Abstract
Chemotherapeutics can induce oxidative stress, inflammation, apoptosis, mitochondrial dysfunction, and abnormalities in neurotransmitter metabolism leading to toxicity. Because there have been no therapeutic strategies developed to target inflammation and oxidative stress, there is a continuing need for new and improved therapy. As a result, there has been increasing interest in complementary and alternative medicine with anticancer potential. Studies have shown that the antioxidant activities and anti-inflammatory effects of citrus fruits are promising natural phytochemicals in the development of new anticancer agents. Tangeretin is a naturally polymethoxylated flavone compound extracted from the citrus peel that has shown significant intestinal absorption and adequate bioavailability, with the added benefit of promoting longevity. In addition, tangeretin is known to exhibit considerable selective toxicity to many types of cancer cell proliferation such as ovarian, brain, blood, and skin cancer. Evidence indicates that tangeretin acts through several mechanisms including growth inhibition, induction of apoptosis, autophagy, antiangiogenesis, and estrogenic-like effects. Furthermore, tangeretin works through mitigating levels of inflammatory mediators in the immune system. Using tangeretin in combination with clinically applied anticancer drugs could be a good strategy for increasing the efficiency of these agents and protecting noncancerous cells from damage caused by chemotherapy. The purpose of this review is to highlight the protective effects of a novel natural product, tangeretin against chemotherapeutic-induced toxicity. The development of chemoprevention strategies can lead to significant health care improvement in cancer survivors. Thus, study outcomes may attract more investigators to conduct tangeretin-related research and find out potentially significant impacts on health care of cancer patients and decreased health problems associated with chemotherapeutics-induced toxicity.
1. Introduction
Cancer is the second-leading cause of death globally. More than 14,000,000 new cases are expected and more than 8,000,000 deaths per year, which is translated as about 13% of deaths worldwide [1]. Cancer chemoprevention is pharmacological intervention from a natural, synthetic, biological, or chemical origin that blocks or reverses carcinogenesis. Many forms of cancer are hardly eradicated through cancer chemotherapies, which unfortunately have serious toxicity [2, 3] and unavoidable consequences associated with cancer therapy [4]. Chemoprevention approaches have generated much expectation and interest as adjuvants as have the chemotherapeutic agents in cancer therapy.
Flavonoids are naturally occurring polyphenols that have shown clearly their selective toxicity to cancer cells. Polyphenols inhibit carcinogen-activating enzymes and have various antioxidant properties [5]. Fruits, vegetables, grains, and traditional medicinal herbs are an abundant source of flavonoids [6,7]. Several epidemiologic studies suggested a protective role of flavonoids on certain cancer types, for instance, lung, breast, colon, and prostate [8, 9]. Citrus fruits are an example of chemopreventive and cochemotherapeutic agents containing flavonoids that are associated with cancer treatment [10]. Tangeretin (4′, 5, 6, 7, 8-pentamethoxyflavone) is a natural polymethoxyflavone (PMF) compound, extracted from citrus peel [11] with more than one mechanism of anticancer activity [12]. In the present review, we postulate, from the current evidence on tangeretin use, its potential use as an agent for cancer prevention and/or chemoprevention.
2. Biological Functions of Tangeretin
Several studies have shown that tangeretin has significant health-enhancing effects. Tangereting has been reported to reduce blood sugar [13–15], inhibit inflammation [16–22], and oxidation [16, 19]. In addition, it acted as an antiviral [23], prevented heart diseases [24], hepatitis [17, 25], kidney dysfunction [19], and hyperlipidemia [26]. Moreover, the capacity for tangeretin is remarkable in some neurological disorders, including Alzheimer's disease [27], epilepsy, and Parkinson's disease [28]. Tangeretin has further been reported to have an anticancer effect in traditional Chinese medicine [29]. Tangeretin has been observed to inhibit the development and progression of various kinds of cancer cells [29–33], as discussed below: the mechanism of tangeretin is summarized in Figure 1.
Figure 1.
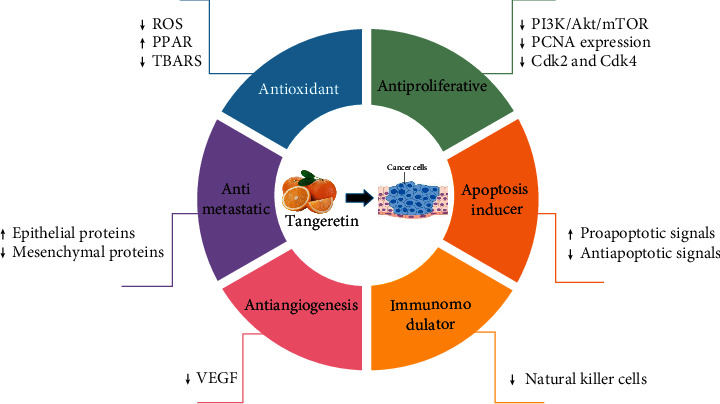
Mechanisms of the anticancer activity of tangeretin. Cdk2, cyclin-dependent kinases 2; Cdk4, cyclin-dependent kinases 4; PCNA, proliferating cell nuclear antigen; PI3K/Akt/mTOR, phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; TBARS, thiobarbituric acid reactive substances; VEGF, vascular endothelial growth factor.
2.1. Potential Role of Tangeretin as an Antioxidant
Oxidative stress plays a critical role in cancer growth and progression. Evidence indicates that, in the initial phase of cancer, oxidative stress is prevalent [34]. Free radicals generated are known to damage lipids, proteins, and DNA in patients receiving chemotherapeutics. Superoxide dismutase, a key antioxidant enzyme is a major target of oxidative damage. Superoxide dismutase is oxidatively modified by carbonylation. The cysteine residue of the superoxide dismutase is oxidized to cysteic acid. Several lines of evidence implicate lipid peroxidation in the progression of cancer. 4-Hydroxy-2-trans-nonenal (HNE), an aldehydic product of membrane lipid peroxidation, is increased due to oxidative stress. Increased DNA oxidation, especially 8-hydroxy-2′-deoxyguanosine (8-OHdG), is present in the tumor due to oxidative stress. Glutathione (reduced form) levels were depleted in cancer and similarly in the breast cancer model [35]. A tangeretin antioxidant effect was found to balance the defense system. Over accumulation of reactive oxygen species result in oxidative stress, resulting in DNA, RNA, and protein oxidation, as well as lipid peroxidation by indirectly promoting cellular glutathione in renal tissues [36] and HepG2 cancer cells [37]. Since the exact cause of oxidative stress pathophysiology remains controversial, several therapeutic strategies have been tried to prevent or slow progression.
2.2. Suppression of Carcinogenesis by Tangeretin
Carcinogenesis is a complicated procedure, including many DNA and non-DNA modifications which ultimately promote the conversion of a normal cell into a cancerous one [38]. The antimutagenic effect of tangeretin on different mutagens such as 2-aminofluorene, benzo[a]pyrene, and nitroquinoline N-oxide was reported using a salmonella/microsome assay. [39]. The antimutagenic effect of tangeretin was further confirmed using the Ames test [40]. Additionally, tangeretin was reported to prevent induced unscheduled DNA synthesis in rat hepatic slices [41]. In vivo studies showed the capability of tangeretin to protect against 7, 12-dimethylbenz[a]anthracene (DMBA) induced breast cancer in rats [33, 36].
2.3. Effect of Tangeretin on Cell Cycle Regulation
The cell cycle is the process by which cells grow and divide. Regulatory proteins control the cell cycle by either tumor suppression of cell growth or death of damaged cells. Cyclin-dependent kinases (CDK) cyclin complexes are the cell cycle protein machinery controlling cell proliferation under specific stimuli. Cancer growth has been associated with defects in CDK as evidence by an in vitro study on COLO 205 human colon cancer. In this study, administration of tangeretin was able to block (G1 phase) by activating the expression of CDK inhibitors p27 and p21 [30]. In another study supporting the anticancer effect of tangeretin on breast cancer cell line (MCF-7), inhibition of cell proliferation was shown to arrest the cell at the G1 phase [42].
2.4. Effect on Apoptosis
Cell death, particularly apoptosis, is critical for balanced cell death and growth to maintain body functions [43]. Cancer causes a defect to occur in any point in apoptotic pathways resulting in malignant cells that will not perish [43]. One example is the reduced expression of p53, a tumor suppressor gene, which alters apoptosis and enhanced carcinogenesis. Tangeretin exerts anticancer activity by inhibiting the growth as well as the progression of cancer cells in both in vitro and in vivo studies. Results demonstrated that tangeretin possessed selective effectiveness against tumor cell lines [44]. In the studies using a colon carcinoma model [30] and HL-60, human promyelocytic leukemia [45], tangeretin treatment significantly evoked apoptosis by enhancing the expression of p53. Similarly, in rats' breasts and a hepatocellular cancer model, ethanol extract from Citrus reticulata (C. reticulata) peels was found to decrease proliferation by activation of p53 expressions in a dose-dependent fashion [46]. Furthermore, the action of tangeretin on the hallmarks of apoptosis has been reported, including B-cell lymphoma-2 gene (Bcl-2) [47, 48], caspases [32, 49], and DNA fragmentation [45, 47] in different cancer cell lines.
2.5. Effect on Angiogenesis and Metastasis
Cancer cell proliferation in addition to metastasis depends on angiogenesis for the adequate supply of oxygen and nutrients [10]. Several proteins have been recognized as angiogenic activators, including bFGF, TGF-α, TGF-β, TNF-α, IL-8, PDGF, G-CSF, PGF, HGF, and vascular endothelial growth factor (VEGF). In neoplastic vascularization, VEGF is considered a main mediator and has an important influence on cancer progression [50]. Some angiogenic proteins can be evoked by hypoxia [51] which triggers the expression of VEGF and its receptor through hypoxia-inducible factor-1α (HIF-1α) [52]. In addition, in silico studies reported the effect of tangeretin on ERK-2 and HIF1- α [10]. Tangeretin has been shown to act on the expression of VEGF to control certain pathological conditions such as tumor angiogenesis and metastasis in human ovarian [53] and lung [47] cancer cells. Further exploration into the mechanisms of TG may reveal promising insights into its underlying anticancer mechanism inducing angiogenesis.
