Abstract
Continued thinning of the atmospheric ozone, which protects the earth from damaging ultraviolet radiation (UVR), will result in elevated levels of UVR reaching the earth’s surface, leading to a drastic increase in the incidence of skin cancer. In addition to promoting carcinogenesis in skin cells, UVR is a potent extrinsic driver of age-related changes in the skin known as “photoaging.” We are in the preliminary stages of understanding of the role of intrinsic aging in melanoma, and the tumor-permissive effects of photoaging on the skin microenvironment remain largely unexplored. In this Review, we provide an overview of the impact of UVR on the skin microenvironment, addressing changes that converge or diverge with those observed in intrinsic aging. Intrinsic and extrinsic aging promote phenotypic changes to skin cell populations that alter fundamental processes such as melanogenesis, extracellular matrix deposition, inflammation, and immune response. Given the relevance of these processes in cancer, we discuss how photoaging might render the skin microenvironment permissive to melanoma progression.
Introduction
For decades cancer has been considered a disease of aging, owing to the increased incidence of cancer among individuals over 60. Concomitantly, the mechanistic hallmarks of aging reflect those of cancer (1), and we are just beginning to understand how age-related changes in noncancerous cells — namely the normal cells of the tumor microenvironment (TME) — impact cancer progression. Recent studies have revealed that chronological aging drives phenotypic changes in the cellular constituents of the stroma and the immune microenvironments that promote tumor progression (2). From these findings, we now understand that we must consider age-related changes in the TME when validating putative therapeutic targets in the preclinical and clinical setting.
To date, the study of aging and the TME has focused predominantly on chronological aging (2–4). However, cellular “aging” occurs not only due to the intrinsic biological changes over time, but also due to extrinsic factors such as pollution, tobacco smoke, and ultraviolet radiation (UVR). Repeated exposure to these factors accelerates the aging process, inducing cells to adopt an aged phenotype in advance of their intrinsic biological clock — a phenomenon known as “premature aging” (5). Repeated exposure to UVR, called photoaging, is of particular importance as it accounts for approximately 80% of facial aging (6). Although the physical features of premature aging due to UVR have been characterized (7), a consensus about the molecular drivers of premature aging and how they are similar to or different from chronological aging is still lacking. Furthermore, preclinical studies have yet to explore how premature aging of the TME due to extrinsic factors impacts tumor progression.
Most of our understanding concerning the effects of premature aging derives from observations about the changes in the skin, which has long been considered the physiological mirror of aging (8, 9). Clinical features of extrinsically driven premature aging include skin thinning, loss of elasticity, aberrant pigmentation, loss of subcutaneous fat in the extremities, and sustained inflammatory response (10, 11). Although there are substantial gaps in our understanding of the molecular underpinnings of these symptoms, they each are characterized by alterations in the skin microenvironment. Notably, modifications to the skin microenvironment due to chronological aging are now considered a contributing factor to the growth and spread of melanoma — the most aggressive form of skin cancer, accounting for the majority of all skin cancer–related deaths (12). These current findings and the known overlaps between the hallmarks of aging and the hallmarks of cancer (Figure 1) lead us to propose that microenvironment changes in the skin due to premature aging can also influence melanoma progression.
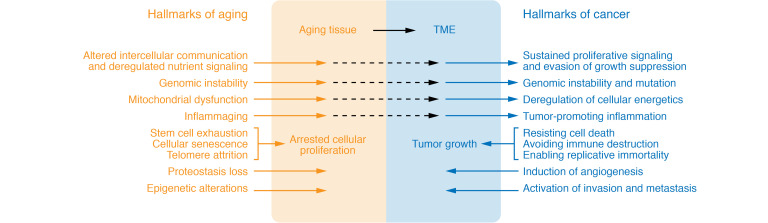
Figure 1. Similar and diverging features of the hallmarks of aging and cancer.
There are many features of the hallmarks of aging, as described in ref. 2 and ref. 197, that can lead to changes in the microenvironment that are permissive of tumor growth. Persistent alterations to intercellular communication and deregulated nutrient signaling that arise with aging can promote proliferative signaling and evasion of growth suppression, both of which are hallmarks of cancer as described in ref. 127. Additional features equivalent between these two processes include genomic instability, deregulated cell energy processes, and chronic inflammation. However, unlike cancer, the aging cell population is also characterized by mechanisms that shorten cellular lifespan, such as stem cell exhaustion, cellular senescence, and telomere attrition. In contrast, cancer cells acquire the ability to constitutively activate prosurvival pathways that resist cell death and allow the transformed cells to avoid immune destruction and to enable replicative immunity. The hallmarks listed in the shaded areas were previously described as unique to aging or cancer.
Clinical and molecular features of photoaging in the skin microenvironment
Chronic exposure to UVR is strongly associated with increased risk of melanoma (13); however, between 2006 and 2015, melanoma incidence decreased among individuals less than 50 years old, likely because of abundant public health campaigns encouraging sun-protective behavior (14). Although declining melanoma incidence occurs in the adolescent and young adult populations, melanoma incidence starkly increases for older adults, reinforcing that patient age is a negative prognostic indicator for melanoma survival (14, 15). Recent studies have sought to determine the effects of the intrinsically aged TME on cancer progression (2, 16), but these effects are likely compounded by the reality that the skin of aging patients has sustained years of UVR exposure. Tissue that has been repeatedly exposed to UVR will show a distinct aging phenotype called photoaging. Pathophysiology of photoaging is clinically recognized by the changes that occur to the UVR-exposed skin, such as actinic elastosis, or wrinkling, and aberrant pigmentation “sun spots,” which also correspond to alterations in the tissue microenvironment.
Each class of UV wavelengths has different energetics, which impacts how UVR is absorbed by the skin microenvironment. Solar UVR consists of low-energy UVA (315–400 nm), medium-energy UVB (280–315 nm), and high-energy UVC (100–280 nm) (17); however, in considering the impact of UVR on the skin microenvironment, UVA and UVB radiation are of primary importance, as solar UVC is completely absorbed by the stratospheric ozone (18, 19). Given the differing energetics between UVA and UVB wavelengths, it is unsurprising that each has a different ability to penetrate the skin. Although both UVA and UVB radiation can penetrate the surface epithelium of the skin (epidermis), the longer UVA wavelength penetrates the deeper layers of the skin (dermis) to a much greater extent than shorter-wavelength UVB radiation (20). The varying ability of UVA and UVB to penetrate layers of the skin poses interesting questions about whether these two UVR wavelengths have differing effects on the skin microenvironment. By comparing the biological impact of repeated skin exposure to UVA and UVB in a mouse model, Wang et al. showed that UVA primarily induced changes in the dermis, promoting oxidative damage in DNA, collagen fiber breakdown, and apoptosis of fibroblasts that led to inflammation (21). In contrast, UVB primarily affected keratinocyte proliferation, induced protein carbonylation, and increased oncogenic risk (21). An important consideration is that this study used nude mice, which are a transgenic mouse model lacking expression of Foxn1, a mutation that leads to the hairless phenotype and other skin defects (22). Given that melanin production in mice has been associated with the delivery of melanin to cells expressing Foxn1 (23), this model may not account for the role of melanin in mediating these changes in the skin microenvironment. Nevertheless, it is evident that repeated exposure to either UVA or UVB has potent effects on the skin microenvironment that can impact aging and cancer progression.
The photoaged microenvironment and melanoma progression
Numerous studies demonstrate how signaling between fibroblasts and melanocytes influences melanoma progression (24–26); however, the role of cell-cell communication between melanocytes and keratinocytes in early tumorigenesis is less frequently discussed. Although in situ melanoma is associated with the longest 5-year survival and lowest risk of reoccurrence (27), the phenotypic shifts and molecular transitions that occur in the tumor cells at this early stage should not be overlooked, as they can also contribute to converting minimally invasive melanoma cells to highly aggressive cells, capable of invading into the skin dermis. Recently, Mescher et al. uncovered a novel tumor-suppressive mechanism that relies upon melanocyte-keratinocyte interaction (28). In this study, keratinocyte-specific expression of Par3, an integral member of the Par3/aPKC/Par6 cell polarity regulatory complex, promoted melanocyte proliferation and differentiation; in contrast, loss of this protein in keratinocytes promoted melanocyte transformation, invasion, and metastasis (28). An alternative explanation for melanoma cell invasion into the dermis identifies keratinocyte expression of Notch ligands as the culprit. In the absence of Notch ligands, MITF, the master transcriptional regulator of melanocyte lineage–related genes, binds to and represses the microRNA miR-222/221 (29); however, as the tumor grows, it encounters distal differentiated Notch ligand–expressing keratinocytes, activating Notch receptor on the transformed melanocytes. When activated, the intracellular domain of Notch interferes with MITF binding to miR-222/221 — an alteration that initiates further invasion (29). In both examples, the tumor-supporting role of keratinocytes in the skin microenvironment depends on a physical interaction between melanocytes and keratinocytes. However, paracrine signals from cells in the TME greatly impact tumor progression (30–33), and thus the role of keratinocytes in melanoma invasion should be further investigated.
