Abstract
Nonalcoholic fatty liver disease (NAFLD) results in the accumulation of fat in the liver and can progress as an inflammatory disorder with considerable vascular endothelial dysfunction known as nonalcoholic steatohepatitis (NASH). Inflammatory signals can trigger the expression of vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells. VCAM-1 is a surface protein that induces adherence and extravasation of monocytes to blood vessels. In this issue of the JCI, Furuta et al. report on their sequencing of RNA transcripts from the livers of mice fed a NASH-inducing diet. VCAM-1 was upregulated in the whole liver as well as liver sinusoidal endothelial cells (LSECs). When the researchers incubated LSECs with palmitate, a toxic lipid, VCAM-1 was upregulated. Notably, inhibiting VCAM-1 in the NASH model reduced VCAM-1 expression, lessened infiltrating macrophages, and mitigated fibrosis. This study connects steatosis to endothelial dysfunction and inflammation and suggests that targeting VCAM-1 may address fibrosis in patients with NASH.
Inflammation underlies nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is a systemic disease that results from multiorgan metabolic dysregulation, tissue inflammation, and organ dysfunction. It is a chronic condition that can progress from steatosis to nonalcoholic steatohepatitis (NASH), manifesting as inflammation, fibrosis, cirrhosis, and hepatocellular carcinoma (1, 2). NAFLD affects nearly one-third of the global adult population (3) and, within a decade, it is estimated it will increase in prevalence by up to 60% (4). In fact, prior to the COVID-19 pandemic, the NAFLD epidemic had center stage as the most concerning disease for those with underlying metabolic risk factors, such as obesity, diabetes, hypertension, and dyslipidemia (5). Indeed, with these shared risk factors for disease susceptibility and severity, it is noteworthy that inflammation underlies both COVID-19 (6) and NAFLD pathogenesis.
Vascular cell adhesion molecule 1 (VCAM-1) is an adhesion molecule that is expressed on endothelial cells and upregulated in response to inflammatory signals (7). VCAM-1 upregulation induces adherence and extravasation of monocytes to vessels (8). Recently, Tong et al. established that serum VCAM-1 levels are increased in patients with COVID-19 (9), perhaps explaining the widespread, systemic vascular inflammation that occurs in infected patients. In this issue of the JCI, Furuta et al. elegantly establish a mechanistic role of VCAM-1 in human and rodent NASH (10).
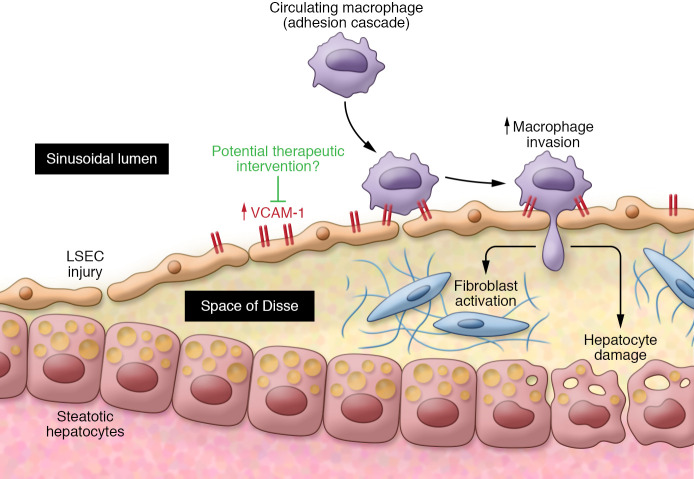
Vascular endothelial dysfunction is an important feature of NASH pathogenesis. Clinical evidence for this vascular endothelial component is revealed in the numerous epidemiologic studies demonstrating that cardiovascular complications are the major causes of morbidity and mortality in patients with NASH (11) and in the portal hypertension that ensues in advanced NASH. Despite the ongoing debate about whether NASH independently promotes endothelial damage in cardiac vessels (12), preclinical data in rats fed a NASH-inducing diet demonstrate that, at least in the liver, endothelial inflammation is an early feature of NASH, preceding both hepatic macrophage cell infiltration and fibrosis (13). Indeed, it is the steatotic hepatocyte that distorts liver sinusoidal endothelial cells (LSECs) and impairs sinusoidal perfusion (14). Sinusoidal injury is followed by macrophage infiltration and adherence to the sinusoidal lining. It is likely that persistence of this cascade leads to progressive steatohepatitis and fibrosis and, conversely, that ameliorating the precipitating endothelial cell dysfunction can remedy NASH fibrosis (Figure 1).
Figure 1. Model of hepatic macrophage infiltrate and fibrosis.
Following distortion and injury of LSECs by steatotic hepatocytes, macrophages infiltrate and adhere to the sinusoidal lining via VCAM-1. The persistence of this cascade likely results in progressive steatohepatitis and fibrosis. Ameliorating the precipitating endothelial cell dysfunction (possibly through VCAM-1) may remedy NASH-related fibrosis.
Recruiting macrophages to the vascular endothelium
While the ultrastructural changes contributing to endothelial dysfunction have been identified, the molecular mechanisms that instigate the recruitment of macrophages to the vascular endothelium had not been characterized previously. Hence, the study by Furuta et al. (10) fills critical knowledge gaps. To reveal factors that associate with transcriptional regulation, the authors sequenced the transcriptome and profiled chromatin regions from the livers of mice fed a high-fat, -fructose, and -cholesterol (FFC) NASH-inducing diet. VCAM-1 was identified as transcriptionally and differentially upregulated, an observation confirmed with targeted gene analysis of whole livers and LSECs derived from NASH mice (10).
