Abstract
Background
Compound Xueshuantong capsule (CXC) and Hexuemingmu tablet (HXMMT) are two important Chinese patent medicines (CPMs) frequently used to treat proliferative diabetic retinopathy (PDR), especially when complicated with vitreous hemorrhage (VH). However, a network pharmacology approach to understand the therapeutic mechanisms of these two CPMs in PDR has not been applied.
Objective
To identify differences in the active ingredients between CXC and HXMMT and to comparatively predict and further analyze the molecular targets shared by these CPMs and PDR. Materials and methods. The differentially expressed messenger RNAs (mRNAs) between normal retinal tissues in healthy individuals and active fibrovascular membranes in PDR patients were retrieved from the Gene Expression Omnibus database. The active ingredients of CXC and HXMMT and the targets of these ingredients were retrieved from the Traditional Chinese Medicine Systems Pharmacology database. The intersections of the CPM (CXC and HXMMT) targets and PDR targets were determined. Then, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed, and the ingredient-target networks, protein-protein interaction networks, and KEGG-target (KEGG-T) networks were constructed.
Results
CXC contains 4 herbs, and HXMMT contains 19. Radix salviae is the only herb common to both. CXC had 34 potential therapeutic targets in PDR, while HXMMT had these 34 and 10 additional targets. Both CPMs shared the following main processes: response to reactive oxygen species and oxidative stress, regulation of blood vessel diameter and size, vasoconstriction, smooth muscle contraction, hemostasis, and blood coagulation. The shared pathways included the AGE-RAGE signaling pathway in diabetic complications, TNF signaling pathway, relaxin signaling pathway, and IL-17 signaling pathway.
Conclusions
Both CXC and HXMMT include components effective at treating PDR and affect the following main processes: response to reactive oxygen species and oxidative stress, regulation of blood vessels, and blood coagulation. Radix salviae, the only herb common to both CPMs, contains many useful active ingredients. The PDR-CXC and PDR-HXMMT networks shared 34 common genes (RELA, HSPA8, HSP90AA, HSP90AB1, BRCA, EWSR1, CUL7, HNRNPU, MYC, CTNNB1, MDM2, YWHAZ, CDK2, AR, FN1, HUWE1, TP53, TUBB, EP300, GRB2, VCP, MCM2, EEF1A1, NTRK1, TRAF6, EGFR, PRKDC, SRC, HDAC5, APP, ESR1, AKT1, UBC, and COPS5), and the PDR-HXMMT network has 10 additional genes (RNF2, VNL, RPS27, COPS5, XPO1, PARP1, RACK1, YWHAB, and ITGA4). The top 5 pathways with the highest gene ratio in both networks were the AGE-RAGE signaling pathway in diabetic complications, TNF signaling pathway, relaxin signaling pathway, IL-17 signaling pathway, and focal adhesion. Additional pathways such as neuroactive ligand-receptor interaction, chemokine signaling pathway, and AMPK signaling pathway were enriched with HXMMT targets. Thus, HXMMT has more therapeutic targets shared by different active ingredients and more abundant gene functions than CXC, which may be two major reasons why HXMMT is more strongly recommended than CXC as an auxiliary treatment for new-onset VH secondary to PDR. However, the underlying mechanisms still need to be further explored.
1. Background
Diabetic retinopathy (DR), a serious complication of diabetes mellitus (DM) caused by microvascular ischemia and hypoxemia, affects approximately 35% of DM patients and an estimated > 90 million people worldwide [1, 2]. The prevalence of proliferative diabetic retinopathy (PDR), a vision-threatening type of DR characterized by retinal neovascular and even vitreous hemorrhage (VH), is nearly 7% [3]. PDR dramatically decreases patients' quality of life and contributes to a massive economic burden. Therefore, it is crucial to develop effective pharmaceutical preparations to treat PDR based on its pathological mechanisms.
For thousands of years, traditional Chinese medicines (TCMs) have been used by Chinese people to treat DM and its complications [4]. Compound Xueshuantong capsule (CXC) and Hexuemingmu tablet (HXMMT) are two important Chinese patent medicines (CPMs) that are frequently used to treat PDR, especially when complicated with VH [5, 6]. According to observations in daily clinical practice and the results of some clinical studies, CXC and HXMMT are crucial auxiliary treatments to eliminate VH secondary to PDR as well as to improve retinal hemodynamics in PDR [6]. Correspondingly, previous experimental studies have shown that CXC and HXMMT may exert protective effects on retinal capillary endothelial cells and nerve cells by regulating multiple pathways [7–9]. However, most CMPs are composed of different types of herbs, and every herb is further composed of multiple active ingredients. Thus, a single CPM may target numerous PDR-related molecules, and the pharmacological mechanisms are complex. Moreover, in clinical practice, HXMMT more effectively eliminates new-onset VH secondary to PDR than HXMMT and is more frequently recommended by TCM doctors for new-onset VH treatment, but its potential mechanisms are poorly understood. Thus, further research is needed to better understand the underlying regulatory and interactive mechanisms of different active ingredients in CXC and HXMMT.
Network pharmacology analysis is a convenient and systematic approach to identify core targets shared by drugs and diseases [10]. It can also be performed to identify potential pathways for disease interventions, providing insight into the complex mechanisms of Chinese herbal formulas used to treat diseases. The aim of our study was to find differences in active ingredients between CXC and HXMMT and to comparatively predict and further analyze the molecular targets shared by these drugs and PDR.
2. Materials and Methods
The general procedure was as follows (described in detail in Section 2.1). First, differentially expressed mRNAs (DEmRNAs) between normal retinal tissues in healthy individuals and abnormal retinal membranes in PDR patients were acquired from the database. The chosen DEmRNAs according to certain criteria were defined as PDR targets (described in detail in Section 2.2). Second, active ingredient screening was, respectively, performed in CXC and HXMMT; thus, the CXC and HXMMT targets were obtained (described in detail in Sections 2.3 and 2.4). Third, the intersections between CXC and PDR targets (CXC-PDR targets) as well as between HXMMT and PDR targets (HXMMT-PDR targets) were determined. Afterward, intersections between CXC-PDR targets and HXMMT-PDR targets were identified. We found that all the CXC-PDR targets were completely included in the HXMMT-PDR targets. Finally, the ingredient-target (I-T) networks, protein-protein interaction (PPI) networks, Kyoto Encyclopedia of Genes and Genomes-target (KEGG-T) networks, and Gene Ontology (GO) analyses were performed for CXC-PDR and HXMMT-PDR targets. A flowchart of the procedure is shown in Figure 1, which provides a detailed description of each step.
Figure 1.