2.6. Estrogenic Effect of Tangeretin
Estrogen has a critical contribution to the growth and differentiation of mammary and uterine cells and, therefore, might provoke cancer development [54]. Generally, estrogen has additional important roles in maintaining lipid level, genital tissue, and bone density [55]. The role of estrogen in preserving bone density is achieved by increasing bone formation and inhibiting the process of bone resorption. Binding estrogen to estrogen receptor (ER) activates or inhibits the copying of specific genes that respond to estrogen. In addition, the complex formation of estrogen-ER might show certain interaction with factors responsible for transcription such as specificity protein 1 (SP-1) [56]. Therefore, targeting the estrogenic receptor may open the door to chemoprevention and a cancer-oriented therapy [57]. Molecules with estrogen-like effects are known to have estrogenic effects, and many phytochemical compounds have been shown to possess certain activities such as phytoestrogens. The association between estrogen and ER may also activate some transcription factors such as c-Myc which later replicates genes and induces cell cycle activation. Treatment with Citrus reticulata was reported to inhibit the proliferation of MCF-7 when combined with doxorubicin, to promote the growth of rat mammary glands and increase rat uterus volume by inducing c-Myc production [10]. Tangeretin has been reported to increase the expression of the powerful invasion suppressor, E-cadherin/catenin [58], which is upregulated by estrogen in breast cancer cells [59]. This finding was confirmed by the ability of tangeretin to inhibit DMBA-induced breast cancer through the modulation of the expression of expressed estrogen and progesterone receptors [33]. Furthermore, the role of tangeretin, to restore bone loss provoked by estrogen, was reported previously in a mixture with other PMF [60].
2.7. Tangeretin Inducing Autophagy Process
Autophagy is a vesicle and lysosome-mediated process that is responsible for removing cytoplasmic waste from the cell and recycling some of its components to maintain hemostasis [61]. In cancer, the autophagic pathway plays a dual role in tumor promotion and suppression. It is believed that basal autophagy is a factor in cancer suppression because of the inability to dilute aberrant components through cell division. Reduced and abnormal autophagy inhibits the degradation of damaged components or proteins in oxidative-stressed cells, leading to cancer growth. Tangeretin derivative 5-acetyloxy-6, 7, 8, 4′-tetramethoxyflavone (5-AcTMF) was reported to induce autophagy in CL1-5 non-small cell lung cancer (NSCLC) by inducing the synthesis of autophagosome as well as increasing the expression of microtubule-associated proteins 1A/1B light chain 3B (LC3-II) and LC3 [62]. These data suggest that tangeretin enhanced autophagy is a key mechanism in the suppression of tumor generation.
2.8. Tangeretin in Inflammation
Chronic inflammation is associated with the onset or progression of many diseases including cancer. Many signaling pathways of inflammation are involved. Inflammatory markers such as interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), nuclear factor kappa-B (NF-κB), and chemokines further enhance pathogenic processes [63]. Polymethoxyflavones were reported to prevent the expression and synthesis of TNF-α and other proinflammatory cytokines. [64]. The anti-inflammatory effect of tangeretin has been reported in different pathological conditions such as allergic rhinitis via stimulating regulatory T cell [65], asthma by regulating phosphoinositide 3-kinase (PI3K) pathway [21], and Respiratory Syncytial Virus-induced inflammation by curbing NF-κB and interleukin-1 beta (IL-1B) [23]. Furthermore, tangeretin alleviated cisplatin-induced renal inflammation via inhibiting NF-κB activation and decreasing its downstream IL-1B and TNF-α [66], as well as iNOS and TNF-α during reinstatement of the anti-inflammatory interleukin-10 (IL-10) [67]. In addition, we have reported previously the ability of tangeretin to decrease cisplatin-induced hepatic inflammation by decreasing TNF-α and boosting IL-10 [17].
3. Absorption and Oral Bioavailability of Tangeretin
Tangeretin has a significant advantage over other chemically relevant flavones as it shows large intestinal absorption and therefore is bioavailable [68]. It is also considered safe when administered orally [69]. The TG kinetics was tested by collecting Hamster's urine and plasma after 35 days of free access to food containing 1% tangeretin. Intestinal absorption of tangeretin was noticeable with respect to the excretion of several metabolites in urine. Animal plasma was almost free of any unchanged tangeretin [68]. Nevertheless, some biologically active botanical chemicals have low bioavailability and poor solubility. In these chemicals, the process of adding an acetyl group to the existing substance is usually used to get a drug derivative that helps improve the uptake and effectiveness of targeted natural molecules. For this reason, the derivative of tangeretin, 5-AcTMF, has been used in numerous studies [62].
4. Safety and Toxicity of Tangeretin
In order to investigate the possibility of oral toxicity, tangeretin was used as a typical compound for safety assessment as it is considered one of the most common PMF that originated from natural sources [70]. In a study carried out by Ting et al. to examine the acute oral toxicity in mice genders, tangeretin was administrated in 1000, 2000, and 3000 mg/kg from a single gavage in an oil suspension. Outcomes were no deaths observed 14 days after administration. However, daily use of low dose tangeretin displayed a U-shaped dose-response curve including hepatic changes. It was concluded that PMF available as a beneficial ingredient in the human diet could be applied safely at different conditions [70]. Consistent with the previous study, Vanhoecke et al. proved the safety of Tangeretin when administrated orally to experimental mice. Evidence included the absence of any major harm to body organs or deterioration in function. These results open the door for additional safety evaluations in humans [69].
Possible genotoxicity of tangeretin has been investigated by Delaney et al., who administered a PMF mixture in vitro using a range of concentrations in five different bacterial strains. Results indicated no mutations detected regardless of ribosomal protein S9 activation. Observed results indicate the safety profile of PMF mixture and excluded any possibility of genotoxicity from in vitro assay systems [71]. However, in another study, Delaney et al. showed a statistically insignificant positive relationship between increasing the concentrations of PMF and spleen weight in sheep red blood cell (SRBC) immunized mice. In mice without immunization, there was no evidence for spleen weight changes [64].
5. Tangeretin-Drug Interactions
In the last two decades, many studies discussed citrus fruit-drug interactions. The simultaneous intake of tangeretin and drugs was reported to have a strong influence on pharmacokinetics. Citrus juice was demonstrated to affect the pharmacokinetics of various kinds of drugs by modulating drug transporters and drug-metabolizing enzymes [72]. For instance, the flavonoid fraction of clementine juice can induce or inhibit a number of human cytochrome enzymes such as CYP3A4 and CYP1A2 [73]. Mandarin juice inhibited CYP3A4, resulting in increased concentration of tacrolimus in patients [72]. The mean felodipine plasma concentration-time area under the curve (AUC) showed to be elevated due to the inhibition of CYP3A4 activity after lime juice consumption [74].
Tangeretin showed significant inhibition on P-gp in (multidrug resistance protein 1) MDR1-MDCKII cells, which might be the reason underlying the increased cell toxicity of paraquat and taxol. In addition, concurrent administration of digoxin with tangeretin has been reported to alter the pharmacokinetics of digoxin [75].
Tangeretin is present in the bitter orange peel of Seville orange, the Seville juice was reported to inhibit intestinal cytochrome P450 (CYP) isozyme 3A4, and P-glycoprotein was shown to affect colchicine metabolism and transport, which can lead to severe toxicity [76]. Sildenafil bioavailability is reported to be improved by Seville orange; this can be contributed to the inhibition of its intestinal first-pass effect of CYP3A4 and/or P-gp [77]. On the other hand, tangeretin was reported as a potent regioselective stimulator causing CYP3A4 induction [73, 78]. This stimulation can alter midazolam metabolism [78]. Thus, flavonoid food contents should be reported to avoid any unpredictable scenarios.
These studies highlight the need for further investigations to confirm the correlation between the in vitro and in vivo results concerning tangeretin-drug interaction.
6. Tangeretin Application
The effect of tangeretin on different cancer types is summarized in Table 1 and detailed below.
Table 1.
Summary of the anticancer effects of tangeretin.
