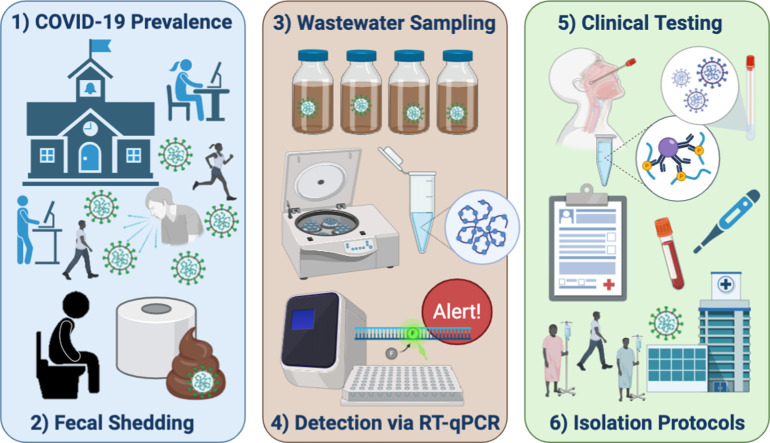
Abstract
Wastewater-based epidemiology has potential as an early-warning tool for determining the presence of COVID-19 in a community. The University of Arizona (UArizona) utilized WBE paired with clinical testing as a surveillance tool to monitor the UArizona community for SARS-CoV-2 in near real-time, as students re-entered campus in the fall. Positive detection of virus RNA in wastewater lead to selected clinical testing, identification, and isolation of three infected individuals (one symptomatic and two asymptomatic) that averted potential disease transmission. This case study demonstrated the value of WBE as a tool to efficiently utilize resources for COVID-19 prevention and response. Thus, WBE coupled with targeted clinical testing was further conducted on 13 dorms during the course of the Fall semester (Table 3). In total, 91 wastewater samples resulted in positive detection of SARS-CoV-2 RNA that successfully provided an early-warning for at least a single new reported case of infection (positive clinical test) among the residents living in the dorm. Overall, WBE proved to be an accurate diagnostic for new cases of COVID-19 with an 82.0% positive predictive value and an 88.9% negative predictive value. Increases in positive wastewater samples and clinical tests were noted following holiday-related activities. However, shelter-in-place policies proved to be effective in reducing the number of daily reported positive wastewater and clinical tests. This case study provides evidence for WBE paired with clinical testing and public health interventions to effectively contain potential outbreaks of COVID-19 in defined communities.
Keywords: Wastewater surveillance, Outbreak prevention, Dormitory, Sewer manhole, University campus
Graphical abstract
1. Introduction
As of February 2021, more than one-hundred million confirmed cases worldwide of the novel Coronavirus Disease, COVID-19 have been reported to the World Health Organization (World Health Organization (WHO), 2020). Individuals infected by the causative agent of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), may develop fever, cough and shortness of breath 2–14 d after exposure, followed by high viral shedding in bodily fluids (Long et al., 2020). Viral shedding by asymptomatic or mild cases of COVID-19 are also common (Gao et al., 2020) with implications in the transmissibility and global burden of COVID-19 (Davies et al., 2020; Widders et al., 2020; Zhao et al., 2020). In particular, asymptomatic transmission of SARS-CoV-2 challenges contact tracing efforts (Peirlinck et al., 2020), although pre-symptomatic transmission has been documented through contact tracing efforts (He et al., 2020; Kimball et al., 2020; Yu et al., 2020). Consequently, infectious disease surveillance such as wastewater-based epidemiology (WBE), provides a response to emerging infections, but containment of COVID-19 has remained a significant challenge (Daughton, 2020; Knoll et al., 2020; Wee et al., 2020).
Initially implemented to detect poliovirus reemergence (Hovi et al., 2001; Manor et al., 1999), WBE utilizes concentrations of SARS-CoV-2 in sewage to monitor population-level COVID-19 cases (Bivins et al., 2020; Daughton, 2018; Sinclair et al., 2008). Currently, WBE is a promising leading indicator to support public health decisions (Bivins et al., 2020; Medema et al., 2020a). Here, WBE was used to detect the presence of COVID-19 in a student dormitory (henceforth Dorm A) at the University of Arizona (UArizona) and was used as the foundation of a strategy to contain potential outbreaks of disease following student re-entry onto campus for the Fall semester of 2020. Upon positive detection of viral RNA in wastewater samples, clinical testing was conducted on every individual living in the dorm. Testing the wastewaters also allowed for evaluation of the effects of an intervention introduced onto the university community, mid-semester, namely a “shelter in place” order imposed on the students.