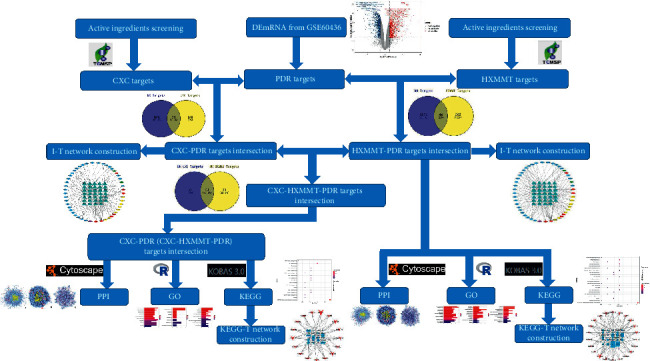
Flowchart of the complete analysis procedure. DEmRNA, differentially expressed messenger RNA; CXC, compound Xueshuantong capsule; PDR, proliferative diabetic retinopathy; HXMMT, Hexuemingmu tablet; I-T, ingredient-target; PPI, protein-protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; KEGG-T, Kyoto Encyclopedia of Genes and Genomes-target.
2.1. Messenger RNA (mRNA) Data Collection and Differential Expression Analysis
The microarray data used in this study were retrieved from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds/). The mRNA expression data were acquired from dataset GSE60436, which contains 3 samples from normal retinal tissues and 3 from active fibrovascular membranes in PDR patients. The raw expression data were first normalized, and analysis of DEmRNAs was then performed using the limma package based on the R language. The criteria for the selection of DEmRNAs were an adjusted P value of <0.05 and a |log2FC| value of >1, and the selected DEmRNAs were defined as PDR targets.
2.2. Active Ingredient Screening
A total of 499 Chinese herbs and 12144 chemical ingredients from the Chinese pharmacopoeia (2010) were registered in the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://tcmspw.com/index.php), a platform that provides pharmacokinetic characteristics and targets of each ingredient in these herbs. Oral bioavailability (OB) and drug-likeness (DL) are two parameters commonly used to screen active ingredients. The OB is the percentage of the orally administered dose of the unchanged drug that enters the systemic blood circulation, and it is an important pharmacokinetic indicator. DL is used to assess whether the ingredients function as known drugs.
Each herb contained in CXC and HXMMT was searched in the TCMSP database, and all ingredients were obtained. According to most traditional Chinese herbs studies [10–12], an ingredient with an OB of ≥30 and a DL of ≥0.18 was considered an active ingredient, and the targets of these ingredients (CXC and HXMMT targets) were retrieved from the database.
2.3. Network Construction
Cytoscape 3.6.1 (http://cytoscape.org/) was used to generate all visual network diagrams, including the I-T, PPI, and KEGG-T networks. The intersections of the formulas' targets (CXC and HXMMT targets) and the PDR targets were determined (CXC-PDR and HXMMT-PDR targets). Accordingly, two I-T networks were constructed. PPI networks were constructed using the Bisogenet 3.0.0 plugin in Cytoscape 3.6.1 based on the following databases: the Database of Interacting Proteins, the Biological General Repository for Interaction Datasets, the Human Protein Reference Database, the IntAct Molecular Interaction Database, the Molecular INTeraction Database and the Binding Database. The CytoNCA plugin was used to perform topological analyses. Degree centrality and betweenness centrality were the measures selected to represent the topological features of each node in the network. Degree centrality represents the number of edges linked by a node, while betweenness centrality represents the proximity of a node to other nodes. KEGG-T networks were constructed after enrichment analyses were performed.
2.4. Functional Enrichment Analysis
GO analyses were performed using the clusterProfiler package based on the R language. The criterion for the selection of GO processes was a P value of <0.05. KEGG pathway analyses were performed using an online biological tool, KEGG Orthology Based Annotation System 3.0 (KOBAS 3.0, http://kobas.cbi.pku.edu.cn). The criteria for the selection of KEGG pathways were a P value of <0.01 and a gene count of ≥3. Visualizations were performed using the ggplot2 R package.
3. Results
3.1. Identification of PDR Targets
Analysis of the microarray dataset GSE60436 showed that 1915 mRNAs (819 upregulated mRNAs and 1096 downregulated mRNAs) were differentially expressed in PDR patients compared with individuals without PDR (Figure 2).
Figure 2.
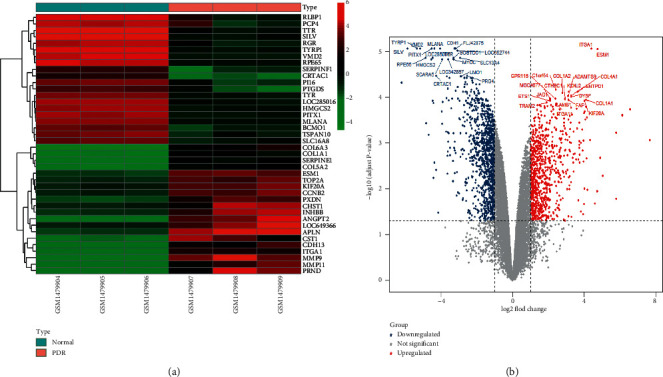
Heatmap (a) and volcano plot (b) of DEmRNAs (PDR targets) in microarray dataset GSE60436. The top 20 downregulated and upregulated DEmRNAs are shown in the heatmap and the volcano plot.
3.2. I-T Network Construction
CXC contains 4 herbs, and HXMMT contains 19 herbs (Table 1). Radix salviae is the only herb common to both CPMs. Through screening the active ingredients, we found 34 potential therapeutic targets in PDR for CXC (Figure 3(a)) and the same 34 and 10 additional therapeutic targets in PDR for HXMMT (Figure 3(b)). The ten additional targets of HXMMT were CYCS, APOD, GOT1, PECAM1, ALDH2, COL1A2, CD300A, PTGER2, CHGA, and CD36. Accordingly, the active ingredients and the potential therapeutic targets were used to construct I-T networks for CXC (Figure 4(a)) and HXMMT (Figure 4(b)). Then, the intersections of the PDR-CXC targets and the PDR-HXMMT targets were determined, and the complete set of potential therapeutic targets of CXC was found to be included among the targets of HXMMT (Figure 3(c)).
Table 1.
| Herbs | |
|---|---|
| Compound Xueshuantong capsule (CXC) | Radix salviae (Danshen), Panax notoginseng (Sanqi), Hedysarum multijugum Maxim. (Huangqi), Figwort root (Xuanshen) |
|
| |
| Hexuemingmu tablet (HXMMT) | Radix salviae (Danshen), Chuanxiong rhizoma (Chuanxiong), Radix paeoniae rubra (Chishao), Gentianae radix et rhizoma (Longdan), Scutellariae radix (Huangqin), Cassiae semen (Juemingzi), Chrysanthemi flos (Juhua), Ecliptae herba (Mohanlian), Equiseti hiemalis herba (Muzei), Pollen typhae (Puhuang), Crataegus pinnatifida Bunge (Shanzha), Prunellae spica (Xiakucao), Rehmanniae radix praeparata (Dihuang), Plantaginis semen (Cheqianzi), Leonuri fructus (Chongweizi), Fructus ligustri lucidi (Nvzhenzi), Curcumae radix (Yujin), Cortex moutan (Mudanpi), Angelicae sinensis radix (Danggui) |
The names in parentheses are the Chinese names of the components.