Melanin synthesis
Skin dyspigmentation is a commonly observed feature of aging. Clinically, these changes are known as solar lentigines (SLs), which are hyperpigmented spots in the skin. Hyperpigmentation of this nature occurs due to increased melanin synthesis by melanocytes, and subsequent increased melanosome transfer and accumulation in keratinocytes (34–36). SL deposits are found in areas of repeated sun exposure and thought to be a manifestation of photoaging (37, 38). Interestingly, nonsolar age-related changes in melanocytes and melanin expression show an inverse association. Hair graying occurs in all individuals independent of sun exposure and results from the progressive loss of hair follicular melanocytes with age (39). Similarly, studies have demonstrated that melanin-producing melanocytes decrease by 10% to 20% per decade (37, 40–42). As a result, sun-protected aged skin has reduced skin pigmentation and tanning response after UV exposure (37, 43, 44). Although more research is needed to discern the solar-induced versus intrinsic aging-related differences in melanin production, given these findings, we can appreciate that aged skin is characterized by both regional increases in melanin deposits and global decreases in melanin synthesis. Notably, the role of melanin in tumor progression has long been debated. The complexity of the issue may be partially attributed to the diversity of melanin subtypes in human skin. Each skin type is characterized by differing ratios of two melanin subtypes, eumelanin and pheomelanin. Furthermore, it has recently been observed that the ratio of expression of each subtype can differentially impact photosensitivity and UV-induced tumorigenesis (45–47). Alternatively, a recent study approached the question from a different perspective, evaluating the impact of melanin on the elastic properties and physical mobility of melanoma cells. Sarna et al. used an in vivo melanoma model to demonstrate that high melanin expression acted as a mechanical inhibitor of invasion and metastasis (48). Although the pigmented cells in this study expressed both melanin subtypes, their findings are supported by an observed clinical association between melanin pigmentation and melanoma aggressiveness, with worse patient survival in amelanotic melanoma (49).
Given that increased melanin production in melanocytes can lead to a stiffer cell body and reduced invasive properties (48), UVR-induced hyperpigmentation in SLs would appear to suppress tumor growth. However, melanogenic factors secreted by cells in the skin microenvironment may promote tumor growth through melanin-independent mechanisms. Research comparing SL lesions with perilesional sun-exposed skin has helped identify putative biomarkers of UVR-induced SLs. These include increases in factors secreted by keratinocytes and fibroblasts, such as stem cell factor (SCF; refs. 50, 51), hepatocyte growth factor (HGF; ref. 51), and sFRP2 (52). Given the hyperpigmented appearance of SLs, it is unsurprising that each of these markers is a potent activator of melanogenesis; however, the regulatory role of these molecular mediators of SLs is not limited to melanin synthesis. For example, in addition to melanogenesis, melanocytes depend significantly on the activation of the c-KIT receptor by its ligand SCF (also known as c-KIT ligand) for growth, differentiation, and migration (53, 54). Aberrant KIT signaling induces tumor growth and metastasis in many tumor types (55–57) due to the ability of cancer cells to utilize SCF/c-KIT signaling to activate oncogenic pathways such as Ras and PI3K (56). KIT inhibitors have recently been explored as a therapeutic target in melanoma (58, 59), as mutations in KIT are potent oncogenic drivers in the acral, mucosal, and chronically sun-damaged subtypes of this disease (60, 61).
Like SCF/c-KIT signaling, HGF signaling is important for melanocytes beyond promoting melanogenesis. Signal transduction through the HGF receptor c-MET protects melanocytes from apoptosis (62) and promotes proliferation and motility (63). Consequently, HGF/scatter factor–transgenic mice have been frequently used as an experimental model to investigate the consequence of chronic UV exposure (64), as well as to study UV-induced melanomagenesis (65, 66). Additionally, HGF/c-MET signaling, which has an established role in carcinogenesis (67), has been explored as a therapeutic target in metastatic melanoma (68, 69). The role of aberrant KIT and c-MET signaling in the progression of some melanoma subtypes calls into question whether keratinocytes and fibroblasts present in UVR-induced SLs are secreting abundant SCF and HGF into the microenvironment that can drive the growth of cancer cells with these mutations.
As mentioned previously, hyperpigmentation of the skin appears as a feature of aged skin that is specific to photoaging rather than chronological aging; however, recent work from our laboratory demonstrated that sFRP2, a soluble moderator of WNT signaling, is abundantly expressed in chronologically aged dermal fibroblasts from patient donors without a tanning history (70), suggesting overlapping mechanisms between intrinsic and extrinsic aging. Exogenous expression of sFRP2 in young fibroblasts induced changes in the TME that accelerated melanoma progression in vitro and in vivo (70). The observed increases in sFRP2-photoaged and chronologically aged skin, as well as its role in cancer progression, spotlights sFRP2 as a melanogenic factor with the potential to accelerate malignant states; nevertheless, further studies are required to fully elucidate how age-related alterations in WNT signaling contribute to melanoma progression.
Senescence/SASP
Senescence is commonly used as a hallmark of chronological aging and has been used both in vitro and in vivo to study aging in fibroblasts (71). Although context-dependent differences between oncology-induced senescence and age-induced senescence exist (2), in either case, the activation of senescence programming has grave implications for the development of malignancies, especially in the elderly population. Previous studies demonstrated decreased fibroblast number and proliferation with age (72–74), thereby suggesting that an aged microenvironment consists of primarily senescent cell populations (2). The senescent cells of a tissue microenvironment are characterized by a senescence-associated secretory phenotype (SASP), which includes secretion of members of the interleukin family (IL-6, IL-8, IL-10) as well as granulocyte-macrophage colony-stimulating factor (GM-CSF; refs. 71, 75, 76), among other factors. Senescent fibroblasts and many of these SASP factors can induce cancer cell proliferation and invasion (26, 77–79). SASP-associated fibroblasts can also undergo substantial changes in metabolic pathways resulting in increased synthesis of nitric oxide, reactive oxygen species (ROS), and high-energy metabolites such as lactate, ketones, and glutamine (80–83). Metabolites such as these have been implicated in regulating the interaction between immune and cancer cells and thus influencing response to anticancer therapies (84). Therefore, we can expect that any stimuli inducing alterations in senescence and SASP in the cellular constituents of the TME will accelerate both aging and cancer progression.
For many years, UVR and photoaging have been considered potent inducers of cellular senescence in both keratinocytes and fibroblasts (85); however, we have yet to understand the ways in which UVR-induced senescence is similar to or different from age-related senescence. The issue becomes further convoluted given the fact that extrinsic factors such as UVR are used to induce senescence in aging studies, as they accelerate the “aging” process, and there are limited methods for studying intrinsic aging and senescence in vitro. Nevertheless, there are similarities and differences that have been discerned. For example, telomere shortening is a commonly identified feature of chronological aging (86). Although telomere shortening was initially suggested to be a shared pathway of both chronological aging and photoaging (87), the issue remains unclear given subsequent contradicting evidence. One study comparing telomere length between sun-exposed and sun-protected epidermal samples failed to observe telomere shortening due to photoaging (88). Conversely, more recent studies have identified UVA-specific irradiation-induced telomere shortening in irradiated fibroblasts (89, 90). Given the fact that telomere shortening is one of the primary hallmarks of aging and a driver of cellular senescence, more work is required to further elucidate the role of telomere shortening in photoaging.
MicroRNA (miRNA) expression is yet another senescence-regulating mechanism that may be different between photoaged and chronologically aged skin. The role of miRNAs in senescence has begun to be understood (91), which is compelling given the recent identification of a unique miRNA signature for photoaged epidermis (92). Srivastava et al. used microarray analysis of biopsies collected from sun-exposed and sun-protected regions of young and aged individuals and found an upregulation of miR-383, -145, and -34a and downregulation of miR-6879, -3648, and -663b in photoaged skin that were not observed in chronological aging (92). The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of target genes of the miRNAs in this signature identified enrichment of many pathways, including differentiation and senescence (92). Given these findings, the genetic outcomes of these UVR-induced miRNA alterations should be further explored and compared with those in recent studies characterizing chronological aging–induced cellular senescence (93). Such a comparative analysis would provide important insights into the senescence mechanisms unique to intrinsic and extrinsic aging.
