Abstract
The enteric nervous system mediates reflexes independently of the brain and spinal cord and transmits signals bidirectionally between the gut and the brain. Hirschsprung disease and chronic intestinal pseudo-obstruction (CIPO) and pediatric CIPO are examples of congenital defects that impair gastrointestinal motility. In this issue of the JCI, Thuy-Linh Le et al. analyzed eight patients with defects in tissue that arose from the neural crest. The patients carried homozygous or heterozygous variants in ERBB3 or ERBB2, which encode transmembrane epidermal growth factor receptors that bind neuroregulin 1 (NRG1). Notably, the genetic variants resulted in loss of function with decreased expression or aberrant phosphorylation of the ERBB3/ERBB2 receptors. Experiments using mice revealed that Erbb3 and Erbb2 were expressed in enteric neuronal progenitor cells. This study is an outstanding example of descriptive observation that begs for mechanistic exploration to reveal precisely how the NRG1/ERBB3/ERBB2 pathway influences ENS development.
Developmental defects that impair gastrointestinal motility
The enteric nervous system (ENS) is the largest collection of neurons in the peripheral nervous system (PNS) and is the only peripheral neuronal unit that can mediate reflexes and integrative neuronal activity independently of input from the brain and/or spinal cord (CNS) (1). The ENS also interacts bidirectionally with the brain and with the enteric microbiome (2). The ENS not only directs the details of bowel behavior, it also acts as an intermediate that translates commands from the brain to the gut and transmits signals emanating from the bowel’s microbiota to the brain. It is, therefore, not surprising that the genes that must act properly and with exquisite timing to complete the jigsaw puzzle of ENS assembly do not always do so impeccably (3, 4). The best known and most studied of the defects of ENS formation is Hirschsprung disease (HSCR, aganglionic megacolon, OMIM 142623) (5, 6). In HSCR, ganglia are congenitally absent from varying lengths of the terminal bowel. The ENS is a neural crest derivative, formed by émigrés that depart primarily from vagal and sacral levels of the neuraxis, and secondarily by Schwann cells that enter the colon with the extrinsic innervation (4, 5, 7). Since HSCR is a member of a diverse set of developmental defects and malignancies that arise in cells of neural crest origin, it is classified as a neurocristopathy (5, 8). Short- and long-segment HSCR and even, rarely, aganglionosis of the whole bowel can occur. The HSCR lesion functionally obstructs the gut because propulsive motility absolutely depends on reflexes and a functionally integrated ENS. The aganglionic bowel is thus narrowed, while the bowel, proximal to the aganglionic segment, dilates despite having functional ganglia.
Modern knowledge of HSCR dates from the 1888 meeting of the German Pediatric Society, when the anatomical details of HSCR in two children who died of the condition were reported by a Danish pediatrician, Harald Hirschsprung (9). Hirschsprung himself later added reports of ten more cases of the disorder that bears his name, and 6389 publications on HSCR, currently listed in PubMed (since 1909), have followed. HSCR is not the only developmental defect that impairs gastrointestinal motility. Another, which is both less common and less studied than HSCR, is chronic intestinal pseudo-obstruction (CIPO) (10). PubMed lists 1042 papers on CIPO, but many of these describe the condition in adults, at least half of whom acquired it secondarily. It has, therefore, been suggested that congenital CIPO in the pediatric population be called PIPO (11). Interest in HSCR and CIPO/PIPO does not stem from the frequency of their occurrence. Neither HSCR nor CIPO/PIPO are exactly epidemic; the incidence of HSCR is approximately 1 in 5000 births, while CIPO/PIPO accounts for about 15% of congenital cases of intestinal failure, which does not occur often (10). An exponential rise in interest in HSCR has occurred since 1994, when three publications in Cell unexpectedly linked missense mutations in the endothelin B gene to HSCR (12–14), and earlier publications that suggested that loss-of-function mutations in RET might give rise to HSCR were confirmed (15). Isolated HSCR is now considered an oligogenic condition in which the most commonly involved genetic defects are abnormalities in RET, either vertically transmitted in the coding sequence or through rare low penetrance noncoding variants. However, at least 25 additional genes have also been linked to HSCR (5, 6, 16, 17). Congenital aganglionosis, identical to that of HSCR, also occurs as a component of monogenic syndromes. The roster of genes that disrupt ENS formation is by no means full; ERBB genes now make their debut in an elegant publication in the current issue of the JCI (18).