Figure 3.
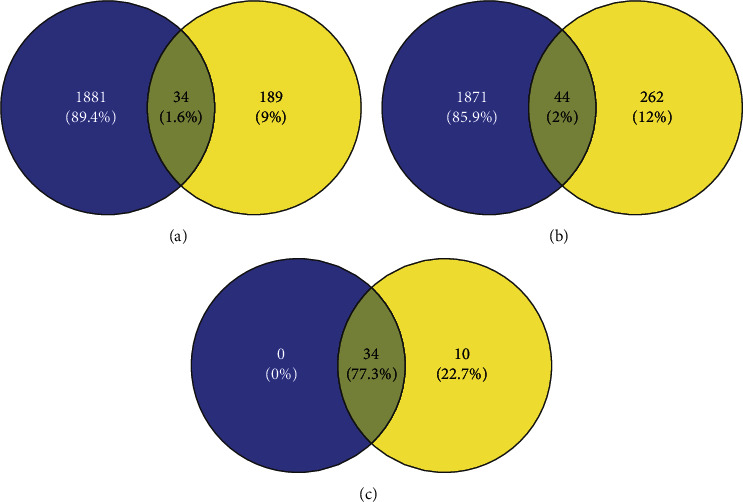
(a) Intersection of compound Xueshuantong capsule (CXC) targets (blue) and proliferative diabetic retinopathy (PDR) targets (yellow). (b) Intersection of Hexuemingmu tablet (HXMMT) targets (blue) and PDR targets (yellow). (c) Intersection of PDR-CXC targets (blue) and PDR-HXMMT targets (yellow).
Figure 4.
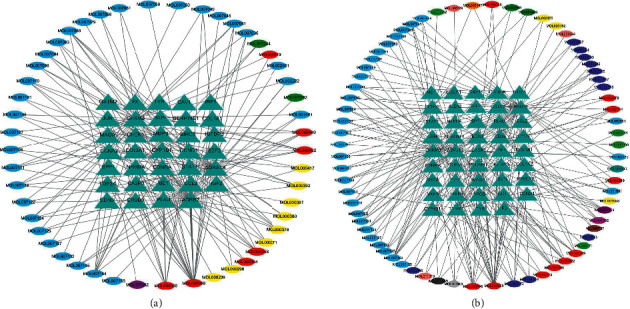
Ingredient-target networks of different Chinese patent medicines. The triangles and circles represent potential therapeutic targets and active ingredients, respectively. (a) Network for the compound Xueshuantong capsule. The blue circles represent active ingredients in Radix salviae, the green circles represent active ingredients in Panax notoginseng, the yellow circles represent active ingredients in Hedysarum multijugum Maxim., the purple circles represent active ingredients in Figwort root, and the red circles represent active ingredients in multiple herbs. (b) Network for the Hexuemingmu tablet. The light blue circles represent active ingredients in Radix salviae, the bluish green circles represent active ingredients in Chuanxiong rhizoma, the light purple circles represent active ingredients in Radix paeoniae rubra, the yellow circles represent active ingredients in Gentianae radix et rhizoma, the dark blue circles represent active ingredients in Scutellariae radix, the light green circles represent active ingredients in Cassiae semen, the pink circles represent active ingredients in Chrysanthemi flos, the dark green circles represent active ingredients in Ecliptae herba, the light gray circles represent active ingredients in Equiseti hiemalis herba, the orange circles represent active ingredients in Fructus ligustri lucidi, the dark purple circles represent active ingredients in Pollen typhae, the dark gray circles represent active ingredients in Crataegus pinnatifida Bunge, the brown circles represent active ingredients in Prunellae spica, and the red circles represent active ingredients in multiple herbs.
A PPI network containing 1825 nodes and 36349 edges was constructed for the set of PDR-CXC targets, while another PPI network containing 2004 nodes and 38863 edges was constructed for the set of PDR-HXMMT targets. The screening parameters were degree centrality and betweenness centrality. The thresholds were set at a degree centrality of ≥61 in the first screen and a betweenness centrality of ≥600 in the second screen. After the first screen, 350 nodes and 10843 edges were included in the PDR-CXC network, while 386 nodes and 11975 edges were included in the PDR-HXMMT network. After the second screen, 39 nodes and 406 edges were included in the PDR-CXC network, while 51 nodes and 628 edges were included in the PDR-HXMMT network. All the key genes in the PDR-CXC network were also included in the PDR-HXMMT network (RELA, HSPA8, HSP90AA, HSP90AB1, BRCA, EWSR1, CUL7, HNRNPU, MYC, CTNNB1, MDM2, YWHAZ, CDK2, AR, FN1, HUWE1, TP53, TUBB, EP300, GRB2, VCP, MCM2, EEF1A1, NTRK1, TRAF6, EGFR, PRKDC, SRC, HDAC5, APP, ESR1, AKT1, UBC, and COPS5). In addition, RNF2, VNL, RPS27, COPS5, XPO1, PARP1, RACK1, YWHAB, and ITGA4 were included only in the PDR-HXMMT network. The topological screening processes of the PPI networks are shown in Figure 5.
Figure 5.
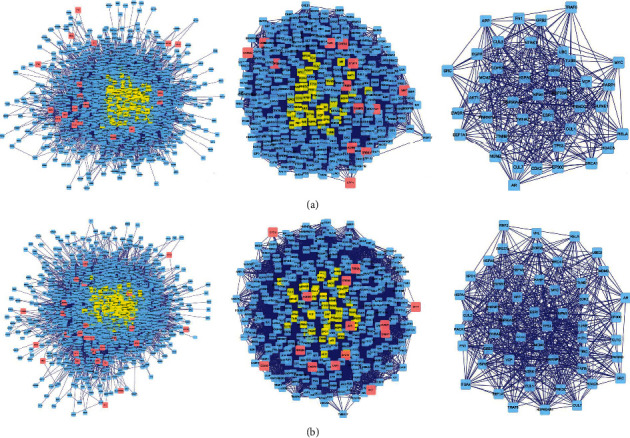
Topological screening process of the protein-protein interaction (PPI) networks. Left: original PPI network; middle: PPI network after the first screen; right: PPI network after the second screen. (a) Screening process of the compound Xueshuantong capsule. (b) Screening process of the Hexuemingmu tablet.
