (conventional); NCCN = National Comprehensive Cancer Network; NSCLC = non-small cell lung cancer; SBRT = stereotactic body radiation therapy.
To augment understanding of potential complicating factors for which single-fraction palliative RT is not strictly evidence-based, we review the frequency of these features at our institution across a breadth of operational definitions supported by the available literature.
Methods and Materials
Data source and study population
Patients seen in consultation for symptomatic bone metastases between March 1, 2007 and July 31, 2013 at the Johns Hopkins Hospital Department of Radiation Oncology were identified using our departmental patient database. Data was queried among patients >18 years of age using International Classification of Diseases, Ninth and Tenth Revision codes for bone site or treatments using <15 fractions.
Study population
The query yielded 424 patients seen in consultation for bone metastases. We limited analysis to patients with pathologically or radiologically confirmed metastatic cancer with dissemination to the bone, resulting in pain or other neurologic sequelae. Owing to infrequent use of stereotactic body radiation therapy during the study period, patients seen in consultation for this approach were excluded. In total, 23 patients were excluded.
Patient and disease characteristics
A review of the electronic medical record was performed for each patient to collect basic demographic information. Site of symptomatic bone metastasis was categorized as spine, hip or pelvis, extremity, chest wall, and skull. If the bone lesion involved more than one site category, the site affected by the majority of the lesion was recorded. Time between consultation for palliative RT and both (1) initial diagnosis with cancer and (2) first diagnosis with any form of metastatic disease were documented in months. Receipt of palliative RT concurrently to any other noncontiguous metastatic site as well as receipt of multiple separate courses of palliative RT during the study period was recorded.
Potential complicating factors were identified on the basis of their inclusion in randomized studies or consensus statements reviewed in Tables 1 or 2, respectively, including:
Prior RT. Treatment with prior definitive or palliative radiation therapy to the current site of symptomatic metastasis was recorded.
Prior surgery. Open and minimally invasive surgical intervention at the current site of symptomatic metastasis at any time before consultation was recorded.
Pathologic fracture. Presence of pathologic fracture was determined by documentation of fracture by attending physicians in radiology, orthopedic surgery, or neurosurgery. Given lack of standardized means for characterizing impending fractures during the study period, only existing fractures were considered. For spine sites, fracture was defined as documentation of loss of vertebral body height, compression fracture, or vertebral body collapse.
Neuraxis compromise. Given a range of definitions used to characterize spinal cord and peripheral nerve compression from Table 1, we documented radiologic evidence of central canal stenosis, neuroforaminal stenosis, or spinal cord edema. Due to the retrospective nature of this study, the presence of documented symptoms associated with these findings was not required. Radiologic evidence was determined by review of computed tomography (CT) and magnetic resonance imaging (MRI) scans performed within 1 month of consultation whenever available. All images were personally reviewed by author SA. When not available, documentation per radiology reports or per clinical notes was used. At a minimum, CT from radiation planning was reviewed when performed. Neuraxis compromise was only considered for spine and medial pelvic sites. To better capture the extent of neuraxis compromise, the Epidural Spinal Cord Compression (ESCC) scale was used. This well-accepted rating system uses axial T2-weighted MRIs to grade the extent of epidural disease on a 6-point scale (0, 1a, 1b, 1c, 2 and 3) for spine lesions above the conus medullaris, as previously described by Bilsky et al.38 Two authors (SA and CE) independently reviewed all cases at risk for potential ESCC, and cases with rating disagreement were reviewed together by these authors to render a final score. Cohen’s kappa statistic for interrater reliability of ECSS was calculated.39 Target sites below the level of the conus or for which neuraxis compromise was due to causes other than epidural disease (eg, retropulsion of bone or herniated discs) were not scored using the ESCC scale. For the purposes of coding, ESCC 0 and 1a were considered to represent no definite central canal stenosis.40
Soft tissue component. The presence of an extraosseous soft tissue component directly extending from the site of bone metastasis was noted. As with neuraxis compromise, this was confirmed via direct review of available CT and MRI images by SA whenever available, with minimum review of the planning CT if performed. In the absence of these studies, radiology reports or clinical notes were used.
For patients seen in consultation for more than one symptomatic site of bone disease, each noncontiguous site was evaluated separately. Noncontiguous sites were defined as those for which RT would be delivered using 2 separate and nonabutting treatment fields. Contiguous sites treated with abutting fields owing to large treatment area were considered as one site. Multiple noncontiguous sites within the same patient were permitted and included in the analysis.
Outcomes analysis
The presence or absence of a complicating feature was evaluated as a binary outcome. When the presence of the features was indeterminate or could not be confirmed by imaging or documentation, the feature was coded as absent. For target sites with prior surgery, no additional radiologically assessed complicating features were coded owing to inability to accurately review imaging in the setting of artifact and postoperative changes. Thus, only prior RT status was documented in patients with prior surgery.