Patients carrying variants in ERBB3 or ERBB2
In this issue of the JCI, Thuy-Linh Le et al. (18) report a comprehensive analysis of eight patients carrying homozygous or heterozygous variants in ERBB3 or ERBB2, which encode transmembrane epidermal growth factor receptors (19). ERBB2 and ERBB3 are members of the erb-b2 receptor tyrosine kinase family, which activate signaling cascades that include PI3K/AKT and ERK. The main ligands of ERBB2 and ERBB3 are neuregulins (NRGs), especially NRG1 (20). Binding of ligand to ERBB2 leads to homo- or heterodimerization of ERBB2 with ERBB3, cross-phosphorylation, and activation of downstream pathways (19). Signaling through ERBB2/ERBB3 is essential for regulating cell proliferation, survival, and differentiation. The patients in the current report with ERBB3/ERBB2 defects presented with a strikingly diverse array of developmental anomalies that involve the neural crest and its derivatives (Figure 1) (18). Among the most prominent of these anomalies were defects that caused intestinal dysmotilities. The defects included rectal aganglionosis, thickened extrinsic nerves in the aganglionic segment, but also atrophy of the outer longitudinal muscle coat of the colon and the presence of ectopic ganglia in the colonic longitudinal muscle layer. In some patients, the immunoreactivity of smooth muscle actin was also deficient in the inner circular smooth muscle of the colon. The pathohistological characterization of the associated disorders were complimented by elegant in vitro experiments, which revealed that the genetic variants are loss-of-function or hypomorphic alleles with decreased expression or aberrant phosphorylation of the ERBB3/ERBB2 receptors. In one patient, HSCR and CIPO actually coexisted, which has not previously been reported.
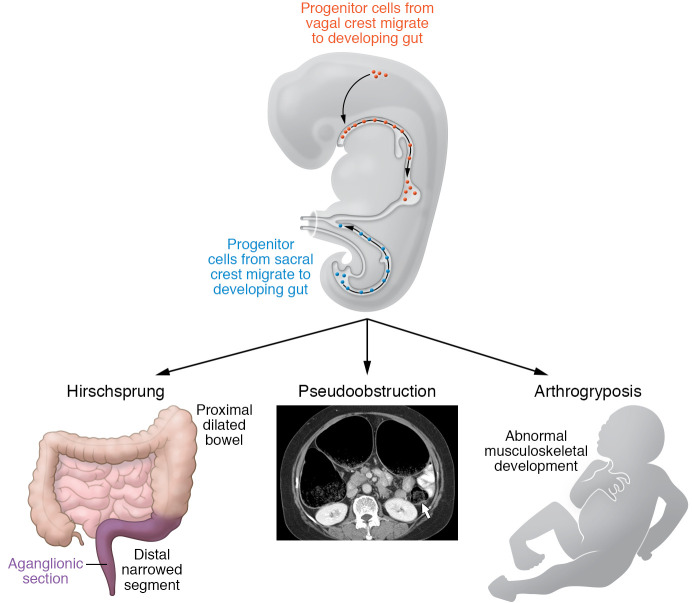
Figure 1. Dysregulation of ERBB2 and ERBB3 signaling as the molecular basis of a diverse array of developmental disorders.
Le et al. (18) described patients with neurocristopathies, developmental disorders arising from the neural crest; the most prominent included congenital aganglionosis of the terminal bowel (HSCR) and CIPO or PIPO. Some individuals had arthrogryposis, possibly due to disordered development of skeletal muscle myotubes. The authors identified variants in ERBB2/ERBB3 genes, which encode transmembrane epidermal growth factor receptors and bind NRG1. ERBB3/ERBB2 was expressed in enteric neuronal progenitor cells, which derive from the neural crest. The loss-of-function ERBB3/ERBB2 variants resulted in reduced expression and altered signaling. The figure was adapted with permission from Rao et al., Hicks et al., and Choi et al. (4, 24, 25).
Clinical considerations
Patient care and counseling might be improved if patients with syndromic HSCR who exhibit dysmotility that persists after surgery to remove the aganglionic bowel were screened for ERBB3/ERBB2 mutations. Thuy-Linh Le et al. (18) were not in a position to clarify how the ERBB3/ERBB2 mutations affected intestinal smooth muscle development that resulted in the abnormalities that they observed. The ENS and smooth muscle can interact during development (21) and Thuy-Linh Le et al. (18) did observe ectopic ganglia in some of the disordered bowel segments. Most clinical cases of CIPO/PIPO, however, are diagnosed as myogenic and linked to smooth muscle–associated genes (FLNA, RAD21, SGOL1, ACTG2, MYH11), although the CIPO/PIPO associated with ERBB3 or ERBB2 variants appears to be neurogenic (18). Conceivably, ERBB3/ERBB2 dysregulation affects gliogenesis in the developing ENS, which might contribute to the ectopic location of ganglia. Enteric ganglia are normally enveloped by glia, and ErbB3 plays a critical role in enteric gliogenesis (22). Smooth muscle can develop independently of the ENS in vivo and in vitro (21). It is therefore difficult to accurately attribute the primary clinical cause of CIPO/PIPO when it is associated with abnormalities of ERBB3 or ERBB2. In contrast, Thuy-Linh Le et al. (18) also observed that some subjects had limited joint flexibility (arthrogryposis), which was likely myogenic and due to ERBB3/ERBB2 signaling abnormalities in developing skeletal muscle myotubes (23).