3.3. GO and KEGG Pathway Analyses
The top 20 or all GO processes (when the number of processes was smaller than 20) in the biological process (BP), cellular component (CC), and molecular function (MF) categories were identified (Figure 6). Both CXC and HXMMT may have therapeutic effects on PDR mainly via the following processes in the BP category: response to reactive oxygen species and oxidative stress, regulation of blood vessel diameter and size, vasoconstriction, smooth muscle contraction, hemostasis, and blood coagulation. Additionally, GO terms, such as positive regulation of blood circulation, negative regulation of cell adhesion, extracellular structure organization, and response to lipopolysaccharide, were enriched in HXMMT targets. In the CC category, the therapeutic targets of both CXC and HXMMT were associated mainly with the terms collagen-containing extracellular matrix, fibrillar collagen trimer, and banded collagen fibril. Moreover, HXMMT targets were enriched in the term platelet alpha granule. In addition, CXC and HXMMT targets overlapped in GO MF terms, most of which were related to protein and enzyme binding and regulation.
Figure 6.
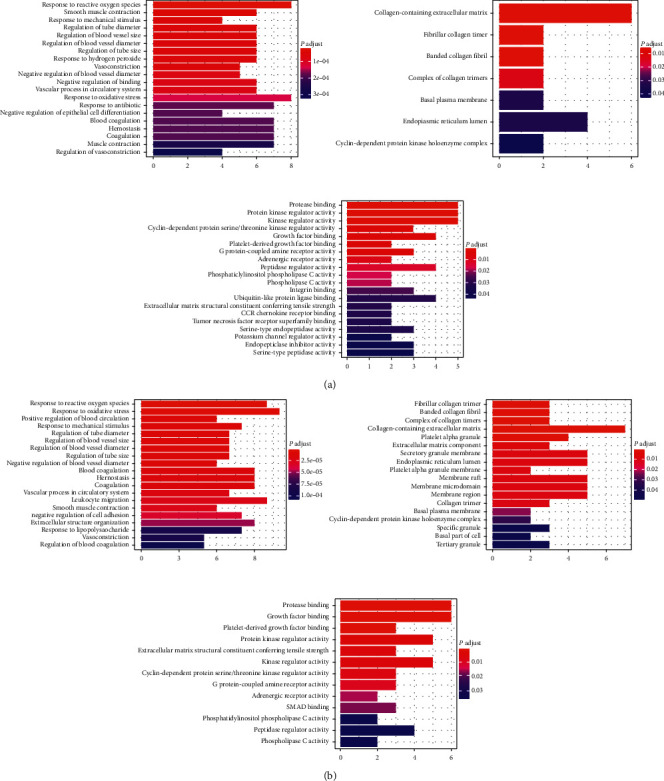
Gene Ontology (GO) analysis results. Left: biological process; middle: cellular component; right: molecular function. (a) GO terms enriched with targets of the compound Xueshuantong capsule. (b) GO terms enriched with targets of the Hexuemingmu tablet.
After KEGG pathway analyses were performed using KOBAS 3.0, possible PDR-related pathways were selected, and visualizations were generated using the ggplot2 R package. All genes and pathways enriched with CXC targets were also enriched with HXMMT targets, and HXMMT had 5 additional target genes: IGFBP3, CYCS, CD36, PTGER2, and COL1A2. The pathway that was the most significantly enriched and had the highest gene ratio in both analyses was the AGE-RAGE signaling pathway in diabetic complications. Other common pathways included the TNF signaling pathway, relaxin signaling pathway, IL-17 signaling pathway, and focal adhesion. Additional pathways, such as neuroactive ligand-receptor interaction, chemokine signaling pathway, and AMPK signaling pathway, were enriched with HXMMT targets. Then, the enriched pathways and their related target genes were used to construct KEGG-T networks for CXC and HXMMT. These results are shown in Figure 7. The most significant pathway and key genes among the PDR treatment targets of CXC and HXMMT are shown in Figure 8.
Figure 7.
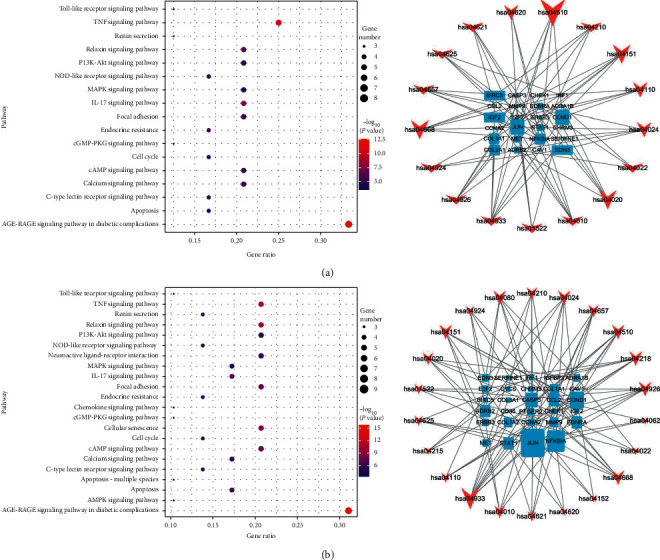
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis results (left) and KEGG-target networks (right). In the right panel, the arrows and squares represent pathways and potential therapeutic targets, respectively. The size of the arrows represents the ratio of the corresponding therapeutic targets, while the size of the therapeutic targets represents the ratio of the corresponding pathways. (a) Pathways enriched with targets of the compound Xueshuantong capsule. (b) Pathways enriched with targets of the Hexuemingmu tablet.
Figure 8.
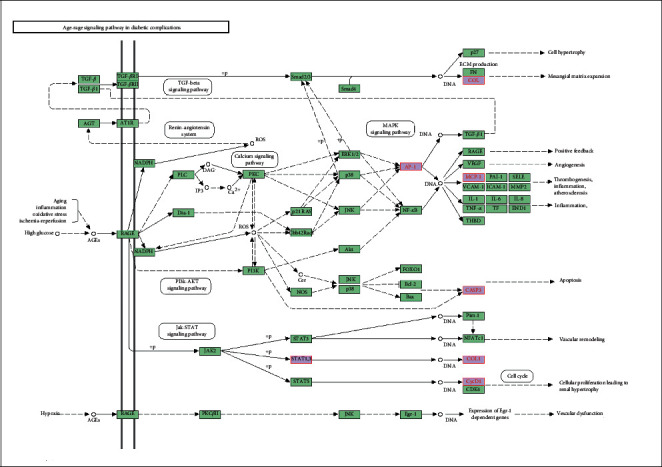
Most significant pathway and key genes (marked in red) among the proliferative diabetic retinopathy treatment targets of the compound Xueshuantong capsule and Hexuemingmu tablet.
4. Discussion
The GO terms enriched with CXC and HXMMT targets were similar and focused mainly on the response to reactive oxygen species and oxidative stress, regulation of blood vessels, and blood coagulation.