Although there are undoubtedly some differences in senescence programs between intrinsic and extrinsically aged skin, Cavinato and Jansen-Dürr recently described that, in both instances, senescence could be attributed to alterations in protein quality mechanisms (94). The photoaging studies presented in this Review show that, as with chronological aging, both decreased proteasome activity and increased autophagic activity are involved in UVR-mediated senescence (94).
Extracellular matrix remodeling
The extracellular matrix (ECM) is an acellular three-dimensional network that serves a dual purpose in tissue homeostasis. This network consists primarily of fibrous proteins that provide the mechanical support necessary to maintain the structural integrity of the tissue. In addition, the structural proteins of the ECM also serve as a scaffolding for the cell, and protein trafficking that occurs during cell-cell communication and differentiation (95–97). The skin ECM consists predominantly of collagen and elastin, which are tightly bound and interwoven with one another by a specialized glycosaminoglycan called hyaluronic acid (HA). The integrity of this structural network decreases during both chronological aging and photoaging, as evidenced by the increased occurrence of skin wrinkling in both cases. Intrinsic aging and photoaging induce collagen fragmentation (98–100) and reductions in collagen density (101–103), as well as reduction of turnover of the collagen cross-linking proteins fibulin, fibrillin, elastin (104–110), and HA (111–114). Although similarities exist in the types of alterations that occur with intrinsic and UVR-mediated extrinsic aging, there are visible differences between chronologically aged and photoaged skin. Chronologically aged skin appears dry, lacks elasticity, and features thin, fine wrinkles (100). In contrast, photoaged skin is characterized by a thickened epidermis that is waxy in appearance and deep, coarse wrinkles (100, 115). The differing appearance of intrinsic and extrinsically aged skin raises questions about potential differences in the molecular mechanisms driving the underlying ECM changes in each process. Unfortunately, given the fact that all skin receives some degree of UVR exposure, many of these questions remain unanswered, as the effects of intrinsic and extrinsic aging on the ECM are often superimposed with one another, making it a challenge to distinguish between purely biological and UVR-related ECM changes with age.
For intrinsic aging, ECM remodeling is primarily attributed to the age-related conferring of SASP by fibroblasts; therefore, alterations to the secretome of fibroblasts, the dermal cells responsible for building the ECM, will ultimately impact ECM deposition. Matrix metalloproteinases (MMPs) are ECM-degrading proteases present in the secretome of SASP-expressing fibroblasts (116, 117), and the activity of many of these SASP-associated MMPs is increased with biological age (117). In addition to increased synthesis of MMPs, aged fibroblasts also show increases in ROS accumulation and DNA damage (118), which may also lead to ECM remodeling (119). Beyond the age-related changes in fibroblast phenotype and function, the molecular mediators of intrinsic ECM remodeling remain mostly unexplored.
Alterations in MMP expression are frequently implicated in UVR-mediated ECM remodeling (120); however, MMP-independent mechanisms of ECM remodeling have also been associated with photoaging. Given the observed degradation of HA in both intrinsically and extrinsically aged ECM, the expression of HA-regulating proteins by fibroblasts has also been investigated in the context of photoaging. Yoshida and Okada describe a newly discovered HA-degrading mechanism mediated by hyaluronan-binding protein involved in hyaluronan depolymerization (HYBID; also known as KIAA119 and CEMIP; ref. 121). In alignment with previous studies that have demonstrated reductions in HA in photoaged skin, this group found that HYBID is overexpressed in photoaged skin and posit this mechanism as an additional explanation for UVR-mediated ECM remodeling (121). Finally, miRNAs and circular RNAs (circRNAs) have also been linked with collagen degradation. The Lai research group recently proposed a novel regulator of collagen production in fibroblasts that are altered in the context of UVR. Their studies have shown that UVA promotes the downregulation of circCOL3A1-859267, which normally binds to and sequesters miR-29c, thereby derepressing the collagen-encoding gene COL1A1 (122). Subsequently, they suggested that downregulation of this circRNA results in miR-29c binding to COL3A1 and inhibition of collagen production (123).
Much of the research pertaining to ECM remodeling and cancer progression focuses on the mechanisms used by invading cancer cells to exploit the ECM for their own benefit (124, 125). However, given the ECM alterations that occur with both aging and photoaging, it is important not to overlook the scenario wherein cancer cells invade a dermal compartment with preexisting alterations to the ECM. Research from our laboratory has demonstrated that the intrinsically remodeled ECM alone can be permissive of melanoma growth and metastasis (126). With these findings, we now understand that ECM remodeling is not only a consequence of malignant cell invasion, but also an age-related change that can increase the aggressiveness of a tumor. Although this realization has generated further interest in studying underlying mechanisms of ECM remodeled as a result of biological aging, given the ECM remodeling shown in photoaging, the mechanisms driving UVR-related ECM degradation are also a pertinent area of research in the context of melanoma.
Inflammation
Inflammation is another systemic alteration that is a hallmark of aging (2) and is associated with tumorigenesis and cancer progression (127). During chronological aging, the body undergoes a progressive increase in systemic low-grade chronic inflammation, a process known as “inflammaging” (2, 128, 129). This aging-induced increase in inflammation is marked by an increase in systemic levels of numerous inflammatory cytokines, including IL-1, IL-6, and IL-33, as well as TNF, IFN-γ, and GM-CSF (130, 131). Given our understanding that microenvironments rich in proinflammatory factors can lead to activation of the stroma, thereby increasing the risk of cancer progression (132), dysregulation of the inflammatory response presents another example of how cancer risk increases with age.
Sunburn, or solar erythema, is the clinical manifestation of the acute response of the skin microenvironment to UVR, and, more specifically, UVB. UVB-induced erythema is caused by the release of proinflammatory cytokines from both keratinocytes and fibroblasts (133–135); however, there are a few different models for the molecular mechanisms that regulate the acute inflammatory response. One study suggests that the response of keratinocytes to UVB is regulated by the tyrosine kinase Src (136), while others have highlighted the NLRP family of inflammasomes as the primary driver (137). Alternatively, Wang et al. used solar-simulated UVR to show that the UV inflammatory response is mediated by cyclooxygenases (COXs; ref. 138). Unlike inflammasomes and COXs, which are known to regulate proinflammatory cytokines, the circadian-based inflammatory response has only recently been explored (139–142) and is not yet fully understood in the context of the skin. Recent work has demonstrated a molecular link between UVR-induced dysregulation of circadian genes and the UVR-mediated inflammatory response in the keratinocytes (143). Park et al. recently observed that UVB downregulated both circadian locomotor output cycles kaput (CLOCK) and tissue inhibitor of metalloprotease 3 (TIMP-3), which concurrently lead to an upregulation of MMP-1 and the proinflammatory cytokines TNF-α and IL-8 (143).
Although these findings suggest putative mediators of UVR-mediated inflammation, the UVR irradiation methods used in these studies do not fully recapitulate the photoaging phenomenon, as each study used single-dose irradiation, with the experimental endpoints within 24–48 hours after this dose. Given the association of sustained inflammation with both aging and cancer, it is crucial to understand whether repeated UVR exposure — and thus the photoaged phenotype — is characterized by chronic, rather than acute, inflammation of the skin microenvironment. Some studies have used mouse models of photoaging to begin to address this question (144, 145). These studies provide preliminary evidence that chronic inflammation of the skin microenvironment may be a feature of photoaging; however, further research is needed to determine whether photoaging recapitulates the “inflammaging” phenotype, and, if so, whether this alteration can facilitate melanoma progression.
UVR-induced immunosuppression in the skin microenvironment
UVR is a potent suppressor of the immune system. UVR-mediated effects are directed through a number of chromophores that elicit their effects predominantly through the mediation of specific leukocytes and cytokines (Figure 2). The first study to link UV-mediated immunosuppression and cancer progression was published in 1974 by Kripke et al. (146). Kripke et al. showed that UV-induced tumors transplanted into irradiated mice continued growing, whereas these tumors were rejected when transplanted into immunocompetent mice, which was later confirmed to be an immune-mediated reaction (147). Further evidence of immune protection against tumor formation in the skin is demonstrated by an increased incidence of skin cancers in transplant patients on immunosuppressive therapies (148–150). Therefore, the combination of UV immunosuppression and photoaging will place individuals chronically exposed to UVR in a significantly increased risk category for melanoma.