| Cell line | Function | Proposed mechanism of action | Reference | |
|---|---|---|---|---|
| Ovarian cancer | OVCAR-3 | Antiangiogenesis | Decrease in VEGF expression. | He et al. [53] |
| A2780/CP70 | ||||
| A2780/CP70 | Cell cycle regulator | Caspase cascade, cell cycle arrest at G(2)-M phase. | Arafa et al. [31] | |
| 2008/C13 | Apoptosis inducer | Downregulation of phospho-Akt along with NF-KB, phospho-GSK-3beta, and phospho-BAD. | ||
|
| ||||
| Gastric cancer | AGS | Apoptosis inducer | Reduction in the MMP. | Dong et al. [32] |
| Upregulation of BAX with p53 activation. | ||||
|
| ||||
| Lung cancer | CL1-5 | Apoptosis inducer | Decreasing expression of Bcl-2, XIAP, and survivin. | Li et al. [16] |
| Disturbance in MMP. | ||||
| Translocation of cytochrome C to the cytosol. | ||||
| H1299 | Antiproliferative | Inhibition of IL-1B-induced COX-2 expression. | Chen et al. [79] | |
| A549 | Apoptosis inducer | Deactivation of NF-κB downstream. | ||
|
| ||||
| Prostate cancer | PC-3 | Antimetastatic | Downregulation of mesenchymal proteins. | Zhu et al. [80] |
| Upregulation of epithelial proteins. | ||||
| Antiproliferative | Downregulation of PI3K/Akt/mTOR pathway. | |||
|
| ||||
| Leukemia | MOLT-4 | Reverse MDR | Inhibition of P-glycoprotein function. | Ishii et al. [81] |
| K562/ADM | Reverse MDR | Inhibition of P-glycoprotein-mediated efflux of [(3)H]vincristine. | Ikegawa et al. [82] | |
| HL-60 | Apoptosis inducer | Lowering DNA content. | Hirano et al. [45] | |
| Cell cycle regulator | Decrease of G1 cells, increase of S and/or G2/M cells. | |||
| L1210 | Antiproliferative | Inhibition of cell growth | Satoh et al. [83] | |
| K562 | ||||
| JCS | Autophagy inducer | Increase in phagocytic activity of the cells. | Mak et al. [84] | |
|
| ||||
| Melanoma | B16F10 | Melanogenesis | Increasing levels of tyrosinase, TRP-1, and ERK 1/2 | Yoon et al. [85] |
| Increasing expression of CREB and MITF | ||||
| JB6 P+ | Anti-inflammatory | Blockage of Akt, MAPKs, and MAPK kinases. | Yoon et al. [86] | |
| Antioxidant | Scavenging ROS. | |||
| B16F10 | Antiproliferative | Inhibition of cell growth. | Rodriguez et al. [87] | |
| SK-MEL-1 | ||||
| HTB43 | Antiproliferative | Greater membrane uptake. | Kandaswami et al. [88] | |
| Kandaswami et al. [89] | ||||
|
| ||||
| Brain cancer | IOMM-Lee | Apoptosis inducer | Enhancing phosphorylation of GSK3β. | Das et al. [90] |
| CH157 MN | Downregulation of TSPAN12, Bcl-XL, and Mcl-1. | |||
| Overexpression of Bax and caspase-3. | ||||
| U-87MG | Cell cycle regulator | Increases G2/M arrest. | Ma et al. [91] | |
| LN-18 | Apoptosis inducer | Modulating PTEN and cell-cycle regulated genes (cyclin-D and cdc-2 mRNA and protein expressions). | ||
| Grade III astrocytoma | Antimetastatic | Downregulation of MMP-2 and MMP-9 | Rooprai et al. [92] | |
|
| ||||
| Breast cancer | DMBA-induced rat mammary carcinoma | Antitumor | Decrease in levels of estradiol, progesterone, and prolactin. | Arivazhagan and Pillai [93] |
| Antioxidant | Decrease in levels of LPSA, TSA, NO, and protein carbonyls. | |||
| Antiproliferative | Reduction in PCNA, COX-2, and Ki-67 markers. | |||
| Cell cycle regulator | Arresting cell division at the G1/S phase via p53/p21 upregulation. | |||
| Antimetastatic | Downregulation of MMP-2, MMP-9, and VEGF. | |||
| MCF-7/6 | Immune-modulator | Reducing the number of NK cells. | Depypere et al. [94] | |
| MCF-7 MDA-MB-435 | Antiproliferative | Blocking cell cycle progression at G1. | Morley et al. [42] | |
| MCF7 MDA-MB-468 | Antiproliferative | Blocking cell cycle progression at G1. | Surichan et al. [12] | |
| CYP1A1/CYP1B1-mediated metabolism to 4′ hydroxy tangeretin. | ||||
| DMBA-induced mammary carcinoma | Antioxidant | Normalizing levels of AST, ALT, ALP, ACP, 5′-ND, γ-GT, and LDH. | Lakshmi and Subramanian [36] | |
| Decrease in TBARS, enzymatic, nonenzymatic antioxidants, phase I and phase II detoxification. | Periyasamy et al. [29] | |||
| DMBA-induced mammary carcinoma | Antioxidant | Normalizing activities of glycolytic enzymes. | Periyasamy et al. [95] | |
| Increasing activities of citric acid cycle and respiratory chain enzyme. | ||||
| Antiproliferative | Downregulation of PCNA expression. | |||
|
| ||||
| Liver cancer | HepG2 | Antioxidant | Suppressing of TAG synthesis and mass. | Kurowska et al. [68] |
| Decreasing activities of DAG acyltransferase and microsomal triglyceride transfer protein. | ||||
| Activating PPAR. | ||||
| REL | Antiproliferative | Upregulation of GJIC. | Chaumontet et al. [96] | |
| Chaumontet et al. [97] | ||||
|
| ||||
| Colorectal cancer | HT29 | Cell cycle regulator | Arresting G2/M phase with reduction in ALDH+. | Silva et.al. [98] |
| COLO 205 | Cell cycle regulator | Blocking cell cycle progression at G1 phase. | Pan et al. [30] | |
| Antiproliferative | Inhibiting the activities of Cdk2 and Cdk4. | |||
| Apoptosis inducer | Increasing in p21, p27, and p53 levels. | |||
6.1. Ovarian Cancer
Ovarian cancer is considered the second most fatal cancer among females in developed regions [99]. Ovarian cancer is difficult to cure because of the resistance that arises towards chemotherapy. Consequently, it was important to identify new and effective chemotherapeutic agents [53]. Despite many women who show a good response to first-line therapy in ovarian cancer, disease recurrence is very common due to resistance to chemotherapeutic agents. Resistance to chemotherapeutic agents in turn is a prime hindrance to improving the diagnosis of ovarian cancer. Subsequently, it is deemed necessary for research regarding ovarian cancer to seek new chemical treatment agents from natural sources [53].
A study conducted by He et al. assessed the impact of tangeretin on the articulation of VEGF and cell proliferation in two different cell lines of ovarian cancer [53]. They reported a modest suppressing effect on cell proliferation for OVCAR-3 and A2780/CP70 cells. Furthermore, tangeretin demonstrated some inhibitory effects on VEGF expression at the OVCAR-3 and A2780/CP-70 cell line [53].
Moreover, the vast majority of ovarian cancer patients are not perfectly treated with the standard therapy of cisplatin [cis-diamminedichloroplatinum(II)] primarily because of the impediment developed with drug resistance [100]. However, when using flavonoids alone, it was able to induce cell death for certain cancer cells while regenerating normal cells [101]. In our study, the potentiality of tangeretin to sensitize resistant ovarian cancer cells to cisplatin was examined and its effect to induce apoptosis was confirmed [31].
6.2. Gastric Cancer
Gastric cancer is considered the second main reason for death associated with cancer over the world [102]. Adenocarcinoma gastric cell line (AGS) is a kind of human gastric mucous cell carcinoma with wild-type p53, which has been used in many studies of antitumor drugs [103]. However, in some cancerous cells, mutation of p53 might lead to p53 inactivation and lose its tumor-suppressive activity [104].
Dong et al. illustrated that AGS when treated with dose-dependent tangeretin, a reduction in the mitochondrial membrane potential (MMP) is shown. A significant manifestation in apoptosis caused by tangeretin is mitochondrial dysfunction [32]. Upregulation of bcl-2-like protein 4 (Bax) activates p53 to induce apoptosis mediated by mitochondria which will contribute to activation of caspase-9 and consequently the downstream caspases in this pathway. Moreover, pifithrin-α (PFT-α), p53 inhibitor, will suppress the expression of p53, p21, caspase-3, and caspase-9, thus, the apoptotic effect that is mediated by tangeretin. In conclusion, data indicated that tangeretin stimulated programmed cell death of AGS cells primarily through dysfunction of mitochondria dependent on p53 as well as external pathways mediated by Fas/FasL [32].
6.3. Lung Cancer
Lung cancer is identified as the main cause of cancer-related mortality in the world. It is classified into three main categories: lung carcinoid tumor, small cell lung cancers (SCLCs), and NSCLCs. The most common lung cancer type is NSCLC which constitutes almost 85% of cases. Concerning the treatment of NSCLC patients, chemotherapy and radiotherapy results are weak due to drug resistance development and the mechanism of cell protection [62].
However, the effect of 5-AcTMF as an anticancer agent on CL1-5 of NSCLC has been examined both in vivo and in the laboratory. Several actions for 5-AcTMF were noticeable, such as suppression of tumor proliferation, arresting G2/M checkpoint that is linked with cell division control 2 (CDC2), and cell division cycle 25C (CDC25C). In addition, there was an elevation in the number of apoptotic cells, activation of caspases pathway, decrease in Bcl-2, XIAP, and survivin expression encouraging the liberation of cytochrome C inside the cytosol and MMP disturbances. Moreover, a curbing effect of 5-AcTMF has been observed on the PI3K/protein kinase B/mammalian target rapamycin (PI3K/Akt/mTOR) as a signaling pathway. Akt is expressed excessively by Akt-cDNA transfection as it is inhibited through 5-AcTMF-mediated programmed cell death and autophagocytosis advocating the activation of apoptosis by suppressing the pathway of Akt. Experimentally, it has been found that the growth of the CL1-5 cell line was delayed in such a manner as to achieve the desired results by 5-acTMF therapy, with no proof of deleterious effects [62].
In another study, Chen et al. investigated the effects of tangeretin on COX-2 expression levels in H1299, a human NSCLC and A549, and lung epithelial carcinoma cells. They found that when the cell is pretreated with tangeretin that inhibition of certain signaling factors is induced by IL-1B. Signaling factors include p38 mitogen-activated protein kinases (p38 MAPKs), c-Jun N-terminal kinase (JNK), and AKT phosphorylation excluding deactivation of downstream NF-κB. The above observations disclose that, with an A549 cell type, the inhibition of tangeretin to IL-1B-induced COX-2 expression is accomplished through deactivation of the NF-κB transcription controller in addition to hindering the process of signaling factors, while not including extracellular signal-regulated kinases [79].
6.4. Prostate Cancer
Prostate cancer is classified in the US as the second common cancer type as well as the second major cause of death in the western world. Almost 25% of male patients diagnosed with cancer in the US of America are confirmed as having prostate cancer. However, the underlying causes of prostate cancer remain unclear as there has been no particular cancer-causing substance discovered [88].