Radix salviae is an important component that is closely related to the response to reactive oxygen species and oxidative stress. In an in vitro model of hypoxia and reoxygenation, Hu's [13] study showed that Radix salviae obviously alleviated cardiomyocyte apoptosis and protected mitochondrial function and cell membrane skeleton integrity in H9c2 cells. In addition, Zhang's experiment in rodents [14] revealed that the Danshen (Radix salviae) dripping pill inhibited apoptosis and exerted neuroprotective effects in the retinas of diabetic rats by increasing the expression of Bcl-2, Bcl-2-associated X, and caspase-3 in diabetic rats. Moreover, according to the I-T networks of CXC and HXMMT, Radix salviae contained a greater number of active ingredients related to potential therapeutic targets in PDR compared with the other herbal components, suggesting that Radix salviae may play a crucial role in PDR treatment. A randomized controlled trial (RCT) performed by Lian and colleagues [5] showed that a Radix salviae-containing Chinese herbal product was effective in treating DR and in delaying the progression from non-PDR to PDR by reducing the area of capillary nonperfusion and degree of vascular leakage. Our study identified tanshinone as one of the most important active ingredients of Radix salviae. According to previous studies, tanshinone exerts protective effects on retinal pigment epithelium and retinal endothelial cells [15–17].
Regarding circulatory-related effects, CXC is considered to be an effective complementary medicine to treat ischemic vascular diseases, such as cerebral infarction and cardiovascular diseases [18, 19]. Lyu's study [18] showed that CXC combined with conventional treatments had better clinical effects than conventional treatments alone. Moreover, a significant reduction in the IL-6 and hs-CRP levels was noticed when CXC was combined with conventional treatments. Regarding CXC and DR, some studies [20, 21] have shown that CXC contributes to the attenuation of streptozotocin- (STZ-) induced retinal lesions, including the amelioration of increases in erythrocyte aggregation, plasma viscosity, and acellular vessel and pericyte loss, by reversing the hyperexpression of vascular endothelial growth factor (VEGF) and intercellular adhesion molecule-1 (ICAM-1) and endothelin-1 (ET-1) and the hypoexpression of pigment epithelium-derived factor (PEDF) and occludin in the retinas of STZ-induced rats. In addition, Liu's study [22] showed that different core bioactive ingredients in CXC had novel therapeutic uses in managing blood circulation. Panaxytriol and ginsenoside Rb1 were related to red blood cell aggregation, while angoroside C was involved in platelet aggregation. Protocatechualdehyde was related to intrinsic clotting activity, while calycosin-7-O-beta-D-glucoside was related to extrinsic clotting activity. In Sun et al.'s study [23], the systolic and diastolic velocity decreased while the resistance and pulsatility index increased in diabetic rat retinas. Furthermore, they also proved that the protective effects of DR were mediated by coagulation cascades and the peroxisome proliferator-activated receptor (PPAR) signaling pathway. Xing et al.'s study [6] showed that CXC mainly affected blood vessels by protecting high glucose-injured retinal vascular endothelial cells via YAP-mediated effects. However, the effect of HXMMT has seldom been evaluated in DR. Indeed, the only study was conducted by Long et al. [7] in rat models of branch retinal vein occlusion (BRVO), which indicated that HXMMT may alleviate retinal edema by regulating the expression of VEGF-α and improving microcirculation. Further studies should be performed to clarify the mechanism of HXMMT.
Utilizing a network pharmacology approach, Piao et al. [11] found that MMP9 and IGF-1 (an IGF family member contained in Radix salviae) may be key therapeutic targets in DR. Consistent with Piao's result, we found that MMP9 was included in both the CXC and HXMMT I-T networks. Matrix metalloproteinases (MMPs) play an important role in the migration, differentiation, and proliferation of cells [24]. Hyperglycemia may increase the activity of MMP9 and, therefore, provides growth space and nutrients for neovascularization by degrading the basement membrane and relaxing the cell structure [25]. A previous study [26] suggested that MMP9 was upregulated in the DM heart and that knockout of MMP9 in the DM was cardioprotective. Activation of MMPs (MMP-2 and MMP-9) in the retina is an early event in DR. Therefore, activated MMPs increased retinal capillary cell apoptosis and mitochondrial damage [27]. In addition, IGF-2, another IGF family member, was included in our networks but is not targeted by Radix salviae. IGF is expressed in many tissues, including the retina, where it is found in cells such as retinal endothelial cells and retinal pigment epithelial cells. IGF is a crucial regulator of cell differentiation and is closely related to blood-retinal barrier breakdown and retinal neovascularization [28, 29]. However, IGF-2 was related to 2 herbal components of CXC (Huangqi and Sanqi) and 9 herbal components of HXMMT (Cheqianzi, Chishao, Huangqin, Mohanlian, Mudanpi, Muzei, Nvzhenzi, Puhuang, and Xiakucao), implying that different active ingredients may share common therapeutic targets. Combined and stronger therapeutic effects may be exerted on a therapeutic target shared by a greater number of active ingredients. HXMMT contains more components than CXC; therefore, HXMMT may have more therapeutic targets. In addition, many of the genes targeted only by HXMMT but not by CXC were related to circulation and blood coagulation. For instance, CYCS was shown to be involved in blood platelet formation and regulatory processes [30, 31]. APOD, a crucial component of lipoproteins that transports lipids and stabilizes the structure of lipoproteins, was found to also be closely related to angiogenesis, a critical pathophysiological process in PDR [32]. PECAM1 was suggested to play an important role in the maintenance of human vascular endothelial barrier integrity and function [33]. Similarly, our topological analysis showed that some genes targeted only by HXMMT had many other functions. For example, YWHAB may perform specific functions in rod photoreceptors [34]. RACK1 may promote the expression of VEGF in endothelial cells and subsequently facilitate angiogenesis [35]. PARP1, activated by reactive oxygen species, was proven to be involved in inflammation, cell death, and retinal disease progression [36, 37]. In summary, its stronger effects at a given dose and more numerous gene targets may be two major reasons why HXMMT is more strongly recommended than CXC by TCM doctors for treating fresh VH secondary to PDR.
Similar to Li et al.'s research [38], the AGE-RAGE signaling pathway and TNF signal pathway were enriched in CXC in our study. HXMMT and CXC shared many pathways in our study, and the AGE-RAGE signaling pathway in diabetic complications was the most significantly enriched pathway. RAGE is expressed in almost all retinal cells. Retinal Müller cells, the major glial cells in the retina, play a critical role in maintaining the structure and normal functions of the retina, and these cells express high levels of RAGE [39]. In addition, Zong et al.'s study [40] demonstrated that RAGE plays an essential role in retinal neurodegeneration induced by diabetes and that early induction of RAGE expression by hyperglycemia in retinal Müller cells contributes to the increased levels of proinflammatory cytokines, including VEGF (a crucial downstream growth factor in angiogenesis) and monocyte chemoattractant protein-1 (MCP-1), both in vivo and in vitro. Moreover, Hirata et al. [41] found that increased production of VEGF secondary to retinal Müller cell activation may account for neovascularization in PDR. Therefore, the AGE-RAGE signaling pathway may not only provide neuroprotection in DR but also participate in crosstalk between neuroprotection and vascular protection. The difference in the enriched genes between CXC and HXMMT was that one additional gene (COL1A2) was included among the HXMMT targets. COL1A2 has seldom been studied in DR; Zou et al.'s research [42] is the only DR study involving COL1A2 to date. Zou revealed that silencing of circular RNA COL1A2 (circCOL1A2) suppresses angiogenesis during PDR progression by regulating the miR-29b/VEGF axis, suggesting that circCOL1A2 and its related genes may be therapeutic targets in DR.