2. Materials and methods
2.1. Wastewater sampling
On March 13, 2020, following the outbreak of the Coronavirus pandemic, UArizona advised students and employees to work remotely. During the summer, UArizona administration assembled a Task Force and Campus Re-Entry Working Groups to prepare for students' safe return. Seven expert teams were created, consisting of COVID-19 clinical testing: thermometry and wellness checks' contact tracing’ healthcare and guidance’ isolation’ data platforms and communication’ and WBE. Utilization of WBE in conjunction with clinical testing was deemed to be critical for the early detection of infections in student dorms.
The WBE expert team hypothesized that surveillance of defined communities in dorms would provide an effective means for identifying new cases of COVID-19 since: 1) each dorm contained a known population; 2) dorm students provide a representation of the overall status of campus health; 3) wastewater samples could be collected from individual buildings; and 4) actionable public health responses could be initiated in the event of positive wastewater detection. Wastewater samples were collected from sewer manholes downstream from each dorm prior to convergence or mixing with other sewer lines, resulting in samples specific to individual buildings with defined communities.
Wastewater samples were collected from a sewer manhole specific to Dorm A, without convergence or mixing from other sewer lines. Grab samples (1 L) were collected from the manhole using a pole/dipper and submerging a sterile Nalgene bottle into the flowing wastewater until it was full. Samples were collected at 8:00 am on August 18 and 20 during the week that students moved into the dorm. Daily samples were collected from August 25–31 to monitor SARS-CoV-2 RNA in wastewater during the first week of classes. On August 26, five samples were collected five minutes apart between 8:30–8:50 am when peak flow occurred indicating maximum restroom usage to determine sample variation during sample collection. On August 27, two more samples were collected from Dorm A, one in the morning (9:30 am) and afternoon (1:15 pm) to ensure the presence or absence of the virus at different times of the day.
Wastewater samples were also collected twice-per-week from sewer manholes to monitor 13 dorms throughout the Fall semester (August 24 to November 20, 2020). Each wastewater sample was collected from a manhole specific to an individual dorm; thus, all wastewater samples were specific to defined communities living in each dorm. There is also the possibility of a guest or non-resident employee contributing to the wastewater, but this occurrence is unlikely early in morning. All samples were transported in a cooler containing ice to the laboratory for immediate processing.
2.2. Virus concentration and recovery
The method for recovery and concentration of SARS-CoV-2 from wastewater was validated and standardized using human coronavirus 229E (HCoV 229E, ATCC VR-740) as a surrogate. Briefly, the initial virus concentration involved stepwise vacuum filtration of 70 mL aliquots through membrane filters of 0.8, 0.65, 0.45 and 0.22 μm pore sizes (EMD Millipore, Billerica, MA) followed by centrifugal ultrafiltration of the filtrate using the CentriconPlus-70 filter, 100 kDa cutoff (EMD Millipore, Billerica, MA). The final concentrate sample volume (0.2 mL to 0.4 mL) was used for RNA extraction as described below.
Matrix spikes were used to evaluate the performance and recovery efficiency of the method for concentration of enveloped viruses from wastewater samples from UArizona dormitories. The surrogate HCoV 229 E was spiked in dormitory sewage at concentrations between 4.64 × 104 to 4.43 × 106 genome copies per mL of wastewater (GC/mL). Aliquots of 0.5 L of raw sewage were spiked and processed 30 min later, following the method described above. Recovery efficiencies (Y) of HcoV 229 E were calculated as follows:
Y: Recovery yield of the concentration method
X: Recovery HCoV-229 E copy number (copies/mL detected vs copies/mL added)
Co: Stock HCoV-229 E copy number added into test water (copies/μL)
V: Added stock HCoV 229 E volume (μL)
It is important to note that matrix spikes were included only in a baseline study in order to evaluate the recovery efficiency of the method. The concentrations of SARS-CoV-2 RNA were not adjusted to the estimated recovery efficiencies.