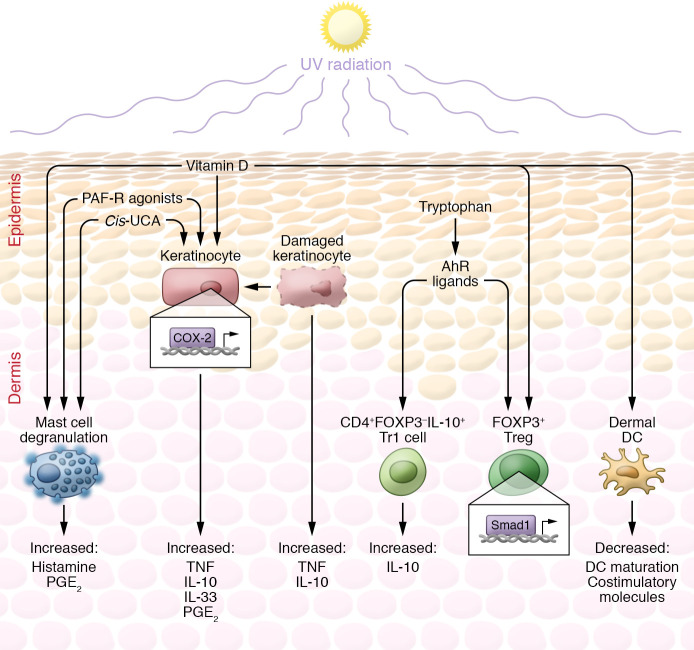
Figure 2. Immunosuppression associated with UV-mediated photoaging.
As a direct result of UVR, several immunosuppressive factors are produced. These include cis-UCA; vitamin D; platelet-activating factor receptor (PAF-R) agonists and platelet-like ligands; and AhR ligands such as FICZ. These factors cause the release of cytokines such as histamine, prostaglandin E2 (PGE2), TNF, and IL-10 (198) and IL-33 (199) from mast cells and keratinocytes and influence the differentiation of CD4+ T cells to an immunosuppressive phenotype. Vitamin D influences the differentiation of monocytes to macrophages and DCs by downregulating the vitamin D receptor, resulting in decreased DC maturation; and by downregulating costimulatory molecules, thus reducing antigen-presenting ability. Vitamin D is also reported to influence immunosuppression via keratinocytes, Tregs, and mast cells. Cis-UCA has potent immunosuppressive effects mediated through numerous dermal cells via the serotonin receptor on, but not limited to, mast cells and keratinocytes. Cis-UCA interacts with dermal mast cells to frequently induce degranulation of both histamine and PGE2. Cis-UCA is able to stimulate the release of TNF-α from keratinocytes, preventing Langerhans cells from migrating to lymph nodes and presenting antigen to T cells; as well as to stimulate production of PGE2. UV-mediated damage to keratinocytes produces PAFs to also release PGE2, as well as IL-10 and TNF. Absorption of UVB by tryptophan produces AhR ligands, including FICZ and TCDD, that signal through keratinocytes and T cells. AhR ligands upregulate COX-2 in keratinocytes, resulting in increased production of PGE2, which influences the recruitment of Tregs. AhR ligands also influence T cells via adaptive and regulatory-like T cells (induced Tregs and Tr1 cells). AhR ligands enhance the production of IL-10 from naive CD4+ T cells. AhR ligands along with TGF-β are also able to induce suppressive T cell phenotypes via the upregulation of FOXP3 either via Smad1 or potentially via direct binding of AhR to the FOXP3 promoter.
Cis-urocanic acid
Urocanic acid (UCA) is a photoreceptor in the stratum corneum that, upon exposure to UVB, is isomerized from trans-UCA to the immunosuppressive imidazole derivative cis-UCA (151). Histidinemic mice (which have less than 10% skin UCA) have impaired contact hypersensitivity (CHS) suppression following UV exposure (152), whereas mice fed a metabolic precursor of UCA have increased suppression (153). Although cis-UCA has been reported to influence other cells through the 5-HT2A serotonin receptor (154), many effects are mediated via mast cells, which themselves have a positive correlation with melanoma aggressiveness as well as sun-exposed skin.
Skin from sun-exposed sites has increased elastosis, which correlates both with the number of mast cells and with age (155). Noonan and Hoffman discovered that suppression of CHS responses required lower doses of UVB in C57BL/6 mice than in BALB/c mice (156). This differing UVB sensitivity between mouse strains is linked to the numbers of dermal mast cells, as the C57BL/6 strain has more dermal mast cells compared with BALB/c mice (157). Further evidence of this interaction is shown by a cis-UCA antibody inhibiting UVB-mediated CHS by 60%, whereas the administration of histamine suppresses CHS (158). Immunosuppressive effects of histamine are not affected by cis-UCA antibodies, suggesting that histamine is a downstream effect of cis-UCA (158). However, antagonists of the histamine receptor inhibited immunosuppression in both UVB- and cis-UCA–induced CHS (158). Genetic knockdown of mast cells renders animals unresponsive to UVB immunosuppression, but once reconstituted with bone marrow–derived mast cell precursors, they become resensitized and susceptible to UVB (157). This finding provides a link between the release of histamine from dermal mast cells, triggered through cis-UCA and UVB-mediated immunosuppression.
Some studies have demonstrated that mast cells gradually decrease with age, whereas exposure to UV increases the number of these cells (159). Alternative studies have suggested that mast cells increase with age (160). However, these data indicate that degranulation of mast cells decreases with age, whereas UVR causes rapid degranulation of histamine and prostaglandin E2 (161). This suggests that UV exposure to aged individuals could not only cause increased mast cells but increase degranulation of immunosuppressive cytokines, promoting a protumor environment.
The aryl hydrocarbon receptor
Although the aryl hydrocarbon receptor (AhR) is not a chromophore itself, downstream signaling from AhR is activated following UVR (162). AhR is widespread among all skin cells and specific immune cells. As well as having a significant influence in UV-mediated immunosuppression, AhR signaling also promotes tumor formation in melanoma (163–165). Moreover, genes associated with BRAF resistance are also regulated by AhR, as combination therapy of BRAF inhibitors and AhR antagonists maintains sensitivity to targeted therapy in melanoma (166). UVB exposure photosynthesizes tryptophan into metabolites of AhR ligands, including the AhR agonist 6-formylindolo[3,2-b]carbazole (FICZ; refs. 167, 168). FICZ has been shown within the skin (169), and sulfate conjugates of FICZ are found in the urine of UV-exposed individuals (170). AhR signaling is particularly critical in UV-mediated immunosuppression, promoting the differentiation of Tregs. Tregs are not only found within the local primary melanoma TME (171, 172) but also are increased in lymph nodes (173) and distant metastatic sites (174). Moreover, the level of Tregs in patients is inversely correlated with patient survival (175, 176). Natural Tregs develop in the thymus, while acquired Tregs develop from naive peripheral CD4+ T cells in response to tolerogenic stimuli (177). FOXP3 is often described as the master regulator of function and development of Tregs; however, CD4+FOXP3–IL-10+ Treg-like Tr1 cells are also suppressive (178, 179). Naive T cells stimulated with an AhR ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), upregulate IL10 and FOXP3 mRNA but not protein, suggesting a Tr1 phenotype (179). These cells also have decreased proliferation and transcription of IFNA1. This differentiation was dependent on AhR signaling, as knockdown of this receptor diminished TCDD-mediated induction of IL-10 as well as the suppressive phenotype (179). Treatment with TCDD alongside TGF-β increased the expression of FOXP3 mRNA and protein. Gene microarray and ChIP data suggest that the upregulation of FOXP3 following TCDD and TGF-β treatment is mediated by Smad1, which binds directly to the FOXP3 promoter (179). AhR-conserved binding sites in the FOXP3 promoter in CD4+ T cells have been reported, suggesting that AhR may also directly upregulate FOXP3 (180). Further evidence of UV-mediated suppression via Treg induction by AhR was demonstrated using the AhR antagonist 3-methoxy-4-nitroflavone, which reduced UV-mediated immunosuppression by decreasing Tregs in mouse models of CHS, whereas an AhR agonist, 4-n-nonylphenol, increased Tregs and suppressed CHS, similarly to UVR (162).