According to the pathway map, the AGE-RAGE signaling pathway in diabetic complications is also closely associated with the PI3K-Akt signaling pathway and VEGF. The PI3K-Akt signaling pathway is one of the most frequently studied pathways in DR [43–45]. The proliferation, migration, and invasion of retinal vascular endothelial cells, retinal pericytes, retinal pigment epithelial cells, and microglial cells can be regulated through this pathway [46–49]. A series of pathophysiological processes, including oxidative stress regulation, inflammatory response regulation, angiogenesis, and neuroprotective regulation, are also involved. Another common and well-known pathway, the TNF signaling pathway, is closely related to inflammation, which is a crucial process in DR progression [50, 51]. Gao's study [51] revealed that hypoxia inducible factor subtype 1α in diabetic retina is likely to play a role in dysfunction and vulnerability related to DR progression via TNF-α.
The genes and pathways mentioned above and whether CXC/HXMMT regulates their functions are summarized in Table 2.
Table 2.
Genes/pathways mentioned in the discussion and whether CXC/HXMMT regulates their functions.
| Genes/pathways | Role in PDR | CXC regulates? | HXMMT regulates? |
|---|---|---|---|
| Bcl-2, Bcl-2-associated X, and caspase-3 | Apoptosis and neuroprotective effects | Yes [14] | Unknown |
| VEGF, ICAM-1, ET-1, PEDF, and occludin | Erythrocyte aggregation, plasma viscosity, acellular vessel, and pericyte loss | Yes [20, 21] | Unknown |
| PPAR signaling pathway | Protective effects | Unknown | Unknown |
| YAP | Protecting retinal vascular endothelial cells | Yes [6] | Unknown |
| MMP9 and IGF-1 | Regulating retinal capillary cell apoptosis/neovascularization | Unknown | Unknown |
| APOD | Neovascularization | Unknown | Unknown |
| PECAM1 | Maintenance of human vascular endothelial barrier integrity | Unknown | Unknown |
| YWHAB | Performing specific functions in rod photoreceptors | Unknown | Unknown |
| RACK1 | Neovascularization | Unknown | Unknown |
| PARP1 | Inflammation, cell death, and retinal disease progression | Unknown | Unknown |
| AGE-RAGE signaling pathway | Neovascularization/neuroprotection | Unknown | Unknown |
| Circular RNA COL1A2/miR-29b/VEGF | Neovascularization | Unknown | Unknown |
| VEGF/PI3K-Akt signaling pathway | Neovascularization | Unknown | Unknown |
| TNF signaling pathway | Inflammation | Unknown | Unknown |
PDR, proliferative diabetic retinopathy; CXC, compound Xueshuantong capsule; HXMMT, Hexuemingmu tablet.
5. Conclusions
Both CXC and HXMMT include components effective in treating PDR and affect the following main processes: response to reactive oxygen species and oxidative stress, regulation of blood vessels, and blood coagulation. Radix salviae, the only herb common to both CPMs, contains many useful active ingredients. The PDR-CXC and PDR-HXMMT networks shared 34 common genes (RELA, HSPA8, HSP90AA, HSP90AB1, BRCA, EWSR1, CUL7, HNRNPU, MYC, CTNNB1, MDM2, YWHAZ, CDK2, AR, FN1, HUWE1, TP53, TUBB, EP300, GRB2, VCP, MCM2, EEF1A1, NTRK1, TRAF6, EGFR, PRKDC, SRC, HDAC5, APP, ESR1, AKT1, UBC, and COPS5), and the PDR-HXMMT network has 10 additional genes (RNF2, VNL, RPS27, COPS5, XPO1, PARP1, RACK1, YWHAB, and ITGA4). The top 5 pathways with the highest gene ratio in both networks were the AGE-RAGE signaling pathway in diabetic complications, TNF signaling pathway, relaxin signaling pathway, IL-17 signaling pathway, and focal adhesion. Additional pathways, such as neuroactive ligand-receptor interaction, chemokine signaling pathway, and AMPK signaling pathway, were enriched with HXMMT targets. Thus, HXMMT has more therapeutic targets shared by different active ingredients and more abundant gene functions than CXC, which may be two major reasons why HXMMT is more strongly recommended than CXC as an auxiliary treatment for new-onset VH secondary to PDR. However, the underlying mechanisms need to be further elucidated.
Acknowledgments
This research was supported by the Natural Science Foundation of Ningbo, China (2015A610311), Ningbo Public Welfare Program (2019C50045), and Clinical Pharmacology Research Foundation of Guangdong Province (2019XQ09).
Abbreviations
- CXC:
Compound Xueshuantong capsule
- HXMMT:
Hexuemingmu tablet
- CPM:
Chinese patent medicines
- PDR:
Proliferative diabetic retinopathy
- VH:
Vitreous hemorrhage
- GO:
Gene ontology
- KEGG:
Kyoto encyclopedia of genes and genomes
- DR:
Diabetic retinopathy
- DM:
Diabetes mellitus
- mRNA:
Messenger RNA
- DEmRNA:
Differentially expressed messenger RNA
- I-T:
Ingredient-target
- PPI:
Protein-protein interaction
- KEGG-T:
Kyoto encyclopedia of genes and genomes-target
- GEO:
Gene expression omnibus
- TCMSP:
Traditional Chinese medicine system pharmacology
- OB:
Oral bioavailability
- DL:
Drug-likeness
- KOBAS:
KEGG orthology based annotation system
- BP:
Biological process
- CC:
Cellular component
- MF:
Molecular function
- STZ:
Streptozotocin
- VEGF:
Vascular endothelial growth factor
- MCP-1:
Monocyte chemoattractant protein-1.
Contributor Information
Hongyan Yao, Email: sowenyao@hotmail.com.
Zijing Li, Email: lizj29@mail.sysu.edu.cn.
Data Availability
No data were used to support this study.
Disclosure
An earlier version of this manuscript has been presented as a preprint following this link: https://www.researchsquare.com/article/rs-55929/v1.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Hongyan Yao and Zijing Li drafted the manuscript and analyzed the data. Danli Xin was responsible for data collection and image editing. Zongyi Zhan collected the data. Zijing Li was responsible for manuscript design.