2.3. Virus detection and quantification
The nucleic acid of both SARS-CoV-2 and HCoV 229 E was extracted from the final concentrate sample volume (0.2 mL) using the QIAGEN QIAmp Viral Mini Kit (Qiagen, Valencia, CA) following the manufacturer's instructions using 2 × 40 μL Buffer AVE for elution of RNA. cDNA synthesis was performed from the extracted RNA using the SuperScript® IV First-Strand Synthesis reverse transcription kit (Invitrogen, Carlsbad, CA) with random hexamers following manufacturer's instructions. Samples were assayed for SARS-CoV-2 using the RT-PCR assays manufactured at Integrated DNA Technologies (IDT, Coralville, IA) for research use only (RUO). RUO kits include all published assays for the nucleocapsid genes N1 and N2 developed by the Centers for Disease Control and Prevention (Table S1). Similarly, samples were assayed for HCoV 229 E using a real-time quantitative reverse transcriptase PCR (RT-qPCR) assay previously developed for the rapid detection and quantitation of this virus in clinical samples (Vijgen et al., 2005).
Real-Time RT-PCR assays were performed using the LightCycler® 480 Instrument II (Roche Diagnostics, Indianapolis, IN). For SARS-CoV-2, reaction mixtures (25 μL) contained 12.5 μL of LightCycler 480 Probes Master (Roche Diagnostics), primers at a final concentration of 500 nM and probes at 125 nM, nuclease-free water plus 5 μL of template cDNA. Cycling conditions included a pre-incubation step of 10 min at 95 °C for activation of FastStar Taq DNA polymerase followed by 45 cycles of amplification (5 s at 95 °C and 30 s at 55 °C). For HCoV 229 E, reaction mixtures (25 μL) contained 12.5 μL of LightCycler 480 Probes Master (Roche Diagnostics) primers (300 nM) and probe (200 nM), nuclease-free water (5 μL) plus 5 μL of viral cDNA. Cycling conditions included activation of DNA polymerase (10 min at 95 °C) and 45 cycles of amplification (15 s at 95 °C and 1 min at 60 °C). Fluorescence data were collected after every cycle and analyzed with LightCycler® 480 Software version 1.5.1.6.2 (Roche Diagnostics). Primers and probes used for detection and quantitative estimation of SARS-CoV-2 RNA and HCoV 229E RNA in wastewater sample concentrates are described in Table S1.
For quantification of SARS-CoV-2 and HCoV-229 E, standard curves were generated based on IDT reported titers, using ten-fold dilutions of a plasmid control from 2 × 105 copies/reaction to 2 × 100 copies/reaction containing the complete nucleocapsid gene from 2019-nCoV (IDT, Coralville, IA) and a gBlock gene fragment (3.82 × 106 copies/reaction to 3.82 × 100 copies/reaction, IDT, Coralville, IA).The Roche system was used based on second-derivative Cq determination and nonlinear fit algorithms. Limits of blank, detection, and quantification for the RT-qPCR assays were determined following standard procedures previously described (Francy et al., 2012) and currently in use in our laboratory. Limit of blank (LoB) are defined as the lowest concentration that can be reported with 95% confidence to be above the concentrations of blanks. The LoB was used to determine the most accurate limit of detection based on 100 reactions. It is the highest apparent concentration expected to be found when replicates of a blank are tested and is determined by calculating the 95th percentile of the Cq values for all the blanks (reagent water containing no target material) for a specific target (CqLoB). This includes the Cq values for no-template controls, extraction blanks, and filter blanks. Limit of detection (LoD) is defined as the lowest concentration that can be detected with 95% confidence that it is a true detection and can be distinguished from the LoB. The LoD was determined by running a series of dilutions of the target with a minimum of 10 replicates per dilution. The dilution with the lowest concentration of known target that met the following requirements was chosen as the LoD: 1) the standard deviation (in Cq values) of the replicates was less than one and 2) all replicates were positive (> LoD). The average Cq value (CqLoD) for this dilution was used to calculate a concentration (copies/rxn) using the standard curve run with the dilution series. Limit of quantification (LoQ) is defined as the lowest concentration that can be accurately quantified. The LoQ was determined using the CqLoD and the standard deviation of CqLoD as previously defined. A Cq value for the LoQ (CqLoQ) was calculated as follows:
where σCqLoD is the standard deviation of the CqLoD for this assay. This CqLoQ was used to calculate a concentration (copies/rxn) using the standard curve run with the dilution series. A summary of the performance of the standard curves for each assay is given in Table S2.