The frequency of each potential complicating feature was first considered individually. We then sought to demonstrate the breath of operational definitions that could constitute a complicated lesion as per the literature cited in Tables 1 and 2. To do so, the frequencies of complicated bone metastases were estimated using definitions derived from all possible combinations of the aforementioned complicating features. When assessed as combinations of features, presence of at least one complicating feature included in the definition was sufficient for coding as a complicated bone metastasis.
For the variables of prior RT, prior surgery, and soft tissue component, one definition (described earlier) was used. For pathologic fracture, 2 definitions were considered: any fracture versus nonspine fractures only. For neuraxis compromise, 3 definitions were considered: all neuraxis compromise, central canal stenosis only, or spinal cord edema only. No study included consideration of neuroforaminal stenosis alone, so this component was not assessed individually. Only one definition of pathologic fracture and neuraxis compromise was included at a time when considering combinations of features.
Statistical analysis
Descriptive statistics were performed for patient and disease characteristics. Associations between potential complicating features and the corresponding target site of symptomatic bone metastasis were analyzed using Fisher exact and analysis of variance tests. Odds ratios for presence of complicated bone metastases as a function of primary cancer site, RT target site, time from diagnosis to consultation, and delivery of RT to other palliative sites were assessed using uni- and multivariable logistic regressions. Given the hypothesis that there may be complex relationships between variables, we made an a priori decision to include all variables in the multivariable assessment regardless of univariable results.
In the case of multiple palliative RT treatments within the same patient to different target bone sites, each target site was considered independently in these analyses.
All statistical tests used a 2-sided α = 0.05 for significance testing. Statistics were performed using Stata version 14.0 (College Station, TX).
This study was approved by the Institutional Review Board of Johns Hopkins Hospital (IRB00125143), with waiver of informed consent.
Results
A total of 686 noncontiguous target sites of symptomatic bone disease were evaluated for 401 patients. Patients were treated at an average of 1.7 sites (standard deviation 1.1) during the study period. Among included patients, primary cancer was 30.6% lung, 19.4% breast, 14.0% prostate, 5.0% leukemia/lymphoma/myeloma, and 31.1% other. Among separate lesions considered, site of symptomatic disease were 49.6% spine, 21.3% hip/pelvis, 17.5% extremity, 8.5% chest wall, and 3.2% skull. Table 3 shows disease features and treatment characteristics by target site.
Table 3.
Characteristics of the target symptomatic bone metastasis by treatment site
| Spine, N = 340 |
Extremity, N = 120 |
Hip/pelvis, N = 146 |
Chest wall, N = 58 |
Skull, N = 22 |
||
|---|---|---|---|---|---|---|
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | P value* | |
| Primary cancer site | ||||||
| Breast | 66 (19.4%) | 21 (17.5%) | 33 (22.6%) | 8 (13.8%) | 5 (22.7%) | - |
| Prostate | 55 (16.5%) | 11 (9.2%) | 20 (13.7%) | 5 (8.6%) | 4 (18.2%) | |
| Leukemia/lymphoma/myeloma | 22 (6.5%) | 5 (4.2%) | 2 (1.4%) | 0 | 5 (22.7%) | |
| Lung | 197 (28.5%) | 37 (30.8%) | 42 (28.8%) | 31 (53.5%) | 3 (13.6%) | |
| Other | 99 (29.1%) | 46 (38.3%) | 49 (33.6%) | 14 (24.1%) | 5 (22.7%) | |
| Time from diagnosis to consultation in months | ||||||
| Time from initial cancer diagnosis | 46.2 (90.5) | 64.4 (141.8) | 61.1 (43.6) | 37.8 (43.6) | 62.5 (93.6) | .398 |
| Time from diagnosis with any metastasis† | 42.3 (162.2) | 41.8 (77.1) | 32.4 (126.8) | 20.7 (26.7) | 50.8 (97.5) | .803 |
| Other palliative RT | ||||||
| >1 concurrent site | 113 (33.2%) | 48 (40.0%) | 53 (36.3%) | 26 (44.8%) | 4 (18.8%) | .139 |
| >1 course in study period | 201 (59.1%) | 80 (66.7%) | 108 (74.0%) | 45 (77.6%) | 15 (68.2%) | .005 |
| Presence of complicating features at the target site | ||||||
| Prior RT | 13 (3.8%) | 4 (3.3%) | 8 (5.5%) | 3 (5.2%) | 2 (9.1%) | .691 |
| Prior surgery | 38 (11.2%) | 13 (10.8%) | 9 (6.2%) | 0 | 1 (4.6%) | .016 |
| Pathologic fracture | 112 (32.9%) | 9 (7.5%) | 14 (9.6%) | 6 (10.3%) | 0 | <.001 |
| Neuraxis compromise‡ | 170 (50.0%) | - | 10 (6.9) | - | - | <.001 |
| Soft tissue component | 134 (39.4%) | 33 (27.5%) | 54 (36.9%) | 32 (55.2%) | 13 (59.1%) | .0004 |
Abbreviations: RT = radiation therapy; SD = standard deviation.