The authors established further that, in NASH, lipids themselves regulated VCAM-1. Namely, the lipotoxic lipid palmitate upregulated VCAM1 gene and protein expression in primary mouse and human LSECs through a mechanism that involved MAPK. MAPK activation occurred through the MAP3K mixed lineage kinase 3 (MLK3), as both pharmacologic and genetic inhibition of MLK3 prevented LSEC VCAM-1 upregulation. Since LSEC incubation with palmitate caused VCAM-1 upregulation, palmitate may directly affect LSEC function through its own bioactive effects on cellular processes and indirectly affect LSEC function through the aforementioned structural derangements that result from hepatocellular steatosis (10).
While several studies corroborate the association of VCAM-1 upregulation with inflammatory diseases (15, 16), the translational relevance of VCAM-1 in NASH was supported by the authors’ investigation. Hepatic VCAM-1 protein was upregulated in NASH patients compared with normal liver and steatotic livers of NAFLD patients. Moreover, VCAM-1 was upregulated in palmitate-incubated primary human LSECs. Indeed, the endothelial cell–derived VCAM-1 promoted hepatic inflammation and fibrosis in NASH. Endothelial cell–specific Vcam1 knockout mice (Vcam1Δend) fed a choline-deficient, high-fat diet for six weeks exhibited reduced expression of several inflammatory markers, reduced macrophage infiltration, as measured by marker F4/80 labeling, and decreased fibrosis, as measured by histology and markers of stellate cell activation (10).
Clinical implications
Notably, blocking VCAM-1 using an antibody failed to influence body weight, energy expenditure, hepatic steatosis, or glucose intolerance. While it does not appear that targeting VCAM-1 alone remedies the constellation of metabolic and hepatic injury in NASH targeting, VCAM-1 did reduce both the hepatic monocyte–derived macrophage infiltrate and fibrosis. The use of AGI-1067 (a VCAM-1 pharmacologic inhibitor that has been used in clinical trials of other diseases) had effects similar to those of the VCAM-1 antibody. The inhibitor additionally improved insulin resistance, perhaps pointing toward an extrahepatic effect of this intervention (10).
The ultimate clinical utility of VCAM-1 has yet to be established in NASH. One hundred seventeen patients with NASH who participated in the Pioglitazone vs. Vitamin E vs. Placebo for the Treatment of Nondiabetic Patients with NASH (PIVENS) trial had paired liver biopsies and serum samples that were analyzed post hoc (17). There was no difference in VCAM-1 levels between those patients who had NASH resolution and those whose NASH persisted after 96 weeks of either vitamin E or pioglitazone therapy. As criteria for NASH resolution require the absence of features of steatohepatitis, it is possible that VCAM-1 levels were associated with improvements in some NASH features, such as fibrosis or inflammation. Hence, targeting VCAM-1 is unlikely to reverse NASH, but VCAM-1 may act as a NASH antifibrotic therapeutic target for patients with fibrosis who have been unsuccessful with dietary and weight loss strategies. This therapy would need to be combined with other therapies that target dysmetabolism and steatosis, the onset of which impairs endothelial function and initiates inflammation.
Nevertheless, Furtura et al. (10) help us understand how patients progress from steatosis to endothelial dysfunction and inflammation. VCAM-1 mediates this transition through an MLK3-dependent process. Because VCAM-1 production is not confined to hepatic LSECs, this protein may affect the systemic inflammation that characterizes NAFLD hepatic disease as well as cardiovascular disease, the source of greatest morbidity and mortality for NAFLD patients.
Acknowledgments
This work was supported by NIH R01 AA026302 and P30 DK0503060.
Version 1. 03/15/2021
Print issue publication
Footnotes
Conflict of interest: RMC has received research support from Intercept Pharmaceuticals Inc. and Merck & Co. Inc.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(6):e147556. https://doi.org/10.1172/JCI147556.
See the related article at Lipid-induced endothelial vascular cell adhesion molecule 1 promotes nonalcoholic steatohepatitis pathogenesis.
References
- 1.Carr RM, et al. Nonalcoholic fatty liver disease: pathophysiology and management. Gastroenterol Clin North Am. 2016;45(4):639–652. doi: 10.1016/j.gtc.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM. Non-alcoholic fatty liver disease — A global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Del Valle DM, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heymann F, Tacke F. Immunology in the liver — from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13(2):88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 8.Walpola PL, et al. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol. 1995;15(1):2–10. doi: 10.1161/01.ATV.15.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Tong M, et al. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis. 2020;222(6):894–898. doi: 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta K, et al. Lipid-induced endothelial vascular cell adhesion molecule 1 promotes nonalcoholic steatohepatitis pathogenesis. J Clin Invest. 2021;131(6):e143690. doi: 10.1172/JCI143690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cusi K, Godinez Leiva E. Cardiovascular risk in patients with nonalcoholic fatty liver disease: looking at the liver to shield the heart. Curr Opin Lipidol. 2020;31(6):364–366. doi: 10.1097/MOL.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, et al. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon? Diabetologia. 2008;51(11):1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 13.Pasarin M, et al. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7(4):e32785. doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCuskey RS, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40(2):386–393. doi: 10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 15.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107(10):1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter RA, et al. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol. 2002;128(1):44–51. doi: 10.1046/j.1365-2249.2002.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey KE, et al. Relationship between resolution of non-alcoholic steatohepatitis and changes in lipoprotein sub-fractions: a post-hoc analysis of the PIVENS trial. Aliment Pharmacol Ther. 2019;49(9):1205–1213. doi: 10.1111/apt.15216. [DOI] [PMC free article] [PubMed] [Google Scholar]