2.4. Clinical testing and public health protocols
UArizona performs two clinical tests for COVID-19 diagnosis: antigen (Sofia SARS Antigen FIA, Quidel, San Diego, CA, USA) via anterior nasal swab and RT-PCR (CDC 2019-nCoV RT-PCR Diagnostic Panel) (Centers for Disease Control and Prevention Division of Viral Diseases, 2020) via nasopharyngeal swab samples. These tests were developed and analytical performance characteristics have been determined by the University of Arizona Genetics Core for Clinical Services. This assay was developed as a Laboratory-Developed Test and has been validated pursuant to the CLIA regulations and is used for clinical purposes. These tests are not yet approved or cleared by the United States Food and Drug Administration (FDA).
Upon arriving on campus, every individual was required to report to a designated COVID-19 testing site and undergo an anterior nasal swab for antigen testing. Individuals were kept on-site until tests results were noted. Each individual was required to test negative before receiving access to the dorm and campus buildings. If a person had a positive COVID-19 test, they were required to isolate for a minimum of 10 days (at home or in a designated isolation dorm) from the onset of symptoms or from the date the sample was taken, per guidelines from the United States Center for Disease Control and Prevention (CDC) (Centers for Disease Control and Prevention (CDC), 2020). If the individual remains symptom-free after 10 days, they can be cleared to return to the dorm; however, if symptoms persist they may be kept in isolation longer.
While living in the dorm, individuals were subject to clinical testing via two routes: Campus Health Services (CHS) or Test All Test Smart (TATS). CHS testing was conducted only on individuals that were experiencing symptoms and reported to the health services building for clinical testing and diagnosis. TATS testing was conducted on every individual living in the dorm upon positive detection of SARS-CoV-2 in wastewater that suggested prevalence of disease among persons in the dorm. Individuals were excluded from TATS testing if they had already reported to CHS for testing on the same day or had proof of recently returning from isolation and no longer experiencing symptoms. In essence, CHS tested individuals that were symptomatic, and TATS tested individuals that were asymptomatic or had not yet reported symptoms. CHS and TATS both utilized anterior nasal swab samples for antigen and/or PCR tests.
3. Results and discussion
The matrix spike assays yield an average recovery of 14 ± 16% which indicated low and highly variable efficiencies of recovery of HCoV 229 E in wastewater as observed in studies with other coronaviruses used for the same purpose (Medema et al., 2020b; Randazzo et al., 2020). Low recoveries of enveloped and non-enveloped viruses in wastewater and variations in the efficiency of the methods are predominantly associated with the complexity of this environmental matrix (Hellmér et al., 2014; Michael-Kordatou et al., 2020).
Upon positive detection of viral RNA in wastewater samples, targeted clinical testing was conducted on residents living within dorms. UArizona performed two clinical testing modalities, antigen (1-hour turnaround) test via anterior nasal swab and RT-PCR (48–72 h turnaround) via nasopharyngeal swab samples. Individuals were subject to clinical testing via CHS and/or TATS throughout the semester.
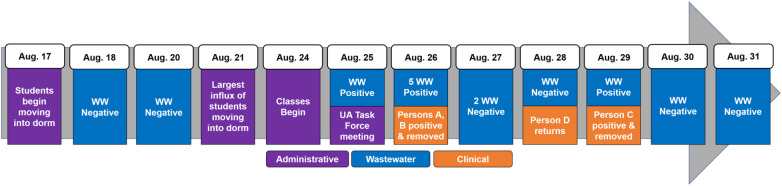
A baseline survey of SARS-CoV-2 RNA in wastewater was conducted prior to the fall semester via wastewater sample collection from multiple dorms between August 18–20, 2020, when some students had already returned for fall semester. No SARS-CoV-2 RNA was detected in these samples. Negative results corresponded with the requirement that all students were determined negative prior to dorm entry. The largest number of students that moved into campus housing occurred on August 21, three days before the start of classes on August 24 (Fig. 1 ). On August 25, wastewater from Dorm A tested positive for SARS-CoV-2 N1 gene (1.61 × 105 copies/L) (Table 1 ). This positive sample triggered an emergency UA Task Force meeting, which supported additional wastewater sampling and clinical testing among Dorm A residents (Fig. 1).
Fig. 1.
Timeline of events at Dorm A. Legend: Dates (left to right) and events (top to bottom) are listed in chronological order. WW = wastewater.
Table 1.