P value as per Fisher exact test for categorical and analysis of variance for continuous variables compared between target sites. No statistical testing was performed on the site of bone metastasis primary cancer site comparison owing to prohibitive number of cells.
Time from first radiologically or pathologically confirmed metastasis in the body to consult, not limited to the target site.
Only sites of spine and medial pelvis considered, N = 346.
Time from diagnosis to consultation by target site
Across patients, mean time between the initial cancer diagnosis and consultation for palliative RT at the target sites was 52 months (standard deviation [SD] 116.1). Mean time between diagnosis with any form of metastatic disease and consultation for palliative RT at the target site was 38.4 months (SD 132.5). There was no significant difference in time from initial diagnosis or metastatic diagnosis to consultation by RT target site (Table 3).
Frequency of individual complicating features
Table 3 also displays the frequency of various complicating features arranged by target site. There were significant differences in prevalence of these features by target site for all factors except for presence of prior RT.
Prior RT. Prior RT was noted in 30 target sites (4.4%). Of all prior RT cases, 43.3% were spine, 13.3% were extremity, 26.7% were hip/pelvis, 10.0% were chest wall, and 6.7% were skull sites.
Prior surgery. Prior surgery was noted in 61 target sites (8.9%). Of all postoperative cases, 62.3% were spine, 21.3% were extremity, 14.8% were hip/pelvis, 0% were chest wall, and 1.6% were skull sites.
Pathologic fracture. Definite pathologic fracture was identified in 141 target lesions (20.6%). Of all fractures, 79.4% were spine, 6.4% were extremity, 9.9% were hip/pelvis, 4.3% were chest wall, and 0% were skull sites.
Neuraxis compromise. Among 346 sites of the spine and medial pelvis considered for this complicating feature, 180 (52.0%) were noted to have definite neuraxis compromise. Figure 1 delineates details of neuraxis compromise. When neuraxis compromise was present, 16.6% of cases were central canal stenosis (without spinal cord edema) only, 18.9% were neuroforaminal stenosis only, 56.7% were both central canal stenosis (without spinal cord edema) and neuroforaminal stenosis, and 8.9% were central canal stenosis with spinal cord edema and neuroforaminal stenosis. Of the sites evaluated for neuraxis compromise, 198 (57.2%) had an MRI amenable to review. Fifty-eight cases involved sites below the conus medullaris or canal stenosis owing to bony retropulsion only; these cases did not receive ESCC scores. Among the remaining 140 cases, 26.1% were scored as 0, 9.3% scored as 1a, 20.0% scored as 1b, 12.9% scored as 1c, 15.0% scored as 2, and 16.4% scored as 3. Kappa for interrater reliability for ESCC between authors SA and CE was 0.882, which is classified as excellent.39
Soft tissue component. A definite soft tissue component was identified in 265 (38.6%) target lesions. Of all lesions with a soft tissue component, 50.2% were spine, 12.5% were extremity, 20.4% were hip/pelvis, 12.1% were chest wall, and 4.9% were skull sites.
Fig. 1.
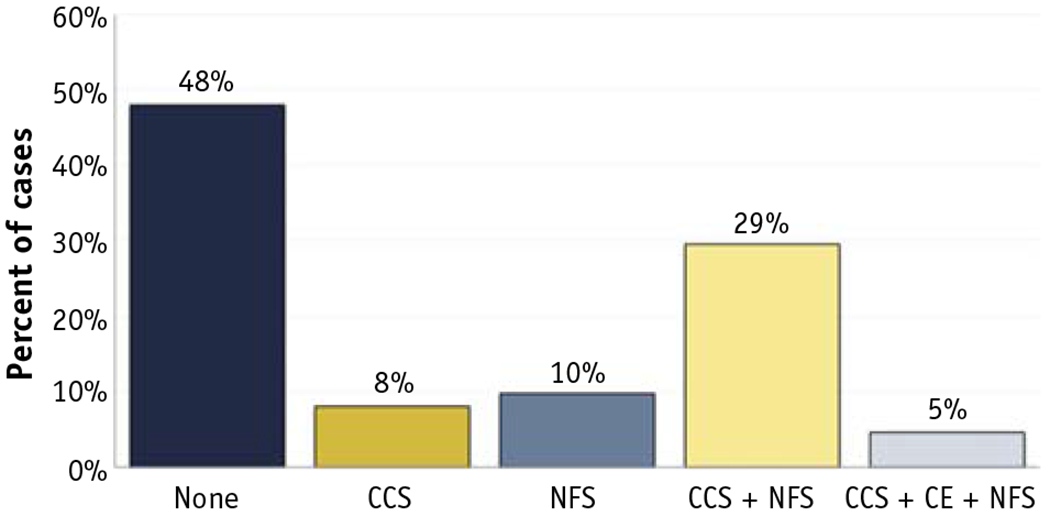
Percent of all target spine and medial pelvis bone metastases with neuraxis compromise, N = 346. Abbreviations: CCS = central canal stenosis; CE = cord edema; NFS = neuroforaminal stenosis.
