Abstract
Leber’s hereditary optic neuropathy (LHON) is the most common mitochondrial disease and in most cases is caused by mutations in mitochondrial DNA–encoded (mtDNA-encoded) respiratory complex I subunit ND1, ND4, or ND6. In this issue of the JCI, Stenton et al. describe biallelic mutations in a nuclear DNA–encoded gene, DNAJC30, establishing recessively inherited LHON (arLHON). Functional studies suggest that DNAJC30 is a protein chaperone required for exchange of damaged complex I subunits. Hallmark mtDNA LHON features were also found in arLHON, including incomplete penetrance, male predominance, and positive response to idebenone therapy. These results extend complex I–deficient phenotypes to include recessively inherited optic neuropathy, with important clinical implications for genetic counseling and therapeutic considerations.
Leber’s hereditary optic neuropathy
Mitochondrial dysfunction can contribute to a wide range of blinding ocular disorders, particularly those involving the highly metabolic retinal ganglion cells that form the optic nerve (1). Both mitochondrial DNA–encoded (mtDNA-encoded) and nuclear DNA–encoded mitochondrial gene mutations can cause ocular disease (2). Leber’s hereditary optic neuropathy (LHON) is the first disease to be associated with mtDNA mutations and continues to be the most commonly inherited mitochondrial disease (3). LHON is characterized by painless and progressive optic nerve degeneration causing central vision loss that typically develops between the ages of 20 and 40 (4). Currently, there is no curative therapy; however, in some cases vision can spontaneously improve (5) and idebenone treatment can also restore vision in some patients (6).
More than 90% of LHON patients of Northern European descent have one of three primary mtDNA missense mutations that encode subunits of respiratory complex I: m.3460G>A (ND1), m.11778G>A (ND4), and m.14484T>C (ND6) (7). Clinically, complex I deficiency is heterogeneous, ranging from severe disorders of childhood that include Leigh syndrome and other diseases with multisystem involvement to single organ presentations, such as isolated optic neuropathy (8). Compared with other complex I phenotypes, LHON due to mtDNA mutations (mtLHON) is a relatively mild disease limited to the eye and with adult onset. The mtDNA mutations are all missense alleles that cause disease primarily when homoplasmic (9), suggesting that, overall, they have modest impact on complex I function. In contrast, mutations involving most of the other complex I subunits cause severe early-onset multisystem disorders (Figure 1 and refs. 8, 10–17).
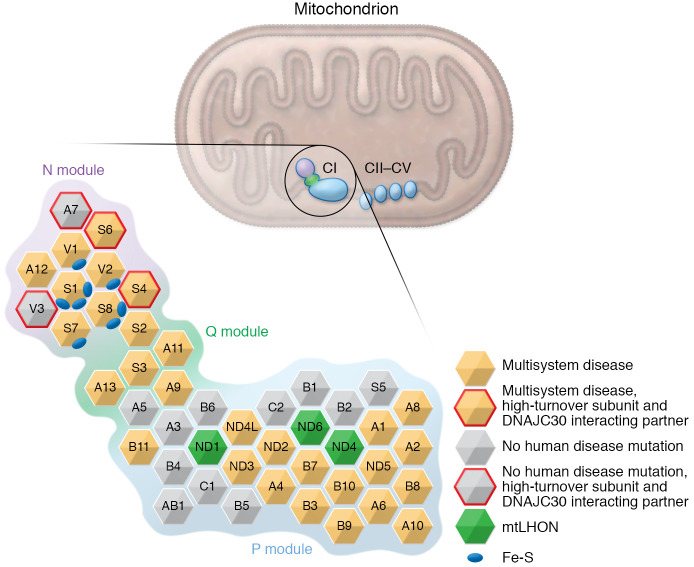
Figure 1. Organization of human mitochondrial respiratory chain complex I subunits.
The 45 complex I (CI) subunits (shown as hexagons) are organized according to the three functional modules (N, electron accepting; Q, ubiquinone reducing; and P, proton pumping). Some gene mutations encode subunits that result in severe early-onset multisystem disease (orange), while other gene mutations encode subunits that cause LHON (green) or have not been associated with a human phenotype (gray). The four DNAJC30 interacting partners identified by Stenton et al. (18) also have a high turnover rate (outlined in red). Figure was modified with permission from Fassone and Rahman (8).
Autosomal recessive inheritance of LHON
In this issue of the JCI, Stenton et al. (18) describe biallelic missense mutations in a nuclear DNA–encoded gene, DNAJC30, in 33 LHON patients from 29 families without disease-causing mtDNA mutations, establishing an autosomal recessive mode of inheritance (arLHON). Using patient-derived cell lines and a DNAJC30-knockout cellular model, the researchers also showed that DNAJC30, previously known to associate with complex I, likely functions as a chaperone protein facilitating the efficient exchange of complex I subunits exposed to reactive oxygen species. Specifically, five subunits located in the complex I N-module (NDUFV3, NDUFS4, NDUFS6, and NDUFA7) and one subunit in the P-module (NDUFA6) had substantially reduced turnover in DNAJC30-deficient cell lines, and four of these subunits were identified as DNAJC30 interacting partners (Figure 1). One patient with DNAJC30 mutations presented with Leigh syndrome; however, the majority of arLHON patients developed only optic nerve disease with generally the same clinical features as those found in mtLHON, including the pathognomonic triad of ophthalmological features: circumpapillary telangiectatic microangiopathy, vessel tortuosity of the central retinal vessels without leakage on fluorescein angiography, and subacute-phase swelling (pseudoedema) of the retinal nerve fiber layer (RNFL) (18).