Wastewater surveillance from manhole samples at Dorm A.
| Date | Time | N1 (copies/L) | N2 (copies/L) |
|---|---|---|---|
| Aug 18 | 8:00 am | Non-detect | Non-detect |
| Aug 20 | 8:00 am | Non-detect | Non-detect |
| Aug 25 | 8:30 am | Non-detect | 1.61 × 105 |
| Aug 26 | 8:30 am | 3.84 × 105 | 1.06 × 106 |
| 8:35 am | 3.74 × 105 | 1.06 × 106 | |
| 8:40 am | Non-detect | 1.06 × 106 | |
| 8:45 am | 1.73 × 105 | 1.06 × 106 | |
| 8:50 am | 3.77 × 105 | 1.06 × 106 | |
| Aug 27 | 9:30 am | Non-detect | Non-detect |
| 1:15 pm | Non-detect | Non-detect | |
| Aug 28 | 9:05 am | Non-detect | Non-detect |
| Aug 29 | 8:00 am | 1.00 × 104 | 9.93 × 105 |
| Aug 30 | 9:15 am | Non-detect | Non-detect |
| Aug 31 | 9:30 am | Non-detect | Non-detect |
Non-detect = below Limit of Detection (LoD).
The next day (August 26, 2020), five wastewater samples were collected once every 5 min between 8:30–8:50 am. Importantly, all five samples yielded virtually identical SARS-CoV-2 concentrations (Table 1), even though an estimated 1000 gal of wastewater had flowed through the sewer during the collection period. This validated the presence for SARS-CoV-2 in the wastewater samples that were representative of the resident population living in the dorm. This also confirmed that SARS-CoV-2 surveillance could be conducted through monitoring sewer systems, which has been utilized for other pathogens (Manor et al., 2014; Sinclair et al., 2008). That same day, 270 antigen tests and 260 PCR tests were conducted on-site at Dorm A (via TATS) among the 311 total residents (Table 2 ). Antigen testing identified one positive individual (Person A) despite demonstrating no symptoms. The other 269 antigen tests were negative. Simultaneously, an additional individual reported experiencing symptoms (Person B) to CHS and tested positive via an antigen test. Person A and Person B were immediately relocated into an isolation facility to prevent viral transmission. One PCR test was inconclusive (Person C) as the Cq was near 40 and retested per CDC guidelines (Centers for Disease Control and Prevention Division of Viral Diseases, 2020). Results of this retest were not available until August 29.
Table 2.
COVID-19 clinical testing results for persons living in Dorm A.
| Date | CHS antigen | CHS PCR | TATS antigen | TATS PCR | Total positive |
|---|---|---|---|---|---|
| Aug 24 | – | 0/1 | – | – | 0 |
| Aug 25 | 0/1 | – | 0/2 | – | 0 |
| Aug 26 | 1/4 | 1/4 | 1/270 | 2/260 | 3 |
| Aug. 27 | 0/1 | 0/1 | 0/26 | 0/21 | 0 |
| Aug 28 | 0/2 | 0/2 | 0/5 | – | 0 |
| Aug 29 | – | – | – | – | 0 |
| Aug 30 | – | – | – | – | 0 |
| Aug 31 | 0/2 | 0/1 | – | – | 0 |
No clinical tests were performed at the dorm prior to August 24, since all individuals tested negative at a designated testing site on-campus prior to entering the dormitory between August 17 and 23. CHS = campus health service; TATS = Test All Test Smart. Dash line (−), no tests conducted. Numerator is number of positive tests. Denominator is total number of tests conducted. On August 28, one individual returned to the dorm after completing isolation protocols.
Over the next two days, antigen tests (34 individuals) and PCR tests (24 individuals) were conducted among individuals not tested on August 26. All tests were negative (Table 2); corresponding wastewater samples from August 27 and 28 were also negative (Table 1), indicating that the source(s) for SARS-CoV-2 had likely been removed from the dorm. However, wastewater analysis on August 29 was positive for both N1 (1.04 × 104 copies/L) and N2 (9.93 × 105 copies/L) genes (Table 1). From the TATS samples conducted on August 26, PCR results were positive for two tests three days later (Table 2), one of which was collected from Person A, who was isolated contemporarily. The other positive result confirmed Person C, who previously had inconclusive results. Person C was immediately relocated into isolation despite being asymptomatic.