Frequency of complicated bone metastases across a range of definitions
For illustrative purposes only, Appendix E1 (available online at https://doi.org/10.1016/j.ijrobp.2019.11.033) shows the percent of cases with at least one complicating feature present across 96 possible definitions created from various combinations of the 8 variables listed. Depending on the definition used, the percent of complicated bone metastases ranged from 2.3% to 67.3%.
Figure 2 shows the percent of cases with at least one complicating feature present across a selection of commonly used definitions of complicated symptomatic bone metastasis cited in randomized studies and census statements. Variable definitions of fracture and neuraxis compromise were included to reflect uncertainty in how these features were specified. The most inclusive definition yielded 67.3% complicated lesions. Conversely, a stricter definition that required spinal cord edema and excluded both vertebral body compression fractures and soft tissue components resulted in classification of 19.1% complicated lesions.
Fig. 2.
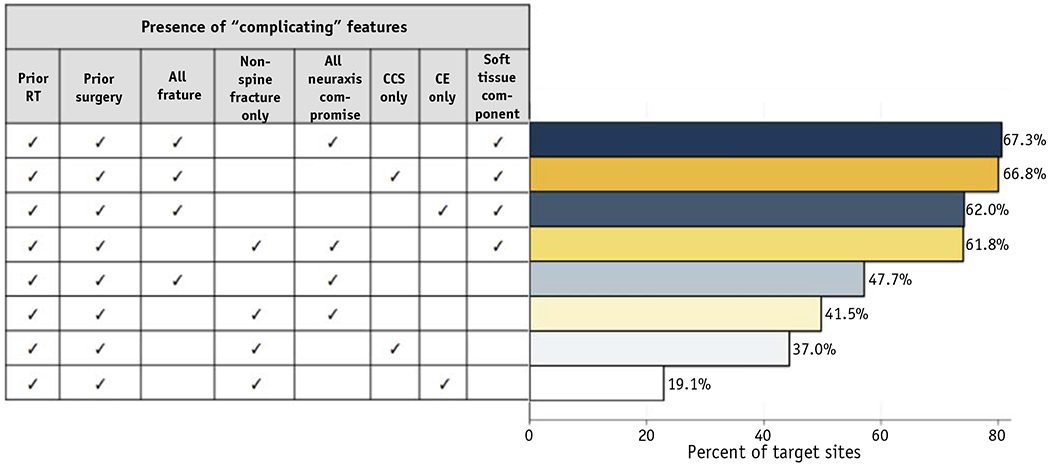
Percent of all target symptomatic bone metastases cases with at least one complicating feature across most common definitions of complicated symptomatic bone metastasis. The checkmarks (✓) indicate that the selected variable was used as part of the operational definition for complicated bone metastasis. Abbreviations: CCS = central canal stenosis; CE = cord edema; RT = radiation therapy.
Odds of complicated metastasis by disease features
Table 4 shows univariable logistic regressions for odds of a complicated symptomatic bone metastasis using the most inclusive definition. On univariable analysis, compared with breast cancer metastases, leukemia/lymphoma/myeloma and other cancer (but not prostate or lung cancer) yielded higher odds of complicated bone metastases. Compared with spine target sites, extremity, hip/pelvis, and chest wall (but not skull) sites had significantly lower odds of complicated bone metastases. Shorter time from initial cancer diagnosis to consultation for palliative bone RT significantly increased the odds of complicated bone metastases, but there was no association between odds of such lesions with time from diagnosis with any metastatic disease to consultation. Odds of having a complicated lesion were significantly higher for patients who received concurrent palliative RT to sites other than the target site and who received multiple courses of palliative RT during the study period. With the exception of time from diagnosis to consultation and receipt of multiple palliative RT courses, all other significant associations persisted on multivariable logistic regressions after controlling for primary cancer site, target symptomatic bone site, time from diagnosis to consultation, and receipt of other palliative RT.
Table 4.
Uni- and multivariable logistic regressions for odds of complicated symptomatic bone metastasis using most inclusive definition* as a function of primary cancer site, target symptomatic bone site, and time from diagnosis to consult
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% confidence interval | P value | Odds ratio | 95% confidence interval | P value | |
| Primary cancer site | ||||||
| Breast | - | - | - | - | - | - |
| Prostate | 1.21 | 0.71-2.08 | .483 | 1.35 | 0.70-2.61 | .368 |
| Leukemia/lymphoma/myeloma | 3.39 | 1.32-8.75 | .011 | 3.80 | 1.21-11.94 | .022 |
| Lung | 1.39 | 0.89-2.18 | .145 | 1.52 | 0.84-2.74 | .169 |
| Other | 2.14 | 1.35-3.40 | .001 | 2.61 | 143-4.75 | .002 |
| Target symptomatic bone site | ||||||
| Spine | - | - | - | - | - | - |
| Extremity | 0.19 | 0.12-0.30 | <.001 | 0.14 | 0.08-0.24 | <.001 |
| Hip/pelvis | 0.26 | 0.17-0.40 | <.001 | 0.32 | 0.19-0.52 | <.001 |
| Chest wall | 0.34 | 0.19-0.61 | <.001 | 0.35 | 0.18-0.68 | .002 |
| Skull | 0.48 | 0.19-1.22 | .123 | 0.31 | 0.11-0.90 | .031 |
| Time from diagnosis to consultation in months | ||||||
| Time from initial cancer diagnosis | 0.99 | 0.99-1.00 | .037 | 0.99 | 0.99-1.00 | .755 |
| Time from diagnosis with any metastasis† | 0.99 | 0.99-1.00 | .084 | 0.99 | 0.99-1.00 | .133 |
| Other palliative RT | ||||||
| >1 concurrent site | 0.52 | 0.37-0.72 | <.001 | 0.58 | 0.37-0.94 | .025 |
| >1 course in study period | 0.52 | 0.36-0.74 | <.001 | 0.68 | 0.40-1.13 | .136 |
Definition includes the presence of at least one of the following features: prior radiation therapy, prior surgery, any facture, any neuraxis compromise, or soft tissue component.
Time from first radiologically or pathologically confirmed metastasis in the body to consult, not limited to the target site.
Discussion
In this retrospective study, we found that complicated symptomatic bone metastases were identified in up to 67% of patients at our institution. However, a breadth of operational definitions for complicated lesions can be deduced from randomized trials and consensus statements. As such, the frequency of complicated lesions varies widely. To our knowledge, this is the first attempt to characterize frequency of complicated bone metastases using granular patient-level data, detailed radiologic review, and a range of definitions for the outcome of interest. Given that such complicated lesions may have been excluded from trials of single- versus multiple-fraction palliative RT, our results lend insight into the clinical applicability of consensus statements when selecting appropriate palliative regimens in our patient population.
Our findings are generally congruent with the sparse literature available describing rates of complicated metastases. Tiwana et al report a similar population-based experience in British Columbia using a definition of complicated bone metastasis including clinical or radiologic features suggestive of actual or impending pathologic fracture or neurologic compromise. According to this definition, complicated lesions were reported in 34% of cases in their large series of 3,200 bone sites treated with palliative RT.41 Similarly, when considering all types of pathologic fractures and neuraxis compromise but no other complicating features, we report a rate of 36.4% (Appendix E1, available online at https://doi.org/10.1016/j.ijrobp.2019.11.033).
Our data show that one of the most frequently encountered complicating features was neuraxis compromise. Furthermore, we found that odds of having a complicated lesion were highest at spine sites. These findings are congruent with data reporting spine as the most common site of bone metastasis,42 with an associated high risk of developing skeletal-related events and resultant decrement to quality of life.43 Notably, neuraxis compromise was among the most complex features to operationalize. In randomized trials, exclusion criteria related to the nervous system ranged from simple notation of “spinal cord compression” to the use of qualifiers such as suspected compression, radiologically confirmed compression, effacement of the cord, or presence of clinical symptoms consistent with compression. Some trials also excluded cases due to clinical/radiologic evidence of cauda equina or peripheral nerve compression (Table 1). In the absence of standardized clinical or radiologic criteria to define neuraxis compromise, we erred on the side of recording radiologic presence of central canal stenosis, neuroforaminal stenosis, or spinal cord edema. Notably, for the subset of cases that could be scored using the ESCC scale, approximately equal thirds of cases had scores of 0 to 1a, 1b to 1c, and 2 to 3, suggesting that a large majority of spine cases may have substantial neuraxis compromise.
Our definitions of neuraxis compromise are associated with notable strengths and limitations. Strengths include its utilization of relatively objective measures and coverage of most of the exclusion criteria from the randomized trials evaluated. Use of a radiologic measure is aligned with current management frameworks used for spinal tumors, such as the MRI-based ESCC scoring criteria.38 Yet unlike the ESCC scoring method, our measure can be determined using CT- or MRI-based imaging, affording greater generalizability. Studies cited in Table 1 did not consistently specify which—if any—imaging modality was required to assess for neuraxis compromise, and our data suggests that MRIs are only completed in 57% of cases in which the target site is the spine or medial pelvis. Although this methodology is more inclusive, it does create the risk of underreporting neuraxis compromise in patients evaluated with CT alone. An additional limitation of our definition is the lack of detail regarding clinical symptoms of neuraxis compromise. Unfortunately, inclusion of such data was limited by the retrospective nature of our study. Another limitation is that the frequency of complicated metastasis varies widely depending on which of our criteria is applied when defining neuraxis compromise. Although a flexible definition enhances applicability over a wider range of cases, it does not permit for a precise classification of which types of neuraxis lesions are best considered complicated.