References
- 1.Suresh R., Yu H., Thoveson A., et al. Loss to follow-up among patients with proliferative diabetic retinopathy in clinical practice. American Journal of Ophthalmology. 2020;215:66–71. doi: 10.1016/j.ajo.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Vujosevic S., Aldington S. J., Silva P., et al. Screening for diabetic retinopathy: new perspectives and challenges. The Lancet Diabetes & Endocrinology. 2020;8(4):337–347. doi: 10.1016/s2213-8587(19)30411-5. [DOI] [PubMed] [Google Scholar]
- 3.Sabanayagam C., Banu R., Chee M. L., et al. Incidence and progression of diabetic retinopathy: a systematic review. The Lancet Diabetes & Endocrinology. 2019;7(2):140–149. doi: 10.1016/s2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 4.Ai X., Yu P., Hou Y., et al. A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomedicine & Pharmacotherapy. 2020;132 doi: 10.1016/j.biopha.2020.110852. [DOI] [PubMed] [Google Scholar]
- 5.Lian F., Wu L., Tian J., et al. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: a randomized, double-blind, placebo-controlled multicenter clinical trial. Journal of Ethnopharmacology. 2015;164:71–77. doi: 10.1016/j.jep.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Xing W., Song Y., Li H., et al. Fufang Xueshuantong protects retinal vascular endothelial cells from high glucose by targeting YAP. Biomedicine & Pharmacotherapy. 2019;120 doi: 10.1016/j.biopha.2019.109470. [DOI] [PubMed] [Google Scholar]
- 7.Long P., Yan W., Liu J., et al. Therapeutic effect of traditional Chinese medicine on a rat model of branch retinal vein occlusion. Journal of Ophthalmology. 2019;2019:13. doi: 10.1155/2019/9521379.9521379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R. L., Wang J. X., Chai L. J., et al. Xueshuantong for injection (lyophilized) alleviates streptozotocin-induced diabetic retinopathy in rats. Chinese Journal of Integrative Medicine. 2020;26(11):825–832. doi: 10.1007/s11655-020-3088-5. [DOI] [PubMed] [Google Scholar]
- 9.An X., Jin D., Duan L., et al. Direct and indirect therapeutic effect of traditional Chinese medicine as an add-on for non-proliferative diabetic retinopathy: a systematic review and meta-analysis. Chinese Medicine. 2020;15:p. 99. doi: 10.1186/s13020-020-00380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L., Li Z., Wang Y., Zhang B., Liu G., Liu J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian decoction in rats with type 2 diabetes mellitus. Journal of Ethnopharmacology. 2020;258 doi: 10.1016/j.jep.2020.112842.112842 [DOI] [PubMed] [Google Scholar]
- 11.Piao C. L., Luo J. L., Jin G., et al. Utilizing network pharmacology to explore the underlying mechanism of Radix Salviae in diabetic retinopathy. Chinese Medicine. 2019;14:p. 58. doi: 10.1186/s13020-019-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piao C., Sun Z., Jin D., et al. Network pharmacology-based investigation of the underlying mechanism of Panax notoginseng treatment of diabetic retinopathy. Combinatorial Chemistry & High Throughput Screening. 2020;23(4):334–344. doi: 10.2174/1386207323666200305093709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu F., Koon C. M., Chan J. Y., Lau K. M., Fung K. P. The cardioprotective effect of Danshen and Gegen decoction on rat hearts and cardiomyocytes with post-ischemia reperfusion injury. BMC Complementary Medicine and Therapies. 2012;12:p. 249. doi: 10.1186/1472-6882-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q., Xiao X., Zheng J., et al. Compound danshen dripping pill inhibits retina cell apoptosis in diabetic rats. Frontiers in Physiology. 2018;9:p. 1501. doi: 10.3389/fphys.2018.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z.-L., Li Y., Liu X.-W., et al. Sodium tanshinone IIA silate alleviates high glucose induced barrier impairment of human retinal pigment epithelium through the reduction of NF-κB activation via the AMPK/p300 pathway. Current Eye Research. 2020;45(2):177–183. doi: 10.1080/02713683.2019.1668419. [DOI] [PubMed] [Google Scholar]
- 16.Qian S., Qian Y., Huo D., Wang S., Qian Q. Tanshinone IIa protects retinal endothelial cells against mitochondrial fission induced by methylglyoxal through glyoxalase 1. European Journal of Pharmacology. 2019;857 doi: 10.1016/j.ejphar.2019.172419. [DOI] [PubMed] [Google Scholar]
- 17.Fan K., Li S., Liu G., Yuan H., Ma L., Lu P. Tanshinone IIA inhibits high glucose-induced proliferation, migration and vascularization of human retinal endothelial cells. Molecular Medicine Reports. 2017;16(6):9023–9028. doi: 10.3892/mmr.2017.7743. [DOI] [PubMed] [Google Scholar]
- 18.Lyu J., Xie Y., Sun M., Zhang L. Efficacy and safety of xueshuantong injection on acute cerebral infarction: clinical evidence and GRADE Assessment. Frontiers in Pharmacology. 2020;11:p. 822. doi: 10.3389/fphar.2020.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S., Chen Y., Wang J., et al. Anti-thrombosis effects and mechanisms by xueshuantong capsule under different flow conditions. Frontiers in Pharmacology. 2019;10:p. 35. doi: 10.3389/fphar.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jian W., Yu S., Tang M., Duan H., Huang J. A combination of the main constituents of Fufang Xueshuantong capsules shows protective effects against streptozotocin-induced retinal lesions in rats. Journal of Ethnopharmacology. 2016;182:50–56. doi: 10.1016/j.jep.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Duan H., Huang J., Li W., Tang M. Protective effects of Fufang Xueshuantong on diabetic retinopathy in rats. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/408268.408268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H., Liang J. P., Li P. B., et al. Core bioactive components promoting blood circulation in the traditional Chinese medicine compound xueshuantong capsule (CXC) based on the relevance analysis between chemical HPLC fingerprint and in vivo biological effects. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112675.e112675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H.-H., Chai X.-L., Li H.-L., et al. Fufang Xueshuantong alleviates diabetic retinopathy by activating the PPAR signalling pathway and complement and coagulation cascades. Journal of Ethnopharmacology. 2021;265 doi: 10.1016/j.jep.2020.113324. [DOI] [PubMed] [Google Scholar]