Interestingly, SARS-CoV-2 RNA was not detected in wastewater on August 27 and 28 while Person C lived in the dorm. Reports have suggested that approximately 50% of COVID-19 patients shed virus in feces (Medema et al., 2020a). Therefore, it is possible that Person C was not shedding virus or had low viral shedding on August 27 and 28. The low viral shedding load justification is supported by the fact that 40 cycles of PCR (Cq = 40) were required for the positive PCR result, which suggested trace amounts of SARS-CoV-2 (Service, 2020). Recent studies have also observed that viral loads from stool samples follow a more erratic pattern than viral loads from the upper respiratory tract (Walsh et al., 2020; Zheng et al., 2020). Importantly, on August 28, another person who was previously infected, Person D, returned to the dorm after following isolation protocols. Reports suggest that viral shedding from the upper respiratory tract may peak in the first week of infection (Cevik et al., 2020); however, low viral shedding in feces can continue for over two weeks after symptoms have ceased (Chen et al., 2020; Park et al., 2020; Walsh et al., 2020; Wang et al., 2020; Zheng et al., 2020). On August 29, viral RNA was detected in wastewater from Dorm A. This positive result was likely due to combined shedding from Person C and Person D. The removal of Person C on August 29 corresponded to lower shedding loads, and ultimately, negative wastewater samples during the following days. Due to negative wastewater samples on August 30 and 31 (Fig. 1; Table 1), no further clinical testing was performed on residents in Dorm A during this timeframe.
The successful use of WBE coupled with targeted clinical testing in Dorm A provided confidence for an early-warning system that can avert COVID-19 outbreaks. Therefore, this program was expanded to monitor 13 dorms during the course of the semester. Specifically, 91 out of 111 (82.0% positive predictive value) positive wastewater samples successfully provided a warning that at least one individual living in the dorm was positive with a SARS-CoV-2 infection (Table 3 ); results were confirmed via a positive clinical test within 4 days of the wastewater sample. This corroborates with a recent report that suggests peak shedding occurs within the first week of illness (Cevik et al., 2020). Also, 185 out of 208 (88.9% negative predictive value) negative wastewater samples concurred with no new positive individuals on dormitory screenings (Table 3). Only 43 out of 319 total wastewater samples were discordant with results from clinical tests (Table 3). Positive wastewater samples not followed with positive clinical tests could be due to infected visitors who used the dorm restroom(s) but were not residents. In addition, on few occasions less than 100% of the dorm residents were tested, due to being out of the building at the time of clinical testing or other reasons.
Table 3.
WBE accuracy as an early-warning diagnostic for new cases of COVID-19.
| Clinical |
|||
|---|---|---|---|
| Positive | Negative | ||
| Wastewater | Positive | 79 | 20 |
| Negative | 25 | 195 | |
Sensitivity (76.0%).
Specificity (90.7%).
Positive predictive value (79.8%).
Negative predictive value (88.6%).
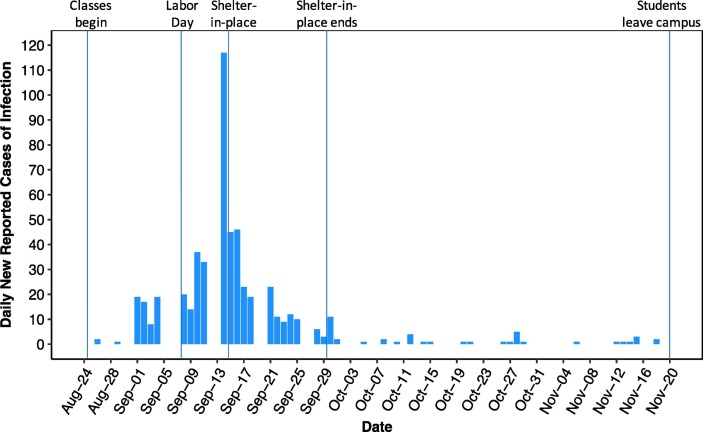
The benefits of the containment strategy are clearly illustrated by Fig. 2 . At the beginning of the semester there were no infections in the monitored dorms. Following campus re-entry, there were clusters of cases during the first 18 days of the semester. This was followed by a dramatic spike in cases beginning September 14, likely due to student behavior around the Labor Day holiday on September 7. Infections resulting from the holiday-related activities would be reflected in new cases being detected likely about five days thereafter (Lauer et al., 2020). Following the spike, the president of UArizona, endorsed a “shelter in place” policy imposed by Pima County on September 15–29, 2020. The effectiveness of this intervention resulted in a dramatic decrease in the number of daily new-reported cases by September 21 (Fig. 2). The decrease in new cases was associated with decreased viral concentrations in the wastewater over this period. Following this intervention, case counts remained remarkably low, and often zero for the rest of September, October, and into November. The vast majority of students left campus the Friday before Thanksgiving, November 20, and did not return to campus. Therefore, there are no WBE results to report for the month of December as the final weeks of courses and exams were conducted remotely online.
Fig. 2.