Zhu et al. assessed tangeretin's effect on two different types of prostate cancer, androgen-insensitive prostate cancer cells (PC-3) and androgen-sensitive human prostate adenocarcinoma cells (LNCaP). Tangeretin showed dosage- and time-dependent inhibition, where the 50% inhibitory concentration (IC50) after 72 hours was noted at 75 μM and 65 μM in both cell lines, respectively [80]. Results of tangeretin treatment in PC-3 clearly exhibited a reduction in mesenchymal proteins containing vimentin, cluster of differentiation 44 (CD44), and cadherin-2 (CDH2). Upregulation was observed with epithelial proteins containing cadherin-1 and cytokeratin-19. An additional result was the suppression of the PI3K/Akt/mTOR pathway. Consequently, it was demonstrated that tangeretin in PC-3 cells stimulated reprogramming of epithelial–mesenchymal transition (EMT) through directing the PI3K/Akt/mTOR pathway to act as the fundamental mechanism of action for inducing toxicity. Therefore, tangeretin offers an unfamiliar approach for prostate cancer treatment [80].
6.5. Leukemia
Tangeretin was observed by Ishii et al. to have an inhibitory effect on cell proliferation, function of P-gp, and significantly affected the cell cycle of human acute T lymphoblastic leukemia (MOLT-4). In addition, tangeretin had an inhibitory effect on cells which exhibit resistance to daunorubicin, a chemotherapeutic agent. However, tangeretin does not stimulate apoptosis [81]. Similarly, the effect of tangeretin on the uptake of [(3)H]vincristine into DOX-resistant human myelogenous leukemia cells (K562/ADM) was tested by Ikegawa et al. Their study found that, by inhibiting the efflux mediated by P-gp for [(3)H]vincristine, that accumulation of chemotherapy drugs occurred within the cells [82]. In contrast to Ishii et al., tangeretin has shown to promote apoptosis in HL-60 cells through DNA fragmentation and reduction of G1 cells along with an increase in the S and/or G2/M cells without any evidence of toxicity towards human peripheral blood mononuclear cells (PBMCs) [45].
The antitumor effect of tangeretin was studied on murine leukemia type P388 in a living organism. The results of that extract proved tangeretin activity in both in vivo and the laboratory. Accordingly, tangeretin showed an inhibitory effect on cell growth in both leukemia L1210 and K562 cell lines [83]. Also, Mak et al. showed the effects of tangeretin on the growth and differentiation of a newly recognized murine myeloid leukemia cell line (WEHI-3B JCS). Both in vitro and in vivo proliferation of JCS leukemic cells which were treated with tangeretin were critically curtailed. However, the rate of survival of rats with JCS tumor cells receiving tangeretin increased [84].
6.6. Melanoma
Melanoma is one of the prevailing malignant tumors s characterized by metastasis. A study done by Martínez et al. showed that Swiss mice that received flavonoid treatment developed suppression for metastasis when compared to an ethanol group at the same index [105]. In another experiment, it was found that treatment with tangeretin 25 μM in B16/F10 murine skin cancer cells catalyzed the production of melanin within cells through activation of melanogenic protein expressions such as tyrosinase, tyrosinase-related protein (TRP)-1, and ERK 1/2. Moreover, CREB and MITF expression was higher in one hour and four hours, respectively. Studies have shown a curative power for tangeretin in skin cancer and the associated depigmentation [85]. In addition, the effect of tangeretin was examined by Yoon et al. using mouse skin epidermal JB6P + cells to prove an inhibitory effect of tangeretin on COX-2 expression as well as the transactivation of NF-κB and activator protein 1. This was accomplished by inhibiting phosphorylation of Akt and MAPKs which include JNK, ERK, p38, and decreased the phosphorylation of MAPK kinases 1/2, 3/6, and 4. In addition, the capability of internal generation ROS was minimized by tangeretin, thus preventing further oxidative stress for healthy cells [86].
According to Rodriguez et al., SK-MEL-1 and B16F10 skin cancer cell lines responded favorably to tangeretin. They indicated that hydroxylated flavonoids with absent double bond C2–C3 lead to loss of effectiveness on both melanoma cell lines. However, tangeretin showed the highest efficacy and this is due to the availability of a minimum of 3 methoxyl groups which provides a more effective antiproliferative effect [87]. Similarly, tangeretin's effects have been studied by Kandaswami et al. in the growth of a human squamous cell carcinoma cell line (HTB43) and have shown that significant cell growth suppression can be attributed to a higher membrane uptake [88,89].
6.7. Brain Cancer
Recurrent meningioma is a rare but serious problem occurring after the failure of standard treatment (surgery and radiation). The current chemotherapies have been considered as regimens with only a slight benefit. Thus, there is an urgent need for effective treatments for meningioma patients who have tried standard therapies but without useful results [90].
Das et al. provided powerful preliminary evidence for the curative effect of tangeretin in IOMM-Lee and CH157MN meningioma cells. They found that tangeretin acts by inducing cell death with phosphorylation of glycogen synthase kinase 3 β (GSK3β) through the suppression of Wnt5/β-catenin pathway. In addition to apoptosis, tangeretin stimulated downregulation processing of the tetraspanin protein (TSPAN12) and survival proteins (Mcl-1 and Bcl-XL), while upregulating apoptotic factors (Bax and caspase-3) [90]. Ma et al. reported similar results for tangeretin-treated U-87 MG and LN-18 cells, as they markedly demonstrated cell growth inhibition and apoptotic effects when compared to nontreated cells. It has been reported that tangeretin acts by the mechanism of modifying phosphatase and tensin homolog (PTEN) together with genes responsible for cell cycle regulation such as cyclin-D, cdc2 mRNA, and protein expressions [91]. However, a study reported by Rooprai et al. shows the effect of tangeretin on different criteria of brain tumor invasion such as expression of matrix metalloproteinase migration, adhesion, and invasion revealing that tangeretin demonstrated a significant downregulation effect of MMP-2 and MMP-9 in the grade 3 astrocytoma. Moreover, in several cell lines such, as anaplastic astrocytoma, ependymoma-a grade II oligoastrocytoma, and glioblastoma multiform, citrus flavonoids showed great inhibition of invasion, migration, and adhesion [92].
6.8. Breast Cancer
At a global level, breast cancer is increasingly alarming as it is the second most common cancer in females. Genetic factors are attributed to only 10% of cases reported with breast cancer, while the most prevalent causes are environmental including diet, which constitutes the most important role in breast cancer prevention [33].
Arivazhagan and Pillai reported that tangeretin can greatly slow antitumor activity through an inhibitory effect on estrogen, progesterone, and prolactin serum level, as well as lipid bound sialic acid (LBSA), total sialic acid (TSA), and levels of nitric oxide and protein carbonyls in tissues of animals with DMBA-induced breast cancer. Furthermore, tangeretin oral treatment decreased signs of tumor cells such as proliferating cell nuclear antigen (PCNA), COX-2, and Ki-67 and affected cell division by upregulating p53/p21 and secondary suppression of metastasis by inhibiting MMP-2, MMP-9, and VEGF [93]. Similarly, it was found that tangeretin therapy in human MCF-7/6 breast cancer cells showed a great anti-invasive as well as antiproliferative effect when tangeretin was applied in vitro. Moreover, a reduction in NK cells was observed [94]. Consistent with the previous studies, tangeretin-treated MDA-MB-468, MDA-MB-435, and MCF-7 cells showed an antiproliferative effect attributed to arresting the cell cycle in G1 phase [12, 42], as well as activation of CYP1 and expression of CYP1A1/CYP1B1 that document the ability of tangeretin to prevent the spread of breast cancer cells by the metabolism-mediated processes through CYP1A1/CYP1B1 and 4′hydroxy tangeretin in both MCF-7 and MDA-MB-468 [12].
Abe et al. pointed out that tangeretin when administered to the mammary gland of a mouse with an induced tumor demonstrated inhibition of atypical hyperplastic lesion and stimulated the programmed death of ductal epithelial cells [106]. However, Morley et al. (2007) disagreed with the ability of tangeretin to procure apoptosis in both MDA-MB-435 and MCF-7 breast cancer cell lines. Rather, they indicated that tangeretin is a cytostatic agent causing inhibition of proliferation with no evidence of programmed cell death [42]. The effectiveness of tangeretin was clearly demonstrated by two studies as a potent suppressor of breast cancer in rats induced by DMBA. Data showed higher performance of the serum enzymes such as liver function biomarkers, alkaline and acid phosphatases, γ-glutamyltransferase (γ-GT), 5′-nucleotidase (5′-ND), and lactate dehydrogenase (LDH) in rats with breast cancer, reduced to levels close to normal by the administration of tangeretin. Moreover, some enzymatic and nonenzymatic antioxidants and thiobarbituric acid reactive substances (TBARS), a byproduct of lipid peroxidation, along with both phases of detoxification showed a significant reduction as a result of tangeretin treatment [29,33]. Lakshmi and Subramanian added to the inhibitory effect of tangeretin in some oxidative stress markers and reported that tangeretin also significantly improved the level of endogenous antioxidants in kidney tissue. This result demonstrates the expression of nuclear factor (erythroid-derived 2)-like 2/Kelch-like ECH-associated protein 1 (Nrf2/Keap1) in renal tissues within the normal range, hence, protecting kidneys efficiently from oxidative damage by DMBA and confirming tangeretin's nature as a nephroprotective agent [36]. Periyasamy et al. demonstrated that tangeretin plays a specific role in regulating the flow of cellular metabolic energy in DMBA-induced breast cancer-bearing rats. However, treated rats with tangeretin exhibited normalization in the level of glycolytic enzymes as well as a significant rise in the activities of the citric acid cycle and respiratory chain enzyme. Moreover, the expression of PCNA was downregulated [95].