The impressive observations of Thuy-Linh Le et al. are essentially descriptive. Mechanistic experiments that reveal exactly how the genetic variants in ERBB3/ERBB2 cause the multitude of defects that Thuy-Linh Le et al. have elegantly documented are now needed. Mechanistic studies will give others in the field something to do. For the moment, Thuy-Linh Le et al. have presented a state-of-the-art analysis and an advance in knowledge (18). The study is also a pleasure to read.
Acknowledgments
MDG is supported by NIH grant NS15547.
Version 1. 03/15/2021
Electronic publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(6):e146389. https://doi.org/10.1172/JCI146389.
References
- 1. Gershon MD. The Second Brain. New York, N.Y.: Harper Collins; 1998. [Google Scholar]
- 2.Giuffrè M, et al. You talking to me? Says the enteric nervous system (ENS) to the microbe. How intestinal microbes interact with the ENS. J Clin Med. 2020;9(11):3705. doi: 10.3390/jcm9113705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosens E, et al. Genetics of enteric neuropathies. Dev Biol. 2016;417(2):198–208. doi: 10.1016/j.ydbio.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Rao M, Gershon MD. Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci. 2018;19(9):552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuckeroth RO. Hirschsprung disease — integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15(3):152–167. doi: 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]
- 6.Amiel J, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45(1):1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 7.Espinosa-Medina I, et al. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc Natl Acad Sci U S A. 2017;114(45):11980–11985. doi: 10.1073/pnas.1710308114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Gaya V, et al. Rare or overlooked? Structural disruption of regulatory domains in human neurocristopathies. Front Genet. 2020;11:688. doi: 10.3389/fgene.2020.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sergi C. Hirschsprung’s disease: historical notes and pathological diagnosis on the occasion of the 100(th) anniversary of Dr. Harald Hirschsprung’s death. World J Clin Pediatr. 2015;4(4):120–125. doi: 10.5409/wjcp.v4.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zenzeri L, et al. Update on chronic intestinal pseudo-obstruction. Curr Opin Gastroenterol. 2020;36(3):230–237. doi: 10.1097/MOG.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 11.Thapar N, et al. Paediatric intestinal pseudo-obstruction: evidence and consensus-based recommendations from an ESPGHAN-led expert group. J Pediatr Gastroenterol Nutr. 2018;66(6):991–1019. doi: 10.1097/MPG.0000000000001982. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda K, et al. Targeted and natural (piebald-lethal) mutation of endothelin-B receptor produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 13.Baynash AG, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79(7):1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 14.Puffenberger EG, et al. A missense mutation of the endothelin-receptor gene in mutagenic Hirschsprung’s disease. Cell. 1994;79(7):1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 15.Romeo G, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367(6461):377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- 16.Klein M, Varga I. Hirschsprung’s disease-recent understanding of embryonic aspects, etiopathogenesis and future treatment avenues. Medicina (Kaunas) 2020;56(11):E611. doi: 10.3390/medicina56110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuckeroth RO. Even when you know everything, there is still more to learn about Hirschsprung disease. Gastroenterology. 2018;155(6):1681–1684. doi: 10.1053/j.gastro.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Thuy-Linh Le Dysregulation of the NRG1-ERBB pathway causes a developmental disorder with gastrointestinal dysmotility in humans. J Clin Invest. doi: 10.1172/JCI145837. [published online January 26, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 20.Meyer D, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124(18):3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- 21.Graham HK, et al. Intestinal smooth muscle is required for patterning the enteric nervous system. J Anat. 2017;230(4):567–574. doi: 10.1111/joa.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalazonitis A, et al. Bone morphogenetic proteins regulate enteric gliogenesis by modulating ErbB3 signaling. Dev Biol. 2011;350(1):64–79. doi: 10.1016/j.ydbio.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks MR, et al. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat Cell Biol. 2018;20(1):46–57. doi: 10.1038/s41556-017-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JS, et al. Colonic pseudoobstruction: CT findings. AJR Am J Roentgenol. 2008;190(6):1521–1526. doi: 10.2214/AJR.07.3159. [DOI] [PubMed] [Google Scholar]
- 25.Kondo D, et al. A novel ZC4H2 gene mutation, K209N, in Japanese siblings with arthrogryposis multiplex congenita and intellectual disability: characterization of the K209N mutation and clinical findings. Brain Dev. 2018;40(9):760–767. doi: 10.1016/j.braindev.2018.05.003. [DOI] [PubMed] [Google Scholar]