- 24.Yadav S. K., Kambis T. N., Kar S., Park S. Y., Mishra P. K. MMP9 mediates acute hyperglycemia-induced human cardiac stem cell death by upregulating apoptosis and pyroptosis in vitro. Cell Death & Disease. 2020;11(3):p. 186. doi: 10.1038/s41419-020-2367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herszenyi L., Hritz I., Pregun I., et al. Alterations of glutathione S-transferase and matrix metalloproteinase-9 expressions are early events in esophageal carcinogenesis. World Journal of Gastroenterology. 2007;13(5):676–682. doi: 10.3748/wjg.v13.i5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prathipati P., Metreveli N., Nandi S. S., Tyagi S. C., Mishra P. K. Ablation of matrix metalloproteinase-9 prevents cardiomyocytes contractile dysfunction in diabetics. Frontiers in Physiology. 2016;7:p. 93. doi: 10.3389/fphys.2016.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowluru R. A., Mishra M. Regulation of matrix metalloproteinase in the pathogenesis of diabetic retinopathy. Progress in Molecular Biology and Translational Science. 2017;148:67–85. doi: 10.1016/bs.pmbts.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Ismayilnajadteymurabadi H., Konukoglu D. The relationship between IGF-2, IGFBP-2, and IGFBP-3 levels in patients suffering from pre-diabetes. Journal of Biological Regulators and Homeostatic Agents. 2018;32(1):63–68. [PubMed] [Google Scholar]
- 29.Clemmons D. R., Sleevi M., Allan G., Sommer A. Effects of combined recombinant insulin-like growth factor (IGF)-I and IGF binding protein-3 in type 2 diabetic patients on glycemic control and distribution of IGF-I and IGF-II among serum binding protein complexes. The Journal of Clinical Endocrinology & Metabolism. 2007;92(7):2652–2658. doi: 10.1210/jc.2006-2699. [DOI] [PubMed] [Google Scholar]
- 30.Ong L., Morison I. M., Ledgerwood E. C. Megakaryocytes from CYCS mutation-associated thrombocytopenia release platelets by both proplatelet-dependent and -independent processes. British Journal of Haematology. 2017;176(2):268–279. doi: 10.1111/bjh.14421. [DOI] [PubMed] [Google Scholar]
- 31.Ledgerwood E. C., Dunstan-Harrison C., Ong L., Morison I. M. CYCS gene variants associated with thrombocytopenia. Platelets. 2019;30(5):672–674. doi: 10.1080/09537104.2018.1543866. [DOI] [PubMed] [Google Scholar]
- 32.Lai C. J., Cheng H. C., Lin C. Y., et al. Activation of liver X receptor suppresses angiogenesis via induction of ApoD. The FASEB Journal. 2017;31(12):5568–5576. doi: 10.1096/fj.201700374r. [DOI] [PubMed] [Google Scholar]
- 33.Ren Q., Ren L., Ren C., Liu X., Dong C., Zhang X. Platelet endothelial cell adhesion molecule-1 (PECAM1) plays a critical role in the maintenance of human vascular endothelial barrier function. Cell Biochemistry and Function. 2015;33(8):560–565. doi: 10.1002/cbf.3155. [DOI] [PubMed] [Google Scholar]
- 34.Inamdar S. M., Lankford C. K., Laird J. G., et al. Analysis of 14-3-3 isoforms expressed in photoreceptors. Experimental Eye Research. 2018;170:108–116. doi: 10.1016/j.exer.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X., Zhu M., Yang X., et al. Inhibition of RACK1 ameliorates choroidal neovascularization formation in vitro and in vivo. Experimental and Molecular Pathology. 2016;100(3):451–459. doi: 10.1016/j.yexmp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Liu S.-Y., Song J.-Y., Fan B., et al. Resveratrol protects photoreceptors by blocking caspase- and PARP-dependent cell death pathways. Free Radical Biology and Medicine. 2018;129:569–581. doi: 10.1016/j.freeradbiomed.2018.10.431. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs K., Vaczy A., Fekete K., et al. PARP inhibitor protects against chronic hypoxia/reoxygenation-induced retinal injury by regulation of MAPKs, HIF1α, Nrf2, and NFκB. Investigative Opthalmology & Visual Science. 2019;60(5):1478–1490. doi: 10.1167/iovs.18-25936. [DOI] [PubMed] [Google Scholar]
- 38.Li H., Li B., Zheng Y. Exploring the mechanism of action compound-Xueshuantong capsule in diabetic retinopathy treatment based on network pharmacology. Evidence-Based Complementary and Alternative Medicine. 2020;2020:12. doi: 10.1155/2020/8467046.8467046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fletcher E., Phipps J., Ward M., Puthussery T., Wilkinson-Berka J. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Current Pharmaceutical Design. 2007;13(26):2699–2712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 40.Zong H., Ward M., Madden A., et al. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE) Diabetologia. 2010;53(12):2656–2666. doi: 10.1007/s00125-010-1900-z. [DOI] [PubMed] [Google Scholar]
- 41.Hirata C., Nakano K., Nakamura N., et al. Advanced glycation end products induce expression of vascular endothelial growth factor by retinal müller cells. Biochemical and Biophysical Research Communications. 1997;236(3):712–715. doi: 10.1006/bbrc.1997.7036. [DOI] [PubMed] [Google Scholar]
- 42.Zou J., Liu K.-C., Wang W.-P., Xu Y. Circular RNA COL1A2 promotes angiogenesis via regulating miR-29b/VEGF axis in diabetic retinopathy. Life Sciences. 2020;256 doi: 10.1016/j.lfs.2020.117888. [DOI] [PubMed] [Google Scholar]
- 43.Zong H., Ward M., Stitt A. W. AGEs, RAGE, and diabetic retinopathy. Current Diabetes Reports. 2011;11(4):244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 44.Giurdanella G., Lazzara F., Caporarello N., et al. Sulodexide prevents activation of the PLA2/COX-2/VEGF inflammatory pathway in human retinal endothelial cells by blocking the effect of AGE/RAGE. Biochemical Pharmacology. 2017;142:145–154. doi: 10.1016/j.bcp.2017.06.130. [DOI] [PubMed] [Google Scholar]
- 45.Chen L., Cui Y., Li B., et al. Advanced glycation end products induce immature angiogenesis in in vivo and ex vivo mouse models. American Journal of Physiology-Heart and Circulatory Physiology. 2020;318(3):H519–h533. doi: 10.1152/ajpheart.00473.2019. [DOI] [PubMed] [Google Scholar]
- 46.Zhou L., Zhang S., Zhang L., Li F., Sun H., Feng J. MiR-199a-3p inhibits the proliferation, migration, and invasion of endothelial cells and retinal pericytes of diabetic retinopathy rats through regulating FGF7 via EGFR/PI3K/AKT pathway. Journal of Receptor and Signal Transduction Research. 2020:1–13. doi: 10.1080/10799893.2020.1783556. [DOI] [PubMed] [Google Scholar]
- 47.Yang X., Huo F., Liu B., et al. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/AKT signaling pathway. Journal of Molecular Neuroscience. 2017;61(4):581–589. doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 48.Ran Z., Zhang Y., Wen X., Ma J. Curcumin inhibits high glucose-induced inflammatory injury in human retinal pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling pathway. Molecular Medicine Reports. 2019;19(2):1024–1031. doi: 10.3892/mmr.2018.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J.-M., Zhang Z.-Z., Ma X., Fang S.-F., Qin X.-H. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Experimental Eye Research. 2020;190 doi: 10.1016/j.exer.2019.107886. [DOI] [PubMed] [Google Scholar]
- 50.Capitão M., Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. Journal of Cellular Biochemistry. 2016;117(11):2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 51.Gao X., Li Y., Wang H., Li C., Ding J. Inhibition of HIF-1α decreases expression of pro-inflammatory IL-6 and TNF-α in diabetic retinopathy. Acta Ophthalmologica. 2017;95(8):e746–e750. doi: 10.1111/aos.13096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