6.9. Liver Cancer
A study reported by Kurowska et al. revealed a significant reduction in the secretion of apolipoprotein B (apoB) and suppression of cholesteryl esters, free cholesterol, and triacylglycerol (TAG) intracellular synthesis upon incubation with tangeretin in human hepatoma cell line HepG2. Cellular triacylglycerol was also decreased in size. These results were correlated with the reduction in microsomal triglyceride transfer protein (MTTP) and diacylglycerol acyltransferase (DGAT) activities. Moreover, tangeretin showed activation of the transcription factor, peroxisome proliferator-activated receptor (PPAR), which is responsible for controlling the oxidation process of fatty acids and triacylglycerol in a positive manner [107].
However, in another study, tangeretin exhibited antagonistic action against the inhibition of gap junctional intercellular communication (GJIC) caused by tumor stimulators such as 3,5,di-tertio-butyl-4-hydroxytoluene (BHT) and 12-O-tetradecanoyl-phorbol-acetate (TPA) in rat liver epithelial cell line (REL) [96, 97].
6.10. Colorectal Cancer
Colorectal cancer (CRC) is one of the leading causes of cancer death in adults and has been linked to many lifestyle-related factors [108]. Silva et al. identified tangeretin as an agent that prevented the spread of colorectal cancer through a different mechanism of action on spheroid cells of HT29 colon cancer cell line. This mechanism includes the antiproliferation effect, disruption of cell cycle (G2/M phase), inhibition of aldehyde dehydrogenase (ALDH+), and a cancer stem cell marker and inducing apoptosis [98]. Similar results have been reported by Fan et al. on intestinal tumor growth of a mouse model for human familial adenomatous polyposis (FAP) that demonstrated further increased apoptosis of intestinal tumors after been fed 0.5% of orange peel extract that is rich in tangeretin [109]. More specifically, Pan et al. illustrated that the antiproliferative effect of tangeretin in COLO 205 was achieved by either modifying the expression of many regulatory proteins that are major for G1 phase, like CDK2 and CDK4, or stimulating the activities of both p21 and p27, cyclin-dependent kinase inhibitors [30].
7. Tangeretin as a Potential Adjuvant Chemotherapeutic Agent
Among available treatment options for cancer, chemotherapy is the most effective therapy for treating a variety of cancers. Chemotherapeutics are mainly classified based on their mechanism of action and their chemical structure. Unfortunately, chemotherapy exhibits undesirable side effects which can lead to dose reduction or even cessation of treatment. Combined chemotherapy may increase the effectiveness of therapeutic chemical agents; this, in turn, permits the use of lower doses of the chemotherapeutic agent and hence reduces toxicity to normal tissues [110, 111]. Increased efficacy can be achieved by combining agents possessing a chemotherapeutic effect that has an additional or synergistic effect, whereas toxicity can be reduced by using agents that have an immunomodulatory effect [112].
Interestingly, many studies have validated the anticancer properties of tangeretin. Bracke et al. in 1999 reported that tangeretin reversed the antiproliferative effect of tamoxifen on tumor cells in human MCF-7/6 mammary adenocarcinoma cells induced in female nude mice [113]. On the other hand, studies on citrus flavonoids have shown that when combined with chemotherapy, tangeretin has significantly increased drug anticancer efficacy on resistant cancer cell lines. Concurrent use of tangeretin with chemotherapeutic agents synergistically stimulated cell death and cell cycle arrest in resistant cancer cells [31].
Published data showed that tangeretin has the ability to sensitize excessive ABCB1 expression cancer cells to chemotherapeutic agents through direct inhibition of ABCB1 transporter activity. This in turn motivated further studies in animals as well as clinical trials for the purpose of overcoming cancer resistance [114]. In a study done by Akao et al. combining tangeretin with 5-demethyl, nobiletin caused cell apoptosis by limiting MMP and raising the assumption that, through the stated combination therapy, an intrinsic process of programmed cell death will be activated by a synergetic effect. These findings suggested the importance of phytochemical combinations for enhancing the cancer-preventive effect [49]. We have reported previously that combination treatments of cisplatin and tangeretin on A2780/CP70 and 2008/C13 cisplatin-resistant human ovarian cancer cells downregulated PI3K/Akt pathway and consequently curbed NF-κB and BAD making resistant cells more prone to the toxic effects of cisplatin. Additionally, the downregulation of GSK-3β accounts for the synergetic effect of cisplatin and tangeretin combination on apoptosis. In line with flow cytometry analysis, this combination treatment also reduced cdc25c gene and cyclin B1 protein levels while p53 was significantly increased. Overall, cisplatin-tangeretin combination therapy provides a novel approach for patients with ovarian cancer and provides an effective treatment regimen [31]. Recent findings from our laboratory indicate that tangeretin has the ability to protect against cisplatin-induced liver and kidney toxicities. This protection was facilitated by modulating different inflammatory mediators, apoptotic, oxidative, and survival signaling pathways [17, 67].
8. Conclusion
In conclusion, it is evident that tangeretin, an extract from citrus peels, has a wide range of positive effects. In our review, we focused on tangeretin as a therapeutic approach in different cancer cell lines with various properties including antioxidant, activating apoptosis, arresting cell cycle, antiangiogenesis, and antiproliferative, which are supported abundantly with evidence-based research detailing the mechanism of action. In addition, using tangeretin in combination with chemotherapeutic agents may be considered as an option for enhancing the efficacy of these agents. Favorable effects of tangeretin presented in this paper encourage the use of this natural agent as a drug with a broad spectrum of medical applications. Further and comprehensive clinical research is needed to prove its beneficial role and effectiveness as a pharmaceutical preparation.
Acknowledgments
This work was supported by a research grant from the Deanship of Graduate Studies and Research, Ajman University, Grant no. 2017-A-PH-03. NT Shurrab is a graduate research assistant supported by the Deanship of Graduate Studies and Research, Ajman University. The authors would like to thank Professor Mark Dougherty, Auburn University, for proofreading the manuscript.
Abbreviations
- 5-ACTMF:
5-Acetyloxy-6, 7, 8, 4′-tetramethoxyflavone
- 5′-ND:
5′-Nucleotidase
- 8-OHdG:
8-Hydroxy-2′-deoxyguanosine
- AGS:
Adenocarcinoma gastric cell line
- ALDH+:
Aldehyde dehydrogenase
- AUC:
Area under the curve
- apoB:
Apolipoprotein B
- Bax:
bcl-2-like protein 4
- Bcl-2:
B-cell lymphoma-2 gene
- Bcl-XL:
B-cell lymphoma-extra large
- bFGF:
Basic fibroblast growth factor
- BHT:
3, 5,di-Tertio-butyl-4-hydroxytoluene
- CD44:
Cluster of differentiation 44
- CDC2:
Cell division control 2
- CDC25 C:
Cell division cycle 25 C
- CDH2:
Cadherin-2
- CDK:
Cyclin-dependent kinases
- CDK2:
Cyclin-dependent kinase 2
- CDK4:
Cyclin-dependent kinase 4
- c-JNK:
c-Jun N-terminal kinase
- CL1-5:
Human lung cancer cell line
- COLO 205:
Human colon cancer
- COX-2:
Cyclooxygenase-2
- CRC:
Colorectal cancer
- CREB:
Cyclic adenosine monophosphate response element binding protein
- DGAT:
Diacylglycerol acyltransferase
- DMBA:
7, 12-Dimethylbenz[a]anthracene
- DOX:
Doxorubicin
- EGF:
Epidermal growth factor
- EMT:
Epithelial-mesenchymal transition
- ER:
Estrogenic receptor
- ERK-2:
Extracellular signal-regulated kinase 2
- G-CSF:
Granulocyte colony-stimulating factor
- GJIC:
Gap junctional intercellular communication
- GSK3β:
Glycogen synthase kinase 3 β
- HepG2:
Human liver cancer cell line
- HGF:
Hepatocyte growth factor
- HIF1-α:
Hypoxia-inducible factor 1-alpha
- HL-60:
Human promyelocytic leukemia
- HNE:
4-Hydroxy-2-trans-nonenal
- HTB43:
Human squamous cell carcinoma cell line
- IC50:
50% inhibitory concentration
- IFN-γ:
Interferon gamma
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- LBSA:
Lipid bound sialic acid
- LC3:
Light chain 3
- LDH:
Lactate dehydrogenase
- LNCaP:
Androgen-sensitive human prostate adenocarcinoma cells
- MCF-7:
Breast cancer cell line
- Mcl-1:
Induced myeloid leukemia cell differentiation protein
- MITF:
Microphthalmia transcription factor
- MMP:
Mitochondrial membrane potential
- MMPs:
Matrix metalloproteinases
- MMP-2:
Matrix metalloproteinase-2
- MMP-9:
Matrix metalloproteinase-9
- MOLT-4:
Human acute T lymphoblastic leukemia
- MTTP:
Microsomal triglyceride transfer protein
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK:
Natural killer
- Nrf2/Keap1:
Nuclear factor (erythroid-derived 2)-like 2/Kelch-like ECH-associated protein 1
- NSCLC:
Non-small cell lung cancer
- P-gp:
P-Glycoproteins
- p38 MAPKs:
p38 mitogen-activated protein kinases
- PBMCs:
Peripheral blood mononuclear cells
- PC-3:
Androgen-insensitive prostate cancer cells
- PCNA:
Proliferating cell nuclear antigen
- PDGF:
Platelet-derived growth factor
- PFT-α:
Pifithrin-α
- PGF:
Placental growth factor
- PI3K/Akt/mTOR:
Phosphatidylinositol 3-kinase/protein kinase B/ mammalian target of rapamycin
- PMF:
Polymethoxyflavone
- PPAR:
Peroxisome proliferator-activated receptor
- PTEN:
Phosphatase and tensin homolog
- SCLCs:
Small cell lung cancers
- SP-1:
Specificity protein 1
- SRBC:
Sheep red blood cells
- TAG:
Triacylglycerol
- TBARS:
Thiobarbituric acid reactive substances
- TGF:
Transforming growth factor
- TH+:
Tyrosine hydroxylase positive
- TNF-α:
Tumor necrosis factor alpha
- TPA:
12-O-Tetradecanoyl-phorbol-acetate
- TRP:
Tyrosinase-related protein
- TSA:
Total sialic acid
- TSPAN12:
Tetraspanin protein
- VEGF:
Vascular endothelial growth factor
- WEHI-3B:
JCS murine myeloid leukemia cell line
- XIAP:
X-linked inhibitor of apoptosis protein
- γ-GT:
γ-Glutamyltransferase.
Data Availability
No data were used to support this study. It is a review article.
Conflicts of Interest
The authors have declared that no conflicts of interest exist.
Authors' Contributions
All the authors contributed equally to this manuscript.
References
- 1.Wiffen P. J., Wee B., Derry S., Bell R. F., Moore R. A. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database of Systematic Reviews. 2017;7 doi: 10.1002/14651858.CD012592.pub2.CD012592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mechetner E., Kyshtoobayeva A., Zonis S, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 1998;4(2):389–398. [PubMed] [Google Scholar]
- 3.Wattanapitayakul S. K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Niumsakul S., Bauer J. A. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clinical Pharmacology Toxicology. 2005;96(1):80–87. doi: 10.1111/j.1742-7843.2005.pto960112.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch F. R., Merrick D. T., Franklin W. A. Role of biomarkers for early detection of lung cancer and chemoprevention. European Respiratory Journal. 2002;19(6):1151–1158. doi: 10.1183/09031936.02.00294102. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S., Afaq F., Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochemical and Biophysical Research Communications. 2001;287(4):914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 6.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutrition and Cancer. 2014;66(2):177–193. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 7.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacognosy Reviews. 2014;8(16):122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qadir M. I., Cheema B. N. Phytoestrogens and related food components in the prevention of cancer. Critical Reviews in Eukaryotic Gene Expression. 2017;27(2):99–112. doi: 10.1615/CritRevEukaryotGeneExpr.2017019473. [DOI] [PubMed] [Google Scholar]
- 9.Costea T., Vlad O. C., Miclea L.-C., Ganea C., Szöllősi J., Mocanu M.-M. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. International Journal of Molecular Sciences. 2020;21(2):p. 401. doi: 10.3390/ijms21020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meiyanto E., Hermawan A., Anindyajati A. Natural products for cancer-targeted therapy: citrus flavonoids as potent chemopreventive agents. Asian Pacific Journal of Cancer Prevention. 2012;13(2):427–436. doi: 10.7314/apjcp.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- 11.Chen F., Ma Y., Sun Z., Zhu X. Tangeretin inhibits high glucose-induced extracellular matrix accumulation in human glomerular mesangial cells. Biomedicine & Pharmacotherapy. 2018;102:1077–1083. doi: 10.1016/j.biopha.2018.03.169. [DOI] [PubMed] [Google Scholar]
- 12.Surichan S., Arroo R. R., Tsatsakis A. M., Androutsopoulos V. P. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4’ hydroxy tangeretin. Toxicology in Vitro. 2018;50:274–284. doi: 10.1016/j.tiv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Sundaram R., Shanthi P., Sachdanandam P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine. 2014;21(6):793–799. doi: 10.1016/j.phymed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim M. S., Hur H. J., Kwon D. Y., Hwang J.-T. Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in C2C12 myotubes and improves glucose tolerance in high-fat diet-induced obese mice. Molecular and Cellular Endocrinology. 2012;358(1):127–134. doi: 10.1016/j.mce.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Miyata Y., Tanaka H., Shimada A., et al. Regulation of adipocytokine secretion and adipocyte hypertrophy by polymethoxyflavonoids, nobiletin and tangeretin. Life Sciences. 2011;88(13-14):613–618. doi: 10.1016/j.lfs.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y. Y., Lee E.-J., Park J.-S., Jang S.-E., Kim D.-H., Kim H.-S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. Journal of Neuroimmune Pharmacology. 2016;11(2):294–305. doi: 10.1007/s11481-016-9657-x. [DOI] [PubMed] [Google Scholar]
- 17.Omar H. A., Mohamed W. R., Arab H. H., Arafa E.-S. A. Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS One. 2016;11(3):p. e0151649. doi: 10.1371/journal.pone.0151649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu Z., Yang B., Zhao H., et al. Tangeretin exerts anti-neuroinflammatory effects via NF-κB modulation in lipopolysaccharide-stimulated microglial cells. International Immunopharmacology. 2014;19(2):275–282. doi: 10.1016/j.intimp.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Zhao Y.-M., Deng Z.-K. Tangeretin ameliorates renal failure via regulating oxidative stress, NF-κB-TNF-α/iNOS signalling and improves memory and cognitive deficits in 5/6 nephrectomized rats. Inflammopharmacology. 2018;26(1):119–132. doi: 10.1007/s10787-017-0394-4. [DOI] [PubMed] [Google Scholar]
- 20.Hagenlocher Y., Feilhauer K., Schäffer M., Bischoff S. C., Lorentz A. Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. European Journal of Nutrition. 2017;56(4):1609–1620. doi: 10.1007/s00394-016-1207-z. [DOI] [PubMed] [Google Scholar]
- 21.Liu L.-L., Li F.-H., Zhang Y., Zhang X.-F., Yang J. Tangeretin has anti-asthmatic effects via regulating PI3K and Notch signaling and modulating Th1/Th2/Th17 cytokine balance in neonatal asthmatic mice. Brazilian Journal of Medical and Biological Research. 2017;50(8):p. e5991. doi: 10.1590/1414-431X20175991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eun S.-H., Woo J.-T., Kim D.-H. Tangeretin inhibits IL-12 expression and NF-κB activation in dendritic cells and attenuates colitis in mice. Planta Medica. 2017;83(06):527–533. doi: 10.1055/s-0042-119074. [DOI] [PubMed] [Google Scholar]
- 23.Xu J.-J., Liu Z., Tang W., et al. Tangeretin from citrus reticulate inhibits respiratory syncytial Virus replication and associated inflammation in vivo. Journal of Agricultural and Food Chemistry. 2015;63(43):9520–9527. doi: 10.1021/acs.jafc.5b03482. [DOI] [PubMed] [Google Scholar]
- 24.Vaiyapuri S., Ali M. S., Moraes L. A., et al. Tangeretin regulates platelet function through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(12):2740–2749. doi: 10.1161/ATVBAHA.113.301988. [DOI] [PubMed] [Google Scholar]
- 25.Akachi T., Shiina Y., Ohishi Y., et al. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against D-galactosamine-induced liver injury in rats. Journal of Nutritional Science and Vitaminology. 2010;56(1):60–67. doi: 10.3177/jnsv.56.60. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y., Vermeer M. A., Bos W., et al. Molecular structures of citrus flavonoids determine their effects on lipid metabolism in HepG2 cells by primarily suppressing apoB secretion. Journal of Agricultural and Food Chemistry. 2011;59(9):4496–4503. doi: 10.1021/jf1044475. [DOI] [PubMed] [Google Scholar]
- 27.Youn K., Yu Y., Lee J., Jeong W.-S., Ho C.-T., Jun M. Polymethoxyflavones: novel β-secretase (BACE1) inhibitors from citrus peels. Nutrients. 2017;9(9):p. 973. doi: 10.3390/nu9090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datla K. P., Christidou M., Widmer W. W., Rooprai H. K., Dexter D. T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport. 2001;12(17):3871–3875. doi: 10.1097/00001756-200112040-00053. [DOI] [PubMed] [Google Scholar]
- 29.Periyasamy K., Baskaran K., Ilakkia A., Vanitha K., Selvaraj S., Sakthisekaran D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7, 12-dimethylbenz(a) anthracene-induced proliferative breast cancer in sprague-dawley rats. Cancer Chemotherapy and Pharmacology. 2015;75(2):263–272. doi: 10.1007/s00280-014-2629-z. [DOI] [PubMed] [Google Scholar]
- 30.Pan M.-H., Chen W. J., Lin-Shiau S. Y., Ho C. T., Lin J. K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23(10):1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 31.Arafa E.-S. A., Zhu Q., Barakat B. M., et al. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Research. 2009;69(23):8910–8917. doi: 10.1158/0008-5472.CAN-09-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y., Cao A., Shi J., et al. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncology Reports. 2014;31(4):1788–1794. doi: 10.3892/or.2014.3034. [DOI] [PubMed] [Google Scholar]
- 33.Lakshmi A., Subramanian S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz(a)anthracene induced mammary carcinoma in experimental rats. Biochimie. 2014;99:96–109. doi: 10.1016/j.biochi.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Thompson H. J. DNA oxidation products, antioxidant status, and cancer prevention. The Journal of Nutrition. 2004;134(11):3186S–3187S. doi: 10.1093/jn/134.11.3186S. [DOI] [PubMed] [Google Scholar]
- 35.Miran T., Vogg A. T. J., Drude N., Mottaghy F. M., Morgenroth A. Modulation of glutathione promotes apoptosis in triple-negative breast cancer cells. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2018;32(5):2803–2813. doi: 10.1096/fj.201701157R. [DOI] [PubMed] [Google Scholar]
- 36.Lakshmi A., Subramanian S. P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7, 12-dimethylbenz[a]anthracene. Toxicology Letters. 2014;229(2):333–348. doi: 10.1016/j.toxlet.2014.06.845. [DOI] [PubMed] [Google Scholar]
- 37.Liang F., Fang Y., Cao W., Zhang Z., Pan S., Xu X. Attenuation of tert-butyl hydroperoxide (t-BHP)-Induced oxidative damage in HepG2 cells by tangeretin: relevance of the nrf2-ARE and MAPK signaling pathways. Journal of Agricultural and Food Chemistry. 2018;66(25):6317–6325. doi: 10.1021/acs.jafc.8b01875. [DOI] [PubMed] [Google Scholar]
- 38.Lechner M., Boshoff C., Beck S. Cancer epigenome. Epigenetics and Cancer, Part A. 2010;70:247–276. doi: 10.1016/B978-0-12-380866-0.60009-5. [DOI] [PubMed] [Google Scholar]
- 39.Calomme M., Pieters L., Vlietinck A., Berghe D. Inhibition of bacterial mutagenesis by citrus flavonoids. Planta Medica. 1996;62(03):222–226. doi: 10.1055/s-2006-957864. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto T., Nishikawa T., Furukawa A., et al. Antimutagenic effects of polymethoxy flavonoids of citrus unshiu. Natural Product Communications. 2017;12(1):23–26. doi: 10.1177/1934578x1701200108. [DOI] [PubMed] [Google Scholar]
- 41.Lake B. G., Beamand J. A., Tredger J. M., Barton P. T., Renwick A. B., Price R. J. Inhibition of xenobiotic-induced genotoxicity in cultured precision-cut human and rat liver slices. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 1999;440(1):91–100. doi: 10.1016/s1383-5718(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 42.Morley K. L., Ferguson P. J., Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Letters. 2007;251(1):168–178. doi: 10.1016/j.canlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 43.Bröker L. E., Kruyt F. A. E., Giaccone G. Cell death independent of caspases: a review. Clinical Cancer Research. 2005;11(9):3155–3162. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 44.Kawaii S., Tomono Y., Katase E., Ogawa K., Yano M. Antiproliferative activity of flavonoids on several cancer cell lines. Bioscience, Biotechnology, and Biochemistry. 1999;63(5):896–899. doi: 10.1271/bbb.63.896. [DOI] [PubMed] [Google Scholar]
- 45.Hirano T., Abe K., Gotoh M., Oka K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. British Journal of Cancer. 1995;72(6):1380–1388. doi: 10.1038/bjc.1995.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krstic J., Galhuber M., Schulz T., Schupp M., Prokesch A. p53 as a dichotomous regulator of liver disease: the dose makes the medicine. International Journal of Molecular Sciences. 2018;19(3):p. 921. doi: 10.3390/ijms19030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roshini A., Jagadeesan S., Arivazhagan L., et al. pH-sensitive tangeretin-ZnO quantum dots exert apoptotic and anti-metastatic effects in metastatic lung cancer cell line. Materials Science and Engineering: C. 2018;92:477–488. doi: 10.1016/j.msec.2018.06.073. [DOI] [PubMed] [Google Scholar]
- 48.Fan S., Xu H., Liu H., Hu Z., Xiao J., Liu H. Inhibition of cancer cell growth by Tangeretin flavone in drug-resistant MDA-MB-231 human breast carcinoma cells is facilitated via targeting cell apoptosis, cell cycle phase distribution, cell invasion and activation of numerous caspases. Journal of the Balkan Union of Oncology. 2019;24(4):1532–1537. [PubMed] [Google Scholar]
- 49.Akao Y., Itoh T., Ohguchi K., Iinuma M., Nozawa Y. Interactive effects of polymethoxy flavones from Citrus on cell growth inhibition in human neuroblastoma SH-SY5Y cells. Bioorganic & Medicinal Chemistry. 2008;16(6):2803–2810. doi: 10.1016/j.bmc.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 50.Ceci C., Atzori M. G., Lacal P. M., Graziani G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: experimental evidence in different metastatic cancer models. International Journal of Molecular Sciences. 2020;21(4):p. 1388. doi: 10.3390/ijms21041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferguson J., De Los Santos Z., Devi N., Van Meir E., Zingales S., Wang B. Examining the structure-activity relationship of benzopyran-based inhibitors of the hypoxia inducible factor-1 pathway. Bioorganic & Medicinal Chemistry Letters. 2017;27(8):1731–1736. doi: 10.1016/j.bmcl.2017.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tirpe A. A., Gulei D., Ciortea S. M., Crivii C., Berindan-Neagoe I. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. International Journal of Molecular Sciences. 2019;20(24):p. 6140. doi: 10.3390/ijms20246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He Z., Li B., Rankin G. O., Rojanasakul Y., Chen Y. C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncology Letters. 2015;9(3):1444–1450. doi: 10.3892/ol.2014.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glaeser M., Niederacher D., Djahansouzi S., et al. Effects of the antiestrogens tamoxifen and raloxifene on the estrogen receptor transactivation machinery. Anticancer Research. 2006;26(1B):735–744. [PubMed] [Google Scholar]
- 55.Jordan V. C. Selective estrogen receptor modulation. Cancer Cell. 2004;5(3):207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 56.Meyer M. R., Haas E., Barton M. Gender differences of cardiovascular disease. Hypertension. 2006;47(6):1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 57.Manni A., Xu H., Washington S., et al. The effects of Tamoxifen and fish oil on mammary carcinogenesis in polyoma middle T transgenic mice. Hormones and Cancer. 2011;2(4):249–259. doi: 10.1007/s12672-011-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong H., Boterberg T., Maubach J., et al. 8-Prenylnaringenin, the phytoestrogen in hops and beer, upregulates the function of the E-cadherin/catenin complex in human mammary carcinoma cells. European Journal of Cell Biology. 2001;80(9):580–585. doi: 10.1078/0171-9335-00190. [DOI] [PubMed] [Google Scholar]
- 59.Blaschuk O. W., Farookhi R. Estradiol stimulates cadherin expression in rat granulosa cells. Developmental Biology. 1989;136(2):564–567. doi: 10.1016/0012-1606(89)90283-2. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto S., Tominari T., Matsumoto C., et al. Effects of polymethoxyflavonoids on bone loss induced by estrogen deficiency and by LPS-dependent inflammation in mice. Pharmaceuticals. 2018;11(1):p. 7. doi: 10.3390/ph11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zare-Shahabadi A., Masliah E., Johnson G. V. W., Rezaei N. Autophagy in alzheimer’s disease. Reviews in the Neurosciences. 2015;26(4):385–395. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y., Guo H., Liu D., Zhao Z. Preservation of renal function by thyroid hormone replacement in elderly persons with subclinical hypothyroidism. Archives of Medical Science. 2016;4(4):772–777. doi: 10.5114/aoms.2016.60965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu X., Tang Y., Hua S. Immunological approaches towards cancer and inflammation: a cross talk. Frontiers in Immunology. 2018;9:p. 563. doi: 10.3389/fimmu.2018.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delaney B., Phillips K., Buswell D., et al. Immunotoxicity of a standardized citrus polymethoxylated flavone extract. Food and Chemical Toxicology. 2001;39(11):1087–1094. doi: 10.1016/s0278-6915(01)00058-8. [DOI] [PubMed] [Google Scholar]
- 65.Xu S., Kong Y.-G., Jiao W.-E., et al. Tangeretin promotes regulatory T cell differentiation by inhibiting notch1/jagged1 signaling in allergic rhinitis. International Immunopharmacology. 2019;72:402–412. doi: 10.1016/j.intimp.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 66.Nazari Soltan Ahmad S., Rashtchizadeh N., Argani H., et al. Tangeretin protects renal tubular epithelial cells against experimental cisplatin toxicity. Iranian Journal of Basic Medical Sciences. 2019;22(2):179–186. doi: 10.22038/ijbms.2018.32010.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arab H. H., Mohamed W. R., Barakat B. M., Arafa E.-S. A. Tangeretin attenuates cisplatin-induced renal injury in rats: impact on the inflammatory cascade and oxidative perturbations. Chemico-Biological Interactions. 2016;258:205–213. doi: 10.1016/j.cbi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Kurowska E. M., Manthey J. A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. Journal of Agricultural and Food Chemistry. 2004;52(10):2879–2886. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- 69.Vanhoecke B. W., Delporte F., Van Braeckel E., et al. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. Vivo. 2005;19(1):103–107. [PubMed] [Google Scholar]
- 70.Ting Y., Chiou Y.-S., Jiang Y., Pan M.-H., Lin Z., Huang Q. Safety evaluation of tangeretin and the effect of using emulsion-based delivery system: oral acute and 28-day sub-acute toxicity study using mice. Food Research International. 2015;74:140–150. doi: 10.1016/j.foodres.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 71.Delaney B., Phillips K., Vasquez C., et al. Genetic toxicity of a standardized mixture of citrus polymethoxylated flavones. Food and Chemical Toxicology. 2002;40(5):617–624. doi: 10.1016/s0278-6915(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 72.Theile D., Hohmann N., Kiemel D., et al. Clementine juice has the potential for drug interactions - in vitro comparison with grapefruit and mandarin juice. European Journal of Pharmaceutical Sciences. 2017;97:247–256. doi: 10.1016/j.ejps.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Weiss J., Gattuso G., Barreca D., Haefeli W. E. Nobiletin, sinensetin, and tangeretin are the main perpetrators in clementines provoking food-drug interactions in vitro. Food Chemistry. 2020;319 doi: 10.1016/j.foodchem.2020.126578.126578 [DOI] [PubMed] [Google Scholar]
- 74.Bailey D., Dresser G. K., Bend J. R. Bergamottin, lime juice, and red wine as inhibitors of cytochrome P450 3a4 activity: comparison with grapefruit juice, Clinical Pharmacology & Therapeutics. 2003;73(6):529–537. doi: 10.1016/S0009-9236(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 75.Bai J., Zhao S., Fan X., et al. Inhibitory effects of flavonoids on P-glycoprotein in vitro and in vivo: food/herb-drug interactions and structure-activity relationships. Toxicology and Applied Pharmacology. 2019;369:49–59. doi: 10.1016/j.taap.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Wason S., DiGiacinto J. L., Davis M. W. Effects of grapefruit and Seville orange juices on the pharmacokinetic properties of colchicine in healthy subjects. Clinical Therapeutics. 2012;34(10):2161–2173. doi: 10.1016/j.clinthera.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Abdelkawy K. S., Donia A. M., Turner R. B., Elbarbry F. Effects of lemon and Seville orange juices on the pharmacokinetic properties of sildenafil in healthy subjects. Drugs in R & D. 2016;16(3):271–278. doi: 10.1007/s40268-016-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Backman J., Maenpaa J., Belle D. J., Wrighton S. A., Kivisto K. T., Neuvonen P. J. Lack of correlation between in vitro and in vivo studies on the effects of tangeretin and tangerine juice on midazolam hydroxylation. Clinical Pharmacology & Therapeutics. 2000;67(4):382–390. doi: 10.1067/mcp.2000.105756. [DOI] [PubMed] [Google Scholar]