Another frequent complicating feature was fracture, which was again ill-defined on the basis of available studies and guidelines. Given high rates of pathologic fracture of the spine among patients with metastatic disease,44 determining whether vertebral body compression should be considered a complicating fracture was particularly problematic. Whereas some of the randomized studies expressly specified exclusion of all nonspine fractures, at least one excluded cervical through thoracic vertebral body collapse only, and most did not specify site of fracture at all. Although there are available radiologic-based guidelines to direct management in this setting, such as the Spinal Instability Neoplastic Score (SINS) criteria,45 the relevance of such ratings to questions regarding single- versus multiple-fraction RT is unknown. As with the definitions used for neuraxis compromise, the decision to consider both (a) all fractures and (b) nonspine fractures only when estimating complicated metastases enhances flexibility but limits precision when determining frequency of complicated lesions. An additional limitation is our inability to include impending fracture, given no standardized definition for this variable in our field.
Although not prevalent in the study population, we prioritized the use of prior RT or surgery at the target site as key to the definition of complicated bone metastases. All of the randomized trials that we analyzed cited prior RT as an exclusion criterion. Conversely, prior surgery was inconsistently specified as cause for exclusion. However, prior surgery is inextricably linked with existing or impending fracture for most bone sites, and it is a key feature for dictating fractionation schemes in both ACR and NCCN guidelines.3–6 As such, both variables were included in all key definitions specified in Fig. 2.
Perhaps most contentious was our decision to include the presence of a soft tissue component as a potential complicating feature. As found by Cheon et al, in their initial analysis,9 we also determined that none of the 29 trials considered in Table 1 excluded cases on the basis of a soft tissue component. However, this feature may contribute to bony instability or fracture, and when present near the neuraxis, it may lead to nervous system compromise. Moreover, presence of a soft tissue component is used to guide fractionation decision as per the NCCN consensus guidelines for non-small cell lung cancer,7 justifying our consideration of this feature.
Importantly, our study offers insight into additional factors associated with the presence of complicated bone metastases such as shorter time from initial cancer diagnosis to consultation for palliative RT and not receiving concurrent palliative RT to other palliative sites. Shorter time between initial diagnosis and the need for palliative RT for a complicated bone metastasis may in part reflect higher symptom burden associated with complicated lesions. Whereas a complicated lesion may require more immediate treatment, an uncomplicated one may be more amenable to attempts to control symptoms through other routes such as systemic therapy and pain medication. Similarly, perhaps patients with complicated lesions are more likely to receive palliative RT to only one site at a time owing to high symptom burden associated with the complicated lesion, precluding attempts to manage less symptomatic lesions. From an optimistic perspective, these data may suggest against the possibility that complicated lesions regularly develop from known uncomplicated bone metastases due to delayed referral patterns to radiation oncology.
Lastly, it should be noted that all cases in our analysis came from a high-volume radiation oncology department, with the majority of patients treated in a tertiary care hospital setting. As such, the frequency of complicated metastases noted may be not be generalizable outside of this context, although similar rates of complicated metastases using the definitions of Tiwana et al,19 are reassuring. Our study question required review of granular, patient-level data, which impaired the ability to use information from multi-institutional or national databases. Similar studies from community-level practices or international institutions would be necessary to characterize frequency of complicated lesions across definitions more broadly.
Because complicated bone metastases may have been excluded from randomized trials comparing single- versus multiple-fraction palliative RT, lack of a consensus definition and high frequency of possible complicating features may contribute to low utilization of single-fraction RT observed in current clinical practice. Despite efforts by campaigns such as Choosing Wisely to encourage use of foreshortened regimens of palliative RT,46 practice patterns suggest persistent use of prolonged palliative RT regimens irrespective of survival.47,48 In the absence of a concrete definition of complicated bone metastases, the data presented offers providers a range of definitions that may be used at their discretion when selecting appropriate fractionation based on patient-specific clinical features. Consensus organizations and cooperative groups may also consider limitations and definitional inconsistencies identified within our study when creating updated palliative RT guidelines and future trials. Institutionally, we have used these definitions to aid in the development of individualized treatment recommendations as part of a decision support tool for managing bone metastases.49
Supplementary Material
Summary.
Neither a consistent definition for nor the prevalence of complicated symptomatic bone metastases has been established. Up to 97 definitions of complicated metastasis can be identified based on the literature. A retrospective review of 686 symptomatic lesions in 401 patients evaluated for radiotherapy for bone metastases was performed. Frequency of complicated metastases ranged from 2% to 67%, depending on the operational definition used.
Acknowledgments
Sources of support: This work was supported by a grant from the National Institutes of Health (5KL2TR001077) to S.A.