DNAJC30-related LHON demonstrates reduced penetrance and male predominance
A consistent feature of mtLHON is reduced penetrance, especially in females, with vision loss experienced in approximately 50% of male and 10% of female carriers. Genetic and environmental factors have been suggested to impact disease penetrance, particularly mitochondrial haplogroup J (19) and smoking (20). Stenton et al. (18) also describe reduced penetrance in the LHON DNAJC30 mutation cohort, suggesting that some of these modifying factors could also influence arLHON pathogenesis. The intriguing effect of sex on penetrance results in a striking male bias that remarkably was also observed in the DNAJC30 LHON cohort (18). A number of hypotheses have been suggested to explain this arLHON male bias, including a modifying X chromosome locus (21), specific effects of estrogen (22), and more frequent adverse exposures in males such as smoking (20). Observing similar sex-related penetrance in both arLHON and mtLHON suggests that modifying factors could impact LHON development regardless of the genetic etiology. The response to idebenone therapy was also similar for mtLHON and arLHON, with arLHON having possibly a somewhat better response (18).
Reduced penetrance and the influence of sex
It is interesting that DNAJC30 arLHON shares important disease features with mtLHON, especially reduced penetrance and male predominance, possibly suggesting that these features relate to complex I ocular disease more generally. Although genotype and phenotype correlations are difficult to validate for complex I deficiency syndromes, among these disorders, reduced penetrance and male predominance appear unique to LHON (8). Involvement of only a single tissue or organ system, such as the eye, is also relatively uncommon in complex I deficiency (8). Beyond the genetic variants known to cause mtLHON, DNAJC30 is the only other complex I–related gene that causes disease limited to the optic nerve.
Mutations in genes encoding three of the high-turnover complex I subunits (NDUFS4, NDUFS6, and NDUFA6) result in severe multisystem disease (Leigh syndrome, lethal neonatal mitochondrial complex I deficiency, and early-onset isolated mitochondrial complex I deficiency, respectively) and one might expect that interrupting complex I subunit repair by loss-of-function DNAJC30 would cause similar early-onset multisystem disorders, rather than the observed milder ocular phenotype. It is possible that other complex I–related proteins or other chaperone proteins (especially those with redundant roles) could limit damage caused by impaired DNAJC30 function and potentially also influence disease penetrance. Stenton et al. (18) investigated patient whole-exome sequencing data sets for rare biallelic variants in genes encoding known complex I subunits and assembly factors as well as rare mtDNA variants but did not identify modifying factors that could explain disease penetrance or male bias. The authors also provided data showing that DNAJC30 expression and protein availability are not factors influencing penetrance or sex effects (18). Haplogroup J, previously suggested to influence mtLHON (19), was also overrepresented in DNAJC30 arLHON cases but does not fully account for penetrance or sex effects. An explanation for the interesting influence of sex on LHON penetrance remains an important area of investigation. Evaluating genetic or environmental factors modifying other complex I deficiency syndromes (23) for a role in LHON could be useful. Additionally, genetic variation in genes impacting complex I function may serve as LHON modifier alleles.
Clinical implications
The discovery that DNAJC30 mutations cause recessively inherited LHON has important clinical implications for disease risk assessment, genetic counseling, and therapeutic considerations. In addition to differences in risk due to heritability, patients with DNAJC30 mutations may have earlier disease onset and a better response to idebenone treatment compared with those with mtDNA mutations. Clinical trials to assess gene-based therapy targeting ND4 in LHON patients with m.11778G>A are underway (24), and we can now consider gene-based approaches targeting DNAJC30. Based on the Stenton et al. results (18), LHON genetic testing should be expanded to include both nuclear DNA and mtDNA sequencing. Although mtDNA and DNAJC30 sequencing would likely reveal a molecular diagnosis for LHON cases from most populations, especially Northern Europeans, some populations have lower LHON mtDNA mutation frequencies (4), and still other genes, perhaps also involved in complex I function, could contribute to LHON disease. LHON genetic testing using mitochondrial DNA sequencing as well as whole-exome or whole-genome sequencing could detect mutations in novel genes as well as modifier alleles potentially contributing to the unresolved etiologies underlying reduced penetrance and sex bias.
Acknowledgments
JLW is supported in part by grants from the NIH (R01EY020928, R01EY022305, R01EY027129, and R01EY031820), and by a National Eye Institute P30 core grant (P30EY014104).
Version 1. 03/15/2021
Electronic publication
Footnotes
Conflict of interest: The author has received research support from Aerpio Pharmaceuticals.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(6):e147734. https://doi.org/10.1172/JCI147734.