Vitamin D
The primary vitamin D precursor in keratinocytes is 7-dehydrocholesterol. Upon UV exposure, 7-dehydrocholesterol is isomerized to pre–vitamin D, which subsequently goes on to the biologically active form 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (181). Vitamin D also influences the immune system to promote immunosuppressive environments (182). Interestingly, numerous myeloid cells, including dendritic cells (DCs) and macrophages, synthesize their own vitamin D and suppress adaptive immune responses (182). These cells express 1α-hydrolase, whereby they can synthesize their own 1,25(OH)2D3 as well as express the vitamin D receptor (VDR), producing a feedback loop (183, 184). Interestingly, as monocytes respond to 1,25(OH)2D3, the differentiation to DCs is inhibited as levels of the VDR decrease, promoting a tolerogenic DC phenotype with a suboptimal antigen-presenting ability (182, 185, 186). This also results in the production of increased macrophage numbers (187). Activation of VDR downregulates DC maturation and costimulatory markers (188, 189). When cultured in vitro independently of professional antigen-presenting cells, 1,25(OH)2D3 induced IL-10–secreting Tregs (190). The ability of these cells to produce proinflammatory cytokines, including IFN-γ, IL-17, and IL-21, was also diminished; moreover, 1,25(OH)2D3 induced the infiltration of a population of functionally suppressive Tregs (191). The influence of vitamin D also stretches beyond myeloid cells. Reports also demonstrate its effects on the expression of microbial pattern recognition receptors such as TLR2 by keratinocytes (192), and show that it decreases UVB-induced skin pathology in mouse models via mast cells (193).
Conclusions and future perspectives
Many molecular alterations that occur in the skin microenvironment with UVR-induced extrinsic aging have the potential to impact tumor progression. To date, photoaging is often viewed as the superimposition of photodamage and aging mechanisms (100, 194) — a presumption that is rooted in the fact that there are many similarities between photoaging and chronological aging hallmarks and processes. However, there are instances, such as with melanin synthesis and senescence, in which the molecular mediators of photoaging diverge substantially from those in classical aging (Figure 3). These findings highlight the critical importance of having clearly defined models for studying chronological aging and photoaging independent of one another.
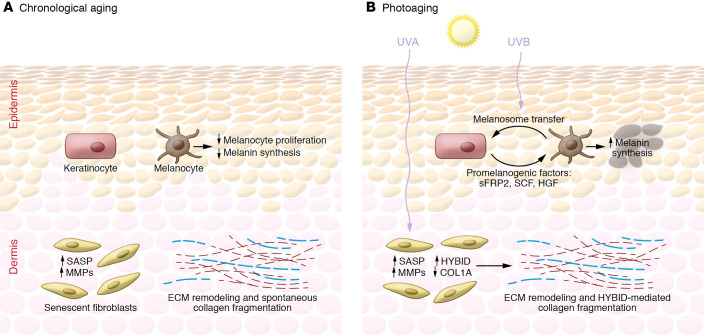
Figure 3. Differences between the chronologically aged and photoaged skin microenvironments.
A summary of key differences between chronologically aged (A) and photoaged (B) skin as described in “The photoaged microenvironment and melanoma progression.” Intrinsic aging is associated with decreases in melanocyte proliferation and melanogenesis, resulting in hypopigmentation of the epidermis. In contrast, UVR-exposed keratinocytes induce melanogenesis, thereby causing regions of hyperpigmentation clinically recognized as “sun spots.” Both the intrinsically aged and the photoaged fibroblasts of the dermal skin layer express SASP and increased production of MMPs, which leads to collagen breakdown and ECM remodeling. The mechanisms driving spontaneous collagen fragmentation in intrinsically aged skin remain largely unknown. However, in photoaged fibroblasts, this process is thought to be mediated by an increased hyaluronic acid depolymerization by HYBID and downregulation of collagen production by altered miRNAs and circRNAs.
The extent to which the mechanisms of extrinsic and intrinsic aging coincide does not detract from the significance of both processes in melanoma progression. In the case of Tregs and keratinocytes, intrinsic and extrinsic aging induces molecular alterations that impact the early stages of melanoma transformation and initial invasion. Alternatively, the constituents of the dermal microenvironment and ECM undergo changes during aging and photoaging that play a substantial role in determining whether melanoma will metastasize to organs beyond the skin.
The links between photoaging and the skin microenvironment are of high importance, specifically for the aged demographic of melanoma patients with a history of repeated sunburn or routine tanning. Additionally, given that molecular response to chronic UVR will diverge depending on inherent, genetically defined skin pigmentation (195, 196), efforts should be made to acquire a much-expanded biorepository of sun-protected and sun-exposed young and aged primary dermal cells from patients of differing degrees of skin pigmentation. Diversification of the primary samples available to study both intrinsic and extrinsic aging will bring us one step closer to obtaining the molecular characterization of photoaging required to identify putative therapeutic targets in the skin microenvironment for cancer patients.
Acknowledgments
ATW, AW, and SMD are supported by NIH grants R01CA174746 and R01CA207935. ATW is also supported by NIH grants P01 CA114046 and R01CA232256, a Bloomberg Distinguished Professorship, and the EV McCollum Endowed Chair.
Version 1. 03/15/2021
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(6):e143763.https://doi.org/10.1172/JCI143763.
References
- 1.Aunan JR, et al. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017;8(5):628–642. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89–106. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullough KD, et al. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed rat liver epithelial cells into the liver. Cancer Res. 1997;57(9):1807–1813. [PubMed] [Google Scholar]
- 4.Sandiford OA, et al. Human aging and cancer: role of miRNA in tumor microenvironment. Adv Exp Med Biol. 2018;1056:137–152. doi: 10.1007/978-3-319-74470-4_9. [DOI] [PubMed] [Google Scholar]
- 5.Krutmann J. Vorzeitige hautalterung durch ultraviolette strahlung und andere umweltnoxen: molekulare grundlagen [Premature skin aging by ultraviolet radiation and other environmental hazards: the molecular basis] Der Hautarzt. 2003;54(9):809–817. doi: 10.1007/s00105-003-0575-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13(3):371–380. doi: 10.1016/j.fsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Sachs DL, et al. Atrophic and hypertrophic photoaging: clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. J Am Acad Dermatol. 2019;81(2):480–488. doi: 10.1016/j.jaad.2019.03.081. [DOI] [PubMed] [Google Scholar]
- 8.Nikolakis G, et al. Skin mirrors human aging. Horm Mol Biol Clin Investig. 2013;16(1):13–28. doi: 10.1515/hmbci-2013-0018. [DOI] [PubMed] [Google Scholar]
- 9.Makrantonaki E, Zouboulis CC. The skin as a mirror of the aging process in the human organism — state of the art and results of the aging research in the German National Genome Research Network 2 (NGFN-2) Exp Gerontol. 2007;42(9):879–886. doi: 10.1016/j.exger.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Tsatsou F, et al. Extrinsic aging: UV-mediated skin carcinogenesis. Dermatoendocrinol. 2012;4(3):285. doi: 10.4161/derm.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruglikov IL, Scherer PE. Skin aging: are adipocytes the next target? Aging (Albany NY) 2016;8(7):1457–1469. doi: 10.18632/aging.100999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, et al. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 13.Rastrelli M, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo (Brooklyn) 2014;28(6):1005–1012. [PubMed] [Google Scholar]
- 14.Paulson KG, et al. Age-specific incidence of melanoma in the United States. JAMA Dermatology. 2020;156(1):57–64. doi: 10.1001/jamadermatol.2019.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balch CM, et al. Age as a predictor of sentinel node metastasis among patients with localized melanoma: an inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patients. Ann Surg Oncol. 2014;21(4):1075–1081. doi: 10.1245/s10434-013-3464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fane M, Weeraratna AT. Normal aging and its role in cancer metastasis. Cold Spring Harb Perspect Med. 2020;10(9):a037341. doi: 10.1101/cshperspect.a037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prüss-üstün A, et al. Solar Ultraviolet Radiation: Global Burden of Disease From Solar Ultraviolet Radiation. WHO; 2006. [Google Scholar]
- 18. Gies P, et al. Solar and ultraviolet radiation. In: Hill D, et al., eds. Prevention of Skin Cancer. Springer, Dordrecht; 2011:21–54. [Google Scholar]
- 19.Amaro-Ortiz A, et al. Ultraviolet radiation, aging and the skin: prevention of damage by topical cAMP manipulation. Molecules. 2014;19(5):6202–6219. doi: 10.3390/molecules19056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77(1):13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 21.Wang PW, et al. Comparison of the biological impact of UVA and UVB upon the skin with functional proteomics and immunohistochemistry. Antioxidants. 2019;8(12):569. doi: 10.3390/antiox8120569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank J, et al. Exposing the human nude phenotype. Nature. 1999;398(6727):473–474. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- 23.Weiner L, et al. Skin as a living coloring book: how epithelial cells create patterns of pigmentation. Pigment Cell Melanoma Res. 2014;27(6):1014–1031. doi: 10.1111/pcmr.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, et al. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22(20):3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 25.Haass NK, et al. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005;18(3):150–159. doi: 10.1111/j.1600-0749.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim E, et al. Senescent fibroblasts in melanoma initiation and progression: an integrated theoretical, experimental, and clinical approach. Cancer Res. 2013;73(23):6874–6885. doi: 10.1158/0008-5472.CAN-13-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrazek AA, Chao C. Surviving cutaneous melanoma: a clinical review of follow-up practices, surveillance, and management of recurrence. Surg Clin North Am. 2014;94(5):989–1002. doi: 10.1016/j.suc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mescher M, et al. The epidermal polarity protein Par3 is a non-cell autonomous suppressor of malignant melanoma. J Exp Med. 2017;214(2):339–358. doi: 10.1084/jem.20160596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golan T, et al. Interactions of melanoma cells with distal keratinocytes trigger metastasis via notch signaling inhibition of MITF. Mol Cell. 2015;59(4):664–676. doi: 10.1016/j.molcel.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Hui L, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Han L, et al. Extracellular vesicles in the tumor microenvironment: therapeutic resistance, clinical biomarkers, and targeting strategies. Med Res Rev. 2017;37(6):1318–1349. doi: 10.1002/med.21453. [DOI] [PubMed] [Google Scholar]
- 32.Najafi M, et al. Tumor microenvironment: interactions and therapy. J Cell Physiol. 2019;234(5):5700–5721. doi: 10.1002/jcp.27425. [DOI] [PubMed] [Google Scholar]
- 33.Al-Zoughbi W, Hoefler G. Tumor macroenvironment: an update. Pathobiology. 2020;87(2):58–60. doi: 10.1159/000502097. [DOI] [PubMed] [Google Scholar]
- 34.Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol. 2003;(12 suppl 2):5–12. doi: 10.1034/j.1600-0625.12.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 35.Aoki H, et al. Gene expression profiling analysis of solar lentigo in relation to immunohistochemical characteristics. Br J Dermatol. 2007;156(6):1214–1223. doi: 10.1111/j.1365-2133.2007.07830.x. [DOI] [PubMed] [Google Scholar]
- 36.Maeda K. Large melanosome complex is increased in keratinocytes of solar lentigo. Cosmetics. 2017;4(4):49. doi: 10.3390/cosmetics4040049. [DOI] [Google Scholar]
- 37.Ortonne JP. Pigmentary changes of the ageing skin. Br J Dermatol. 1990;122(suppl 35):21–28. doi: 10.1111/j.1365-2133.1990.tb16121.x. [DOI] [PubMed] [Google Scholar]
- 38.Goorochurn R, et al. Biological processes in solar lentigo: insights brought by experimental models. Exp Dermatol. 2016;25(3):174–177. doi: 10.1111/exd.12937. [DOI] [PubMed] [Google Scholar]
- 39.Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology. 2009;1(2):83–93. doi: 10.4103/0974-7753.58550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snell RS, Bischitz PG. The melanocytes and melanin in human abdominal wall skin: a survey made at different ages in both sexes and during pregnancy. J Anat. 1963;97(3):361–76. [PMC free article] [PubMed] [Google Scholar]
- 41.Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philos Trans R Soc Lond B Biol Sci. 1967;252(779):447–485. [Google Scholar]
- 42.Gilchrest BA, et al. Effects of aging and chronic sun exposure on melanocytes in human skin. J Invest Dermatol. 1979;73(2):141–143. doi: 10.1111/1523-1747.ep12581580. [DOI] [PubMed] [Google Scholar]
- 43.Hawk JL. Photosensitivity in the elderly. Br J Dermatol. 1990;122(suppl 35):29–36. doi: 10.1111/j.1365-2133.1990.tb16122.x. [DOI] [PubMed] [Google Scholar]
- 44.Shlivko IL, et al. Complex assessment of age-specific morphofunctional features of skin of different anatomic localizations. Ski Res Technol. 2013;19(1):e85–e92. doi: 10.1111/j.1600-0846.2012.00613.x. [DOI] [PubMed] [Google Scholar]
- 45.Wenczl E, et al. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol. 1998;111(4):678–682. doi: 10.1046/j.1523-1747.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 46.Premi S, et al. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347(6224):842–847. doi: 10.1126/science.1256022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delinasios GJ, et al. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci Rep. 2018;8(1):423. doi: 10.1038/s41598-017-18924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarna M, et al. Melanin presence inhibits melanoma cell spread in mice in a unique mechanical fashion. Sci Rep. 2019;9(1):1–9. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas NE, et al. Comparison of clinicopathologic features and survival of histopathologically amelanoticand pigmented melanomas a population-based study. JAMA Dermatol. 2014;150(12):1306–1314. doi: 10.1001/jamadermatol.2014.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hattori H, et al. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J Invest Dermatol. 2004;122(5):1256–1265. doi: 10.1111/j.0022-202X.2004.22503.x. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs D, et al. Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br J Dermatol. 2010;163(5):1020–1027. doi: 10.1111/j.1365-2133.2010.09946.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim M, et al. Secreted frizzled-related protein 2 (sFRP2) functions as a melanogenic stimulator: the role of sFRP2 in UV-induced hyperpigmentary disorders. J Invest Dermatol. 2016;136(1):236–244. doi: 10.1038/JID.2015.365. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida H, et al. Review: melanocyte migration and survival controlled by SCF/c-kit expression. J Investig Dermatol Symp Proc. 2001;6(1):1–5. doi: 10.1046/j.0022-202x.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 54.Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003;16(3):287–296. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 55.Roskoski R. Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337(1):1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 56.Liang J, et al. The C-Kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9(5):435–443. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babaei MA, et al. Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells. Drug Des Devel Ther. 2016;10:2443–2459. doi: 10.2147/DDDT.S89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng D, Carvajal RD. KIT as an oncogenic driver in melanoma: an update on clinical development. Am J Clin Dermatol. 2019;20(3):315–323. doi: 10.1007/s40257-018-0414-1. [DOI] [PubMed] [Google Scholar]
- 59.Slipicevic A, Herlyn M. KIT in melanoma: many shades of gray. J Invest Dermatol. 2015;135(2):337–338. doi: 10.1038/jid.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtin JA, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 61.Beadling C, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 62.et al. Up-regulation of MET expression by α-melanocyte-stimulating hormone and MITF allows hepatocyte growth factor to protect melanocytes and melanoma cells from apoptosis. J Biol Chem. 2007;282(19):14140–14147. doi: 10.1074/jbc.M611563200. [DOI] [PubMed] [Google Scholar]
- 63.Matsumoto K, et al. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991;176(1):45–51. doi: 10.1016/0006-291X(91)90887-D. [DOI] [PubMed] [Google Scholar]
- 64.Noonan FP, et al. Accelerated ultraviolet radiation-induced carcinogenesis in hepatocyte growth factor/scatter factor transgenic mice. Cancer Res. 2000;60(14):3738–3743. [PubMed] [Google Scholar]
- 65.Noonan FP, et al. Neonatal sunburn and melanoma in mice. Nature. 2001;413(6853):271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 66.Noonan FP, et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat Commun. 2012;3:884. doi: 10.1038/ncomms1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demkova L, Kucerova L. Role of the HGF/c-MET tyrosine kinase inhibitors in metastasic melanoma. Mol Cancer. 2018;17(1):26. doi: 10.1186/s12943-018-0795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Czyz M. HGF/c-MET signaling in melanocytes and melanoma. Int J Mol Sci. 2018;19(12):3844. doi: 10.3390/ijms19123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaur A, et al. SFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–254. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campisi J, Robert L. Cell senescence: role in aging and age-related diseases. Aging Facts Theor. 2014;39:45–61. doi: 10.1159/000358899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varani J, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunin G, et al. Age changes in the number and proliferation of fibroblasts in the human skin. Adv Gerontol. 2011;24(1):43–47. [PubMed] [Google Scholar]
- 74.Andrew W, et al. Changes with advancing age in the cell population of human dermis. Gerontology. 1964–1965;10:1–19. doi: 10.1159/000211369. [DOI] [PubMed] [Google Scholar]
- 75.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coppé JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawrenson K, et al. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia. 2010;12(4):317–325. doi: 10.1593/neo.91948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67(7):3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 80.Pavlides S, et al. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9(17):3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinez-Outschoorn UE, et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43(7):1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balliet RM, et al. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10(23):4065–4073. doi: 10.4161/cc.10.23.18254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Outschoorn UE, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9(17):3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wegiel B, et al. Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Front. Oncol. 2018;8:284. doi: 10.3389/fonc.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Debacq-Chainiaux F, et al. UV, stress and aging. Dermatoendocrinol. 2012;4(43):236–240. doi: 10.4161/derm.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Victorelli S, Passos JF. Telomeres and cell senescence — size matters not. EBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kosmadaki MG, Gilchrest BA. The role of telomeres in skin aging/photoaging. Micron. 2004;35(3):155–159. doi: 10.1016/j.micron.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Sugimoto M, et al. Telomere length of the skin in association with chronological aging and photoaging. J Dermatol Sci. 2006;43(1):43–47. doi: 10.1016/j.jdermsci.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Ma HM, et al. Human skin fibroblast telomeres are shortened after ultraviolet irradiation. J Int Med Res. 2012;40(5):1871–1877. doi: 10.1177/030006051204000526. [DOI] [PubMed] [Google Scholar]
- 90.Yin B, Jiang X. Telomere shortening in cultured human dermal fibroblasts is associated with acute photodamage induced by UVA irradiation. Postepy Dermatol Alergol. 2013;30(1):13–18. doi: 10.5114/pdia.2013.33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suh N. MicroRNA controls of cellular senescence. BMB Rep. 2018;51(10):493–499. doi: 10.5483/BMBRep.2018.51.10.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srivastava A, et al. Identification of chronological and photoageing-associated microRNAs in human skin. Sci Rep. 2018;8(1):12990. doi: 10.1038/s41598-018-31217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avelar RA, et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020;21(1):91. doi: 10.1186/s13059-020-01990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp Gerontol. 2017;94:78–82. doi: 10.1016/j.exger.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 95.Frantz C, et al. The extracellular matrix at a glance. J Cell Sci. 2010;123(pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24(5):830–833. doi: 10.1016/j.copbio.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Theocharis A, et al. Extracellular matrix: a functional scaffold. In: Karamanos NK, ed. Extracellular Matrix: Pathobiology and Signaling. De Gruyter; 2012:3–20. [Google Scholar]
- 98.Fisher GJ, et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174(1):101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fisher GJ, et al. Ageing: collagenase-mediated collagen fragmentation as a rejuvenation target. Br J Dermatol. 2014;171(3):446–449. doi: 10.1111/bjd.13267. [DOI] [PubMed] [Google Scholar]
- 100.Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. doi: 10.1101/cshperspect.a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fisher GJ, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 102.Panwar P, et al. Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem. 2015;290(38):23291–23306. doi: 10.1074/jbc.M115.644310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Savic S, et al. The structural characteristics of photoageing in mice caused by the effects of ultraviolet A radiation. Folia Morphol. 2020;79(3):548–556. doi: 10.5603/FM.a2019.0119. [DOI] [PubMed] [Google Scholar]
- 104.Sephel GC, Davidson JM. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol. 1986;86(3):279–285. doi: 10.1111/1523-1747.ep12285424. [DOI] [PubMed] [Google Scholar]
- 105.Aziz J, et al. Molecular mechanisms of stress-responsive changes in collagen and elastin networks in skin. Skin Pharmacol Physiol. 2016;29(4):190–203. doi: 10.1159/000447017. [DOI] [PubMed] [Google Scholar]
- 106.Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986;4(3):433–446. doi: 10.1016/S0733-8635(18)30806-4. [DOI] [PubMed] [Google Scholar]
- 107.Braverman IM, Fonferko E. Studies in cutaneous aging. I. The elastic fiber network. J Invest Dermatol. 1982;78(5):434–443. doi: 10.1111/1523-1747.ep12507866. [DOI] [PubMed] [Google Scholar]
- 108.Amano S. Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures. Exp Dermatol. 2016;25(suppl 3):14–19. doi: 10.1111/exd.13085. [DOI] [PubMed] [Google Scholar]
- 109.Weihermann AC, et al. Elastin structure and its involvement in skin photoageing. Int J Cosmet Sci. 2017;39(3):241–247. doi: 10.1111/ics.12372. [DOI] [PubMed] [Google Scholar]
- 110.Watson REB, et al. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J Invest Dermatol. 1999;112(5):782–787. doi: 10.1046/j.1523-1747.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 111.Dai G, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 2007;171(5):1451–1461. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tzellos TG, et al. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol. 2009;18(12):1028–1035. doi: 10.1111/j.1600-0625.2009.00889.x. [DOI] [PubMed] [Google Scholar]
- 113.Papakonstantinou E, et al. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauhala L, et al. Low dose ultraviolet b irradiation increases hyaluronan synthesis in epidermal keratinocytes via sequential induction of hyaluronan synthases has1-3 mediated by P38 and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling. J Biol Chem. 2013;288(25):17999–18012. doi: 10.1074/jbc.M113.472530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yin R, et al. Skin Photoaging. IOP Concise Physics; 2015; 1–10. [Google Scholar]
- 116.Ghosh K, Capell BC. The senescence-associated secretory phenotype: critical effector in skin cancer and aging. J Invest Dermatol. 2016;136(11):2133–2139. doi: 10.1016/j.jid.2016.06.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freitas-Rodríguez S, et al. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864(11 pt A):2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 118.Davalli P, et al. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chittiboyina S, et al. Microenvironment-cell nucleus relationship in the context of oxidative stress. Front Cell Dev Biol. 2018;6:23. doi: 10.3389/fcell.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pittayapruek P, et al. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshida H, Okada Y. Role of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization), alias KIAA1199/CEMIP, in hyaluronan degradation in normal and photoaged skin. Int J Mol Sci. 2019;20(22):5804. doi: 10.3390/ijms20225804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peng Y, et al. Circular RNA profiling reveals that circCOL3A1-859267 regulate type I collagen expression in photoaged human dermal fibroblasts. Biochem Biophys Res Commun. 2017;486(2):277–284. doi: 10.1016/j.bbrc.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 123.Peng Y, et al. circCOL3A1-859267 regulates type I collagen expression by sponging miR-29c in human dermal fibroblasts. Eur J Dermatol. 2018;28(5):613–620. doi: 10.1684/ejd.2018.3397. [DOI] [PubMed] [Google Scholar]
- 124.Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36(3):171–198. doi: 10.1007/s10585-019-09966-1. [DOI] [PubMed] [Google Scholar]
- 125.Mohan V, et al. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200. doi: 10.1016/j.semcancer.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 126.Kaur A, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9(1):64–81. doi: 10.1158/2159-8290.CD-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 128.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 129.Fulop T, et al. The integration of inflammaging in age-related diseases. Semin Immunol. 2018;40:17–35. doi: 10.1016/j.smim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 130.Zinger A, et al. Cancer and aging — the inflammatory connection. Aging Dis. 2017;8(5):311–627. doi: 10.14336/AD.2016.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23(11):1966–1971. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Imokawa G. Epithelial–mesenchymal interaction mechanisms leading to the over-expression of neprilysin are involved in the UVB-induced formation of wrinkles in the skin. Exp Dermatol. 2016;25(suppl 3):2–13. doi: 10.1111/exd.13083. [DOI] [PubMed] [Google Scholar]
- 134.Kim Y, et al. The pathogenic role of interleukin-22 and its receptor during UVB-induced skin inflammation. PLoS One. 2017;12(5):e0178567. doi: 10.1371/journal.pone.0178567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoshizumi M, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32(11):1405–1411. doi: 10.1016/j.cellbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 136.Han SM, et al. Src kinase mediates UV-induced TRPV1 trafficking into cell membrane in HaCaT keratinocytes. Photodermatol Photoimmunol Photomed. 2018;34(3):214–216. doi: 10.1111/phpp.12363. [DOI] [PubMed] [Google Scholar]
- 137.Hasegawa T, et al. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem Biophys Res Commun. 2016;477(3):329–335. doi: 10.1016/j.bbrc.2016.06.106. [DOI] [PubMed] [Google Scholar]
- 138.Wang P, et al. Cyclooxygenases mediate early induction of interleukin-6 expression by solar ultraviolet irradiation in human skin. J Dermatol Sci. 2017;87(2):201–203. doi: 10.1016/j.jdermsci.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida K, et al. Involvement of the circadian rhythm and inflammatory cytokines in the pathogenesis of rheumatoid arthritis. J Immunol Res. 2014;2014:282495. doi: 10.1155/2014/282495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Castanon-Cervantes O, et al. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185(10):5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McAlpine CS, Swirski FK. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res. 2016;119(1):131–141. doi: 10.1161/CIRCRESAHA.116.308034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Comas M, et al. A circadian based inflammatory response – implications for respiratory disease and treatment. Sleep Sci Pract. 2017;1(1):1–19. doi: 10.1186/s41606-016-0006-z. [DOI] [Google Scholar]
- 143.Park S, et al. TIMP3 is a CLOCK-dependent diurnal gene that inhibits the expression of UVB-induced inflammatory cytokines in human keratinocytes. FASEB J. 2018;32(3):1510–1523. doi: 10.1096/fj.201700693R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma MR, et al. Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH1 and Balb/c mice. Photochem Photobiol. 2011;87(3):690–698. doi: 10.1111/j.1751-1097.2011.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yoshida M, et al. Persistent inflammation in photo-aged skin. J Dermatol Sci. 2017;86(2):e81–e82. [Google Scholar]
- 146.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53(5):1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 147.Ullrich SE, Kripke ML. Mechanisms in the suppression of tumor rejection produced in mice by repeated UV irradiation. J Immunol. 1984;133(5):2786–2790. [PubMed] [Google Scholar]
- 148.Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–20. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 149.Jensen P, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40(2 pt 1):177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 150.Hartevelt MM, et al. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49(3):506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 151.Noonan FP, et al. Mechanism of systemic immune suppression by UV irradiation in vivo. II. The UV effects on number and morphology of epidermal Langerhans cells and the UV-induced suppression of contact hypersensitivity have different wavelength dependencies. J Immunol. 1984;132(5):2408–2416. [PubMed] [Google Scholar]
- 152.De Fabo EC, et al. Further evidence that the photoreceptor mediating UV-induced systemic immune suppression is urocanic acid. J Invest Dermatol. 1983;80(4):319 [Google Scholar]
- 153.Reilly SK, Da Fabo EC. Dietary histidine increases mouse skin urocanic acid levels and enhances UVB-induced immune suppression of contact hypersensitivity. Photochem Photobiol. 1991;53(4):431–438. doi: 10.1111/j.1751-1097.1991.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 154.Walterscheid JP, et al. Cis-uronic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5HT2A receptor. Proc Natl Acad Sci U S A. 2006;103(46):17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grimbaldeston MA, et al. Mast cells in photodamaged skin: what is their role in skin cancer? Photochem Photobiol Sci. 2006;5(2):177–183. doi: 10.1039/B504344A. [DOI] [PubMed] [Google Scholar]
- 156.Noonan FP, Hoffman HA. Susceptibility to immunosuppression by ultraviolet B radiation in the mouse. Immunogenetics. 1994;339(1):29–39. doi: 10.1007/BF00171794. [DOI] [PubMed] [Google Scholar]
- 157.Hart PH, et al. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187(12):2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hart PH, et al. Histamine involvement in UVB- and cis-urocanic acid-induced systemic suppression of contact hypersensitivity responses. Immunology. 1997;91(4):601–608. doi: 10.1046/j.1365-2567.1997.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pilkington SM, et al. Aged human skin accumulates mast cells with altered functionality that localize to macrophages and vasoactive intestinal peptide-positive nerve fibres. Br J Dermatol. 2019;180(4):849–858. doi: 10.1111/bjd.17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim MS, et al. Acute exposure of human skin to ultraviolet or infrared radiation or heat stimuli increases mast cell numbers and tryptase expression in human skin in vivo. Br J Dermatol. 2009;160(2):393–402. doi: 10.1111/j.1365-2133.2008.08838.x. [DOI] [PubMed] [Google Scholar]
- 161.Gilchrest BA, et al. The human sunburn reaction: histologic and biochemical studies. J Am Acad Dermatol. 1981;5(4):411–422. doi: 10.1016/S0190-9622(81)70103-8. [DOI] [PubMed] [Google Scholar]
- 162.Navid F, et al. The aryl hydrocarbon receptor is involved in UVR-Induced immunosuppression. J Invest Dermatol. 2013;133(12):2763–2770. doi: 10.1038/jid.2013.221. [DOI] [PubMed] [Google Scholar]
- 163.Villano CM, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol Appl Pharmacol. 2006;210(3):212–224. doi: 10.1016/j.taap.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 164.Barretina J, et al. Addendum: The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;565(7738):E5–E6. doi: 10.1038/s41586-018-0722-x. [DOI] [PubMed] [Google Scholar]
- 165.Lv JW, et al. Pan-cancer genomic analyses reveal prognostic and immunogenic features of the tumor melatonergic microenvironment across 14 solid cancer types. J Pineal Res. 2019;66(3):e12557. doi: 10.1111/jpi.12557. [DOI] [PubMed] [Google Scholar]
- 166.Corre S, et al. Sustained activation of the Aryl hydrocarbon receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat Commun. 2018;9(1):4775. doi: 10.1038/s41467-018-06951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei YD, et al. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem Biol Interact. 1999;118(2):127–140. doi: 10.1016/S0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 168.Rannug A, et al. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262(32):15422–15427. doi: 10.1016/S0021-9258(18)47743-5. [DOI] [PubMed] [Google Scholar]
- 169.Schallreuter KU, et al. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 1: epidermal H2 O2 /ONOO–-mediated stress abrogates tryptophan hydroxylase and dopa decarboxylase activities, leading to low serotonin and maltonin levels. FASEB J. 2012;26(6):2457–2470. doi: 10.1096/fj.11-197137. [DOI] [PubMed] [Google Scholar]
- 170.Wincent E, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans [3,2-b]carbazole is present in humans [3,2-b]carbazole is present in humans. J Biol Chem. 2009;284(5):2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 171.Mougiakakos D, et al. Intratumoral forkhead box p3-positive regulatory t cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. 2010;116(9):2224–2233. doi: 10.1002/cncr.24999. [DOI] [PubMed] [Google Scholar]
- 172.Kugel CH, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347–5356. doi: 10.1158/1078-0432.CCR-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jandus C, et al. Selective accumulation of differentiated FOXP3+ CD4+ T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother. 2008;57(12):1795–1805. doi: 10.1007/s00262-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mourmouras V, et al. Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. Br J Dermatol. 2007;157(3):531–539. doi: 10.1111/j.1365-2133.2007.08057.x. [DOI] [PubMed] [Google Scholar]
- 175.Knol AC, et al. Prognostic value of tumor-infiltrating Foxp3+ T-cell subpopulations in metastatic melanoma. Exp Dermatol. 2011;20(5):430–434. doi: 10.1111/j.1600-0625.2011.01260.x. [DOI] [PubMed] [Google Scholar]
- 176.Jiang H, et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer. 2015;136(10):2352–2360. doi: 10.1002/ijc.29297. [DOI] [PubMed] [Google Scholar]
- 177.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 178.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 179.Gandhi R, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat Immunol. 2010;11(9):846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Quintana FJ, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 181.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 182.Hart PH, et al. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011;11(9):584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 183.Bouillon R. Vitamine D et la santé globale [Vitamin D and human health] Press Medicale. 2009;38(1):3–6. doi: 10.1016/j.lpm.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 184.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321(2):103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dam TN, et al. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatology Symp Proc. 1996;1(1):72–77. [PubMed] [Google Scholar]
- 186.Griffin MD, et al. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270(3):701–708. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 187.Baeke F, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 188.Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 189.Hewison M, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 190.Khoo AL, et al. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134(4):459–468. doi: 10.1111/j.1365-2567.2011.03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jeffery LE, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183(9):5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Biggs L, et al. Evidence that vitamin D3 promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med. 2010;207(3):455–463. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laga AC, Murphy GF. The translational basis of human cutaneous photoaging: on models, methods, and meaning. Am J Pathol. 2009;174(2):357–360. doi: 10.2353/ajpath.2009.081029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Del Bino S, et al. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci. 2018;19(9):2668. doi: 10.3390/ijms19092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.López-Otín C, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Walterscheid JP, et al. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195(2):171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Byrne SN, et al. The immune-modulating cytokine and endogenous alarmin interleukin-33 is upregulated in skin exposed to inflammatory UVB radiation. Am J Pathol. 2011;179(1):211–222. doi: 10.1016/j.ajpath.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]