Abstract
Wnt signaling plays a critical role in craniofacial patterning, as well as tooth and bone development. Rspo2 and Rspo3 are key regulators of Wnt signaling. However, their coordinated function and relative requirement in craniofacial development and odontogensis are poorly understood. We showed that in zebrafish rspo2 and rspo3 are both expressed in osteoprogenitors in the embryonic craniofacial skeleton. This is in contrast to mouse development, where Rspo3 is expressed in osteoprogenitors while Rspo2 expression is not observed. In zebrafish, rspo2 and rspo3 are broadly expressed in the pulp, odontoblasts and epithelial crypts. However, in the developing molars of the mouse, Rspo3 is largely expressed in the dental follicle and alveolar mesenchyme while Rspo2 expression is restricted to the tooth germ. While Rspo3 ablation in the mouse is embryonic lethal, zebrafish rspo3-/- mutants are viable with modest decrease in Meckel’s cartilage rostral length. However, compound disruption of rspo3 and rspo2 revealed synergistic roles of these genes in cartilage morphogenesis, fin development, and pharyngeal tooth development. Adult rspo3−/− zebrafish mutants exhibit a dysmorphic cranial skeleton and decreased average tooth number. This study highlights the differential functions of Rspo2 and Rspo3 in dentocranial morphogenesis in zebrafish and in mouse.
Subject terms: Model vertebrates, Gene expression analysis
Introduction
The Wnt signaling pathway plays a major role in skeletal patterning and differentiation during embryonic development, and in maintaining postnatal bone homeostasis1–3. Impairment and potentiation of Wnt signaling affects overall bone mass and density1–3. Canonical β-catenin mediated Wnt signaling directly regulates osteoblast differentiation and activity and likely has indirect effects on osteoclasts during bone metabolism4. Moreover, a study reported the direct negative influence of canonical Wnt/β-catenin signaling on osteoclast development using in vitro cell models and in vivo mouse studies5. During embryogenesis, canonical Wnt/β-catenin signaling is highly active in the first pharyngeal arch as well as in multiple craniofacial regions in mouse, chicken and zebrafish6–8. Wnt signaling is involved in regulating skeletogenic neural crest cells, such as the subdivision of each pharyngeal arch into dorsal and ventral elements in zebrafish during craniofacial development9. In addition, Wnt signaling plays a role in mediating regional specification in the vertebrate face7. The identification of modulators of Wnt signaling during development and homeostasis of adult skeletal tissues may lead to new insights into disease etiology and identify potential targets for therapeutic mediation.
Human genome wide association studies revealed many regulators of canonical Wnt signaling that are involved in regulating bone metabolism10,11. The R-spondin (Rspo) family of secreted proteins includes four members (Rspo1-4) in the thrombospondin type 1 repeat (TSR1)-containing protein superfamily that have been shown to potentiate the canonical Wnt/β-catenin pathway12,13. RSPO proteins modulate Wnt signaling through interactions with the LGR4-6 receptors, leading to stabilization of Frizzled and LRP5/6 at the cell membrane, and through regulation of the ubiquitin ligases ZNFR3 and RNF43 that degrade Frizzled receptors13,14. Rspo2 and Rspo3 also have been shown to augment Wnt/β-catenin signaling independent of LGRs by binding to heparin sulfate proteoglycans15. Rspo genes are essential for normal development and have been shown to regulate skeletal patterning during development16. In particular, Rspo2 has been shown to be essential for limb patterning17. Additionally, several GWAS conducted in humans have associated RSPO2 and RSPO3 with bone mineral density10,18.
Rspo3 was identified as a candidate gene that contributes to cleft lip/palate and dental anomalies19. Rspo3 was also reported to have a critical role in mouse placental development17. However, since mouse embryos lacking Rspo3 function die at E10.5 due to placenta and vascular defects, this precluded analysis of its role during later embryonic development17. Conditional ablation of the Rspo3 in limb mesenchymal cells caused modest delay in limb growth during development20. Rspo3 and Rspo2 double mutant mice however developed severe hindlimb truncations, suggesting a redundant function of these genes20. The function of Rspo3 during craniofacial morphogenesis has yet to be defined21.
Wnt/β-catenin pathway also plays a critical role in tooth development and can affect craniofacial development more broadly22,23. Tooth formation initiates from the interactions between the dental epithelial layer and the underlying mesenchyme24,25. Mice have a single set of dentition (monophyodont) that consists of continuously erupting incisors, and three molars in single row on both sides of the upper and lower jaws that do not exhibit continuous growth or replacement26,27. The zebrafish dentition is more numerous, unlike in the mouse, zebrafish teeth exhibit continuous replacement throughout life (polyphyodont)28. Despite these differences, the molecular and cellular mechanisms regulating tooth development are highly conserved between zebrafish and mammals28. Therefore, studies in zebrafish can provide novel insights into the regulation of craniofacial structures that can complement the mouse.
Here, we focused on the roles of Rspo2 and Rspo3 in regulating dental and craniofacial development. We utilized RNAscope probes to gain high resolution images of Rspo2 and Rspo3 gene expression in zebrafish and mouse. We examined the genetic requirement of rspo2 and rspo3 in zebrafish development, using complementary CRISPR/Cas9-mediated targeted mutagenesis. Using these approaches, we revealed roles for rspo2 and rspo3 in tooth development and in morphogenesis of the craniofacial complex.
Results
Rspo2 and Rspo3 are expressed in the craniofacial complex and in the perichondrium and osteoprogenitor cells during zebrafish craniofacial morphogenesis
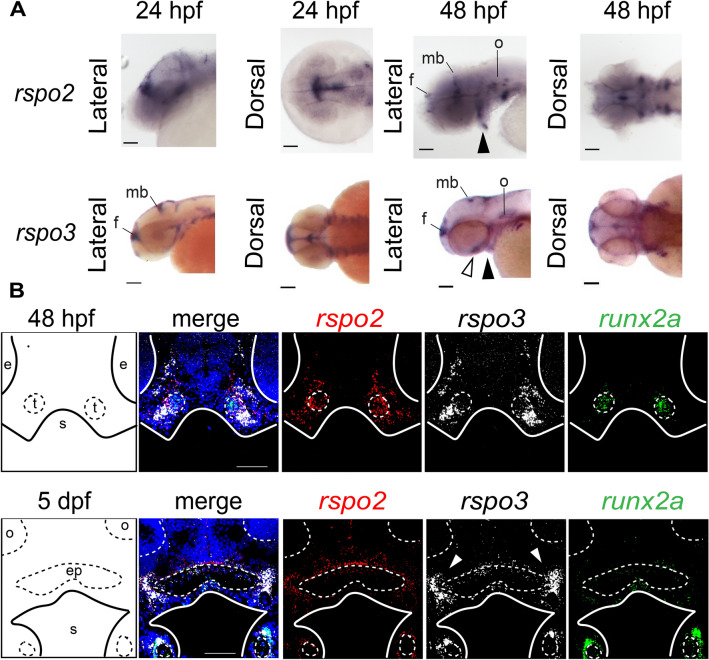
Gene expression patterns of rspo2 and rspo3 during zebrafish embryogenesis were delineated by whole-mount RNA in situ hybridization (WISH). rspo2 and rspo3 transcripts were detected in the brain, otic vesicle, and endodermal pouches at 24 h post-fertilization (hpf) and as well as in regions consistent with the ethmoid plate and Meckel’s cartilage at 48 hpf (Fig. 1A). Using RNAscope in situ hybridization, we identified diffuse rspo3 transcript expression throughout the mesenchyme with concentrated expression in cells that circumscribe the pre