See the related article at Impaired complex I repair causes recessive Leber’s hereditary optic neuropathy.
References
- 1.Kamel K, et al. Mitochondrial dysfunction in ocular disease: Focus on glaucoma. Mitochondrion. 2017;35:44–53. doi: 10.1016/j.mito.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Wiggs JL. Mitochondrial genetics and optic neuropathy. Annu Rev Vis Sci. 2015;1:97–124. doi: 10.1146/annurev-vision-082114-035651. [DOI] [PubMed] [Google Scholar]
- 3.Gorman GS, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77(5):753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundaramurthy S, et al. Leber hereditary optic neuropathy-new insights and old challenges. Graefes Arch Clin Exp Ophthalmol. doi: 10.1007/s00417-020-04993-1. [published online November 13, 2020]. [DOI] [PubMed] [Google Scholar]
- 5.Newman NJ, et al. Visual outcomes in Leber hereditary optic neuropathy patients with the m.11778G>A (MTND4) mitochondrial DNA mutation. J Neuroophthalmol. 2020;40(4):547–557. doi: 10.1097/WNO.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 6.Klopstock T, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(pt 9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackey DA, et al. Primary pathogenic mtDNA mutations in multi generation pedigrees with Leber hereditary optic neuropathy. Am J Hum Genet. 1996;59(2):481–485. [PMC free article] [PubMed] [Google Scholar]
- 8.Fassone E, Rahman S. Complex I deficiency: clinical features, biochemistry and molecular genetics. J Med Genet. 2012;49(9):578–590. doi: 10.1136/jmedgenet-2012-101159. [DOI] [PubMed] [Google Scholar]
- 9. Yu-Wai-Man P, Chinnery. Leber hereditary optic neuropathy. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. University of Washington; 1993–2021. [Google Scholar]
- 10.Pitceathly RD, et al. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 2013;3(6):1795–1805. doi: 10.1016/j.celrep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rahden VA, et al. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am J Hum Genet. 2015;96(4):640–650. doi: 10.1016/j.ajhg.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angebault C, et al. Mutation in NDUFA13/GRIM19 leads to early onset hypotonia, dyskinesia and sensorial deficiencies, and mitochondrial complex I instability. Hum Mol Genet. 2015;24(14):3948–3955. doi: 10.1093/hmg/ddv133. [DOI] [PubMed] [Google Scholar]
- 13.Friederich MW, et al. Mutations in the accessory subunit NDUFB10 result in isolated complex I deficiency and illustrate the critical role of intermembrane space import for complex I holoenzyme assembly. Hum Mol Genet. 2017;26(4):702–716. doi: 10.1093/hmg/ddw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alston CL, et al. Bi-allelic mutations in NDUFA6 establish its role in early-onset isolated mitochondrial complex I deficiency. Am J Hum Genet. 2018;103(4):592–601. doi: 10.1016/j.ajhg.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piekutowska-Abramczuk D, et al. NDUFB8 mutations cause mitochondrial complex I deficiency in individuals with Leigh-like encephalomyopathy. Am J Hum Genet. 2018;102(3):460–467. doi: 10.1016/j.ajhg.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatsuka Y, et al. A homozygous variant in NDUFA8 is associated with developmental delay, microcephaly, and epilepsy due to mitochondrial complex I deficiency. Clin Genet. 2020;98(2):155–165. doi: 10.1111/cge.13773. [DOI] [PubMed] [Google Scholar]
- 17.Correia SP, et al. Severe congenital lactic acidosis and hypertrophic cardiomyopathy caused by an intronic variant in NDUFB7. Hum Mutat. doi: 10.1002/humu.24173. [published online January 27, 2021]. [DOI] [PubMed] [Google Scholar]
- 18.Stenton SL, et al. Impaired complex I repair causes recessive Leber’s hereditary optic neuropathy. J Clin Invest. 2021;131(6):e138267. doi: 10.1172/JCI138267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson G, et al. Clinical expression of Leber hereditary optic neuropathy is affected by the mitochondrial DNA-haplogroup background. Am J Hum Genet. 2007;81(2):228–233. doi: 10.1086/519394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano L, et al. Cigarette toxicity triggers Leber’s hereditary optic neuropathy by affecting mtDNA copy number, oxidative phosphorylation and ROS detoxification pathways. Cell Death Dis. 2015;6:e2021. doi: 10.1038/cddis.2015.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar SP, et al. Evidence for a novel X-linked modifier locus for Leber hereditary optic neuropathy. Ophthalmic Genet. 2008;29(1):17–24. doi: 10.1080/13816810701867607. [DOI] [PubMed] [Google Scholar]
- 22.Pisano A, et al. Targeting estrogen receptor beta as preventive therapeutic strategy for Leber’s hereditary optic neuropathy. Hum Mol Genet. 2015;24(24):6921–6931. doi: 10.1093/hmg/ddv396. [DOI] [PubMed] [Google Scholar]
- 23.Jain IH, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352(6281):54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman NJ, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. doi: 10.1016/j.ophtha.2020.12.012. [published online January 12, 2021]. [DOI] [PubMed] [Google Scholar]