Disclosures: S.A. received grants from Elekta, AB, nonfinancial support from Angiodynamics, and personal fees from Allegheny Health Network, all outside the submitted work. J.W. received personal fees from Allegheny Health Network and is an editor for the International Journal of Radiation Oncology*Biology*Physics. T.S. received nonfinancial support from Emmanuel Merck, Darmstadt Serono, personal fees and nonfinancial support from Allergan, all outside the submitted work. T.M. has received a grant from the Radiation Oncology Institute, outside the submitted work and is involved in a health-related start-up called Oncospace, Inc, which is not affiliated with this project. T.D. is the President of the American Society for Radiation Oncology and has received nonfinancial support from Elekta, Sanofi-Aventis, and Varian, Inc.
Footnotes
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.11.033.
References
- 1.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112–124. [DOI] [PubMed] [Google Scholar]
- 2.Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract Radial Oncol 2017;7:4–12. [DOI] [PubMed] [Google Scholar]
- 3.Kim EY, Chapman TR, Ryu S, et al. ACR appropriateness criteria ® non-spine bone metastases. J Palliat Med 2014;18:11–17. [DOI] [PubMed] [Google Scholar]
- 4.Lo SS-M, Lutz ST, Chang EL, et al. ACR appropriateness criteria ® spinal bone metastases. J Palliat Med 2012;16:9–19. [DOI] [PubMed] [Google Scholar]
- 5.Lo SS-M, Ryu S, Chang EL, et al. ACR appropriateness criteria ® metastatic epidural spinal cord compression and recurrent spinal metastasis. J Palliat Med 2015;18:573–584. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN: Central Nervous System Cancers V1. 2019. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Published 2019. Accessed January 10, 2019.
- 7.NCCN: Non-Small Cell Lung Cancer. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Published 2019. Accessed January 10, 2019.
- 8.NCCN: Prostate cancer. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published 2019. Accessed February 10, 2019.
- 9.Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases radiation trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol 2015;4:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez Bayard L, Salas Buzón M, del C, Angulo Paín E, de Ingunza Barzón L. Radiation therapy for the management of painful bone metastases: Results from a randomized trial. Reports Pract Oncol Radiother 2014;19:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumder D, Chatterjee D, Bandyopadhyay A, Mallick SK, Sarkar SK, Majumdar A. Single fraction versus multiple fraction radiotherapy for palliation of painful vertebral bone metastases: A prospective study. Indian J Palliat Care 2012;18:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkbride P, Warde P, Panzarella T, Aslanidis J, McKenzie M, Sun A. A randomised trial comparing the efficacy of a single radiaton fraction with fractionated radiation therapy in the palliation of skeletal metastases. Int J Radial Oncol 2000;48:185. [Google Scholar]
- 13.Altundag MB, Ucer AR, Calikoglu T, Guran Z. Single (500 cGy, 800 cGy) and multifraction (300x10 cGy) radiotherapy schedules in the treatment of painful bone metastases. THOD - Turk Hematol Derg 2002. [Google Scholar]
- 14.Amouzegar-Hashemi F, Behrouzi H, Kazemian A, Zarpak B, Haddad P. Single versus multiple fractions of palliative radiotherapy for bone metastases: A randomized clinical trial in Iranian patients. Curr Oncol 2008;15:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badzio A, Senkus-Konefka E, Jereczek-Fossa BA, et al. 20 Gy in five fractions versus 8 Gy in one fraction in palliative radiotherapy of bone metastases. A multicenter randomized study. Nowotwory 2003. [Google Scholar]
- 16.Yarnold JR. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol 1999; 52:111–121. [PubMed] [Google Scholar]
- 17.Cole DJ. A randomized trial of a single treatment versus conventional fractionation in the palliative radiotherapy of painful bone metastases. Clin Oncol 1989;1:59–62. [DOI] [PubMed] [Google Scholar]
- 18.El-Shenshawy H, Kandeel A, El-Essawy S. The effect of a single fraction compared to multiple fractions radiotherapy on painful bone metastases with evaluation of computed tomography bone density in osteolytic bone metastases. Bull Alex Fac Med 2006;42:439. [Google Scholar]
- 19.Foro P, Algara M, Reig A, Lacruz M, Valls A. Randomized prospective trial comparing three schedules of palliative radiotherapy. Preliminary results. Oncol 1998. [Google Scholar]
- 20.Foro Arnalot P, Fontanals AV, Galcerán JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008;89:150–155. [DOI] [PubMed] [Google Scholar]
- 21.Gaze MN, Kelly CG, Kerr GR, et al. Pain relief and quality of life following radiotherapy for bone metastases: a randomised trial of two fractionation schedules. Radiother Oncol 1997;45:109–116. [DOI] [PubMed] [Google Scholar]
- 22.Hamouda WE, Roshdy W, Teema M. Single versus conventional fractionated radiotherapy in the palliation of painful bone metastases. Gulf J Oncolog 2007;1:35–41. [PubMed] [Google Scholar]
- 23.Harstell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798–804. [DOI] [PubMed] [Google Scholar]
- 24.Howell DD, James JL, Hartsell WF, et al. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013;119:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaasa S, Brenne E, Lund JA, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy×1) versus multiple fractions (3 Gy×10) in the treatment of painful bone metastases. Radiother Oncol 2006;79:278–284. [DOI] [PubMed] [Google Scholar]
- 26.Sande TA, Ruenes R, Lund JA, et al. Long-term follow-up of cancer patients receiving radiotherapy for bone metastases: Results from a randomised multicentre trial. Radiother Oncol 2009;91:261–266. [DOI] [PubMed] [Google Scholar]
- 27.Kagei K, Suzuki K, Shirato H, Nambu T, Yoshikawa H, Irie G. A randomized trial of single and multifraction radiation therapy for bone metastasis: a preliminary report. Gan No Rinsho 1990;36:2553–2558. [PubMed] [Google Scholar]
- 28.Koswig S, Budach V. Recalcification and pain relief following radiotherapy for bone metastases. A randomized trial of 2 different fractionation schedules (10 × 3 Gy vs 1 × 8 Gy). Strahlentherapie und Onkol 1999;175:500–508. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol 1998;47:233–240. [DOI] [PubMed] [Google Scholar]
- 30.Özsaran Z, Yalman D, Anacak Y, Esassolak M, Haydaroğlu A. Palliative radiotherapy in bone metastases: Results of a randomized trial comparing three fractionation schedules. J BUON 2001. [Google Scholar]
- 31.Price P, Hoskin PJ, Easton D, Austin D, Palmer SG, Yarnold JR. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol 1986;6:247–255. [DOI] [PubMed] [Google Scholar]
- 32.Roos DE, Turner SL, O’Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother Oncol 2005;75:54–63. [DOI] [PubMed] [Google Scholar]
- 33.Safwat E, El-Nahas T, Metwally H, Abdelmotgally R, Kassem N. Palliative fractionated radiotherapy for bone metastases clinical and biological assessment of single versus multiple fractions. J Egypt Natl Canc Inst 2007;19:21–27. [PubMed] [Google Scholar]
- 34.Sarkar. Multiple and single fraction palliative radiotherapy in bone secondaries a prospective study. Indian J Radiol Imaging 2002. [Google Scholar]
- 35.Steenland E, Leer J, Van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101–109. [DOI] [PubMed] [Google Scholar]
- 36.Van Der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: A further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys 2004;59:528–537. [DOI] [PubMed] [Google Scholar]
- 37.Meeuse JJ, van der Linden YM, van Tienhoven G, et al. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: results from the Dutch Bone Metastasis Study. Cancer 2010;116: 2716–2725. [DOI] [PubMed] [Google Scholar]
- 38.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13: 324–328. [DOI] [PubMed] [Google Scholar]
- 39.McHugh ML. Interrater reliability: the kappa statistic. Biochem medica 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 40.Quraishi NA, Arealis G, Salem KMI, Purushothamdas S, Edwards KL, Boszczyk BM. The surgical management of metastatic spinal tumors based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J 2015;15:1738–1743. [DOI] [PubMed] [Google Scholar]
- 41.Tiwana MS, Barnes M, Yurkowski E, Roden K, Olson RA. Incidence and treatment patterns of complicated bone metastases in a population-based radiotherapy program. Radiother Oncol 2016;118: 552–556. [DOI] [PubMed] [Google Scholar]
- 42.Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of Spine Metastasis. Int J Surg Oncol 2011;2011:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: A randomized, placebo-controlled trial. J Clin Oncol 1999;17:846–854. [DOI] [PubMed] [Google Scholar]
- 44.Lad SP, Patil CG, Lad EM, Boakye M. Trends in pathological vertebral fractures in the United States: 1993 to 2004. J Neurosurg Spine 2007;7:305–310. [DOI] [PubMed] [Google Scholar]
- 45.Fox S, Spiess M, Hnenny L, Fourney DR. Spinal Instability Neoplastic Score (SINS): Reliability Among Spine Fellows and Resident Physicians in Orthopedic Surgery and Neurosurgery. Glob Spine J 2017;7: 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ASTRO. Ten Things Physicians and Patients Should Know. Choosing Wisely. https://www.choosingwisely.org/societies/american-society-for-radiation-oncology/. Published 2018. Accessed January 4, 2019.
- 47.Fischer-Valuck BW, Baumann BC, Apicelli A, et al. Palliative radiation therapy (RT) for prostate cancer patients with bone metastases at diagnosis: A hospital-based analysis of patterns of care, RT fractionation scheme, and overall survival. Cancer Med 2018;7:4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellsworth SG, Alcorn SR, Hales RK, McNutt TR, DeWeese TL, Smith TJ. Patterns of care among patients receiving radiation therapy for bone metastases at a large academic institution. Int J Radiat Oncol Biol Phys 2014;89:1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcorn SR, Fiksel J, Hu C, et al. Pilot Assessment of the BMET Decision Support Platform: A Tool to Improve Provider Survival Estimates and Selection of Prognosis-Appropriate Treatment for Patients with Symptomatic Bone Metastases. Int J Radiat Oncol 2019;105:S47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