Daily new-reported cases of SARS-CoV-2 infections in the 13 dorms.
4. Conclusions
The combined strategy of utilizing WBE coupled with targeted clinical testing was critical in COVID-19 containment. WBE strategies averted potential transmission from at least three students, which allowed the university to remain open, and even establish limited in-person classes for students. The data from this study provide evidence that gatherings of students, typified by the surge of cases after Labor Day, may have resulted in increased COVID-19 incidence. It should be noted that findings validate WBE as an important tool that can and should be used in tandem with diagnostics and infection prevention practices to intervene in COVID-19 transmission, and ultimately save lives.
CRediT authorship contribution statement
Walter W. Betancourt: Methodology, Supervision, Writing – original draft. Bradley W. Schmitz: Formal analysis, Investigation, Writing – original draft. Gabriel K. Innes: Formal analysis, Writing – reviewing and editing. Kristen M. Pogreba Brown: Investigation, Resource. Sarah M. Prasek: Formal analysis, Resource. Erika R. Stark: Resource. Aidan R. Foster: Resource. Ryan S. Sprissler: Resource. David T. Harris: Resource. Samendra P. Sherchan: Resource, Writing – reviewing & editing. Charles P. Gerba: Resource. Ian L. Pepper: Formal analysis, Funding acquisition, Project administration, Visualization, Supervision, Investigation, Writing – original draft.
Walter Betancourt and Bradley Schmitz are co-first authors and contributed equally.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank the UArizona Task Force, Amy Glicken, and Jeff Bliznick for their contributions.
Funding
Financial support for the study was provided by the University of Arizona Campus Re-Entry Task Force.
Disclaimers
The IRB at the University of Arizona reviewed the study and verified that all data was de-identified and complied with the Human Subjects Protection Program.
Editor: Adrian Covaci
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.146408.
Appendix A. Supplementary data
Supplementary tables
References
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., De Los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., Van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., Van Der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2020. Duration of Isolation and Precautions for Adults With COVID-19 | CDC [WWW Document]. URL https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html (accessed 11.11.20).
- Centers for Disease Control and Prevention Division of Viral Diseases . 2020. CDC 2019-Novel Coronavirus (2019-nCoV) Real-time RT-PCR Diagnostic Panel. (CDC EUA) [Google Scholar]
- Cevik M., Tate M., Lloyd O., Maraolo A.E., Schafers J., Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. The Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Monitoring wastewater for assessing community health: sewage chemical-information mining (SCIM) Sci. Total Environ. 2018 doi: 10.1016/j.scitotenv.2017.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Pearson C.A.B., Quilty B.J., Kucharski A.J., Gibbs H., Clifford S., Gimma A., van Zandvoort K., Munday J.D., Diamond C., Edmunds W.J., Houben R.M.G.J., Hellewell J., Russell T.W., Abbott S., Funk S., Bosse N.I., Sun Y.F., Flasche S., Rosello A., Jarvis C.I., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020 doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- Francy D.S., Stelzer E.A., Bushon R.N., Brady A.M.G., Williston A.G., Riddell K.R., Borchardt M.A., Spencer S.K., Gellner T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 2012 doi: 10.1016/j.watres.2012.04.044. [DOI] [PubMed] [Google Scholar]
- Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., Ma K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis a virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014 doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovi T., Stenvik M., Partanen H., Kangas A. Poliovirus surveillance by examining sewage specimens. Quantitative recovery of virus after introduction into sewerage at remote upstream location. Epidemiol. Infect. 