- 79.Chen K.-H., Weng M.-S., Lin J.-K. Tangeretin suppresses IL-1β-induced cyclooxygenase (COX)-2 expression through inhibition of p38 MAPK, JNK, and AKT activation in human lung carcinoma cells. Biochemical Pharmacology. 2007;73(2):215–227. doi: 10.1016/j.bcp.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 80.Zhu W. B., Xiao N., Liu X. J. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncology Letters. 2018;15(1):433–440. doi: 10.3892/ol.2017.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishii K., Tanaka S., Kagami K., et al. Effects of naturally occurring polymethyoxyflavonoids on cell growth, p-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia cells. Cancer Investigation. 2010;28(3):220–229. doi: 10.3109/07357900902744486. [DOI] [PubMed] [Google Scholar]
- 82.Ikegawa T., Ushigome F., Koyabu N., et al. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Letters. 2000;160(1):21–28. doi: 10.1016/s0304-3835(00)00549-8. [DOI] [PubMed] [Google Scholar]
- 83.Satoh Y., Tashiro S., Satoh M., Fujimoto Y., Xu J.-Y., Ikekawa T. Studies on the bioactive constituents of aurantii fructus immaturus. Yakugaku Zasshi. 1996;116(3):244–250. doi: 10.1248/yakushi1947.116.3_244. [DOI] [PubMed] [Google Scholar]
- 84.Mak N. K., Wong-Leung Y. L., Chan S. C., Wen J., Leung K. N., Fung M. C. Isolation of anti-leukemia compounds from Citrus reticulata. Life Sciences. 1996;58(15):1269–1276. doi: 10.1016/0024-3205(96)00088-4. [DOI] [PubMed] [Google Scholar]
- 85.Yoon H. S., Ko H. C., Kim S. S., et al. Tangeretin triggers melanogenesis through the activation of melanogenic signaling proteins and sustained extracellular signal- regulated kinase in B16/F10 murine melanoma cells. Natural Product Communications. 2015;10(3):389–392. doi: 10.1177/1934578x1501000304. [DOI] [PubMed] [Google Scholar]
- 86.Yoon J. H., Lim T.-G., Lee K. M., Jeon A. J., Kim S. Y., Lee K. W. Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. Journal of Agricultural and Food Chemistry. 2011;59(1):222–228. doi: 10.1021/jf103204x. [DOI] [PubMed] [Google Scholar]
- 87.Rodriguez J., Yáñez J., Vicente V., et al. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: relationship between structure and activity. Melanoma Research. 2002;12(2):99–107. doi: 10.1097/00008390-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 88.Kandaswami C., Perkins E., Drzewiecki G., Soloniuk D. S., Middleton E., Jr Differential inhibition of proliferation of human squamous cell carcinoma, gliosarcoma and embryonic fibroblast-like lung cells in culture by plant flavonoids. Anti-cancer Drugs. 1992;3(5):525–530. doi: 10.1097/00001813-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 89.Kandaswami C., Perkins E., Soloniuk D. S., Drzewiecki G., Middleton E. Antitproliferative effects of citrus flavonoids on a human squamous cell carcinoma in vitro. Cancer Letters. 1991;56(2):147–152. doi: 10.1016/0304-3835(91)90089-z. [DOI] [PubMed] [Google Scholar]
- 90.Das A., Miller R., Lee P., et al. A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway. Tumor Biology. 2015;36(9):7027–7034. doi: 10.1007/s13277-015-3388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma M., Omar H. R., Ebrahimi G., Campos M. The association between ESR and CRP and systemic hypertension in sarcoidosis. International Journal of Hypertension. 2016;2016:8. doi: 10.1155/2016/2402515.2402515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rooprai H. K., Kandanearatchi A., Maidment S. L., et al. Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathology and Applied Neurobiology. 2001;27(1):29–39. doi: 10.1046/j.0305-1846.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- 93.Arivazhagan L., Pillai S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7, 12-dimethylbenz (α) anthracene-induced rat mammary carcinoma. The Journal of Nutritional Biochemistry. 2014;25(11):1140–1153. doi: 10.1016/j.jnutbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Depypere H. T., Bracke M. E., Boterberg T., et al. Inhibition of tamoxifen’s therapeutic benefit by tangeretin in mammary cancer. European Journal of Cancer. 2000;36(S4) doi: 10.1016/s0959-8049(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 95.Periyasamy K., Sivabalan V., Baskaran K., Kasthuri K., Sakthisekaran D. Cellular metabolic energy modulation by tangeretin in 7, 12-dimethylbenz(a) anthracene-induced breast cancer. Journal of Biomedical Research. 2016;30(2):134–141. doi: 10.7555/JBR.30.20150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chaumontet C., Droumaguet C., Bex V., Heberden C., Gaillard-Sanchez I., Martel P. Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells. Cancer Letter. 1997;114(1-2):207–210. doi: 10.1016/s0304-3835(97)04664-8. [DOI] [PubMed] [Google Scholar]
- 97.Chaumontet C., Suschetet M., Honikman‐Leban E., et al. Lack of tumor‐promoting effects of flavonoids: studies on rat liver preneoplastic foci and onin vivoandin vitrogap junctional intercellular communication. Nutrition and Cancer. 1996;26(3):251–263. doi: 10.1080/01635589609514482. [DOI] [PubMed] [Google Scholar]
- 98.Silva I., Estrada M. F., Pereira C. V., et al. Polymethoxylated flavones from orange peels inhibit cell proliferation in a 3D cell model of human colorectal cancer. Nutrition and Cancer. 2018;70(2):257–266. doi: 10.1080/01635581.2018.1412473. [DOI] [PubMed] [Google Scholar]
- 99.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 100.Stewart J. J., White J. T., Yan X., et al. Proteins associated with Cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Molecular & Cellular Proteomics. 2006;5(3):433–443. doi: 10.1074/mcp.M500140-MCP200. [DOI] [PubMed] [Google Scholar]
- 101.Walle T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Seminars in Cancer Biology. 2007;17(5):354–362. doi: 10.1016/j.semcancer.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao J., Senthil M., Ren B., et al. IRF-1 transcriptionally upregulates PUMA, which mediates the mitochondrial apoptotic pathway in IRF-1-induced apoptosis in cancer cells. Cell Death & Differentiation. 2010;17(4):699–709. doi: 10.1038/cdd.2009.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jiang X.-H., Wong B. C.-Y., Lin M. C.-M., et al. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-κB activation in gastric cancer cells. Oncogene. 2001;20(55):8009–8018. doi: 10.1038/sj.onc.1204981. [DOI] [PubMed] [Google Scholar]
- 104.Goldstein I., Marcel V., Olivier M., Oren M., Rotter V., Hainaut P. Understanding wild-type and mutant p53 activities in human cancer: new landmarks on the way to targeted therapies. Cancer Gene Therapy. 2011;18(1):2–11. doi: 10.1038/cgt.2010.63. [DOI] [PubMed] [Google Scholar]
- 105.Martínez C., Vicente V., Yáñez J, et al. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histology and Histopathology. 2005;20(4):1121–1129. doi: 10.14670/HH-20.1121. [DOI] [PubMed] [Google Scholar]
- 106.Abe S., Fan K., Ho C.-T., Ghai G., Yang K. Chemopreventive effects of orange peel extract (OPE) II. OPE inhibits atypical hyperplastic lesions in rodent mammary gland. Journal of Medicinal Food. 2007;10(1):18–24. doi: 10.1089/jmf.2006.0215. [DOI] [PubMed] [Google Scholar]
- 107.Kurowska E. M., Manthey J. A., Casaschi A., Theriault A. G. Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004;39(2):143–151. doi: 10.1007/s11745-004-1212-8. [DOI] [PubMed] [Google Scholar]
- 108.Maris J. M., Matthay K. K. Molecular biology of neuroblastoma. Journal of Clinical Oncology. 1999;17(7):p. 2264. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 109.Fan K., Kurihara N., Abe S., Ho C.-T., Ghai G., Yang K. Chemopreventive effects of orange peel extract (OPE) I. OPE inhibits intestinal tumor growth in ApcMin/+ mice. Journal of Medicinal Food. 2007;10(1):11–17. doi: 10.1089/jmf.2006.0214. [DOI] [PubMed] [Google Scholar]
- 110.Sharma G., Tyagi A. K., Singh R. P., Chan D. C. F., Agarwal R. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Research and Treatment. 2004;85(1):1–12. doi: 10.1023/B:BREA.0000020991.55659.59. [DOI] [PubMed] [Google Scholar]
- 111.Tyagi A. K., Agarwal C., Chan D. C., Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncology Reports. 2004;11(2):493–499. [PubMed] [Google Scholar]
- 112.Olson R. D., Headley M. B., Hodzic A., Walsh G. M., Wingett D. G. In vitro and in vivo immunosuppressive activity of a novel anthracycline, 13-deoxy, 5-iminodoxorubicin. International Immunopharmacology. 2007;7(6):734–743. doi: 10.1016/j.intimp.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bracke M. E., Depypere H. T., Boterberg T., et al. Influence of tangeretin on tamoxifen’s therapeutic benefit in mammary cancer. JNCI Journal of the National Cancer Institute. 1999;91(4):354–359. doi: 10.1093/jnci/91.4.354. [DOI] [PubMed] [Google Scholar]
- 114.Feng S.-L., Yuan Z.-W., Yao X.-J., et al. Tangeretin, a citrus pentamethoxyflavone, antagonizes ABCB1-mediated multidrug resistance by inhibiting its transport function. Pharmacological Research. 2016;110:193–204. doi: 10.1016/j.phrs.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study. It is a review article.