2001 doi: 10.1017/S0950268801005787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball, A., Hatfield, K.M., Arons, M., James, A., Taylor, J., Spicer, K., Bardossy, A.C., Oakley, L.P., Tanwar, S., Chisty, Z., Bell, J.M., Methner, M., Harney, J., Jacobs, J.R., Carlson, C.M., McLaughlin, H.P., Stone, N., Clark, S., Brostrom-Smith, C., Page, L.C., Kay, M., Lewis, J., Russell, D., Hiatt, B., Gant, J., Duchin, J.S., Clark, T.A., Honein, M.A., Reddy, S.C., Jernigan, J.A., Baer, A., Barnard, L.M., Benoliel, E., Fagalde, M.S., Ferro, J., Smith, H.G., Gonzales, E., Hatley, N., Hatt, G., Hope, M., Huntington-Frazier, M., Kawakami, V., Lenahan, J.L., Lukoff, M.D., Maier, E.B., McKeirnan, S., Montgomery, P., Morgan, J.L., Mummert, L.A., Pogosjans, S., Riedo, F.X., Schwarcz, L., Smith, D., Stearns, S., Sykes, K.J., Whitney, H., Ali, H., Banks, M., Balajee, A., Chow, E.J., Cooper, B., Currie, D.W., Dyal, J., Healy, J., Hughes, M., McMichael, T.M., Nolen, L., Olson, C., Rao, A.K., Schmit, K., Schwartz, N.G., Tobolowsky, F., Zacks, R., Zane, S., 2020. Asymptomatic and Presymptomatic SARS-CoV-2 infections in residents of a Long-term care skilled nursing facility — King County, Washington, March 2020. MMWR. Morb. Mortal. Wkly. Rep. 69, 377–381. doi:10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed]
- Knoll R.L., Klopp J., Bonewitz G., Gröndahl B., Hilbert K., Kohnen W., Weise K., Plachter B., Hitzler W., Kowalzik F., Runkel S., Zepp F., Winter J., Cacicedo M.L., Gehring S. Containment of a large SARS-CoV-2 outbreak among healthcare workers in a pediatric intensive care unit. Pediatr. Infect. Dis. J. 2020 doi: 10.1097/INF.0000000000002866. [DOI] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Manor Y., Handsher R., Halmut T., Neuman M., Bobrov A., Rudich H., Vonsover A., Shulman L., Kew O., Mendelson E. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian authority. J. Clin. Microbiol. 1999 doi: 10.1128/jcm.37.6.1670-1675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor Y., Shulman L.M., Kaliner E., Hindiyeh M., Ram D., Sofer D., Moran-Gilad J., Lev B., Grotto I., Gamzu R., Mendelson E. Intensified environmental surveillance supporting the response to wild poliovirus type 1 silent circulation in Israel, 2013. Eurosurveillance. 2014 doi: 10.2807/1560-7917.ES2014.19.7.20708. [DOI] [PubMed] [Google Scholar]
- Medema G., Been F., Heijnen L., Petterson S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: opportunities and challenges. Curr. Opin. Environ. Sci. Heal. 2020 doi: 10.1016/j.coesh.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020 doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Lee C.-W., Park D.-I., Woo H.-Y., Cheong H.S., Shin H.C., Ahn K., Kwon M.-J., Joo E.-J. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirlinck M., Linka K., Sahli Costabal F., Bhattacharya J., Bendavid E., Ioannidis J.P.A., Kuhl E. Visualizing the invisible: the effect of asymptomatic transmission on the outbreak dynamics of COVID-19. Comput. Methods Appl. Mech. Eng. 2020 doi: 10.1016/j.cma.2020.113410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service, R.F., 2020. A call for diagnostic tests to report viral load. Science (80-. ). doi: 10.1126/science.370.6512.22. [DOI] [PubMed]
- Sinclair R.G., Choi C.Y., Riley M.R., Gerba C.P. Pathogen surveillance through monitoring of sewer systems. Adv. Appl. Microbiol. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Moës E., Maes P., Duson G., Van Ranst M. Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. J. Clin. Microbiol. 2005 doi: 10.1128/JCM.43.11.5452-5456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., Ahern S., Carty P.G., O’Brien K.K., O’Murchu E., O’Neill M., Smith S.M., Ryan M., Harrington P. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zheng J., Guo L., Yao H., Wang L., Xia X.D., Zhang W. Fecal viral shedding in COVID-19 patients: clinical significance, viral load dynamics and survival analysis. Virus Res. 2020 doi: 10.1016/j.virusres.2020.198147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Goh J.Q., Yeo D.W.T., Gan W.H., Chua Y.Y., Wijaya L., Tan T.T., Tan B.H., Ling M.L., Venkatachalam I. Containment of COVID-19 cases among healthcare workers: the role of surveillance, early detection, and outbreak management. Infect. Control Hosp. Epidemiol. 2020 doi: 10.1017/ice.2020.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widders A., Broom A., Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect. Dis. Heal. 2020 doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2020. WHO Coronavirus Disease (COVID-19) Dashboard [WWW Document]. URL https://covid19.who.int/ (accessed 12.12.20).
- Yu P., Zhu J., Zhang Z., Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.J., Lu X.X., Deng Y. Bin, Tang Y.J., Lu J.C. COVID-19: asymptomatic carrier transmission is an underestimated problem. Epidemiol. Infect. 2020 doi: 10.1017/S0950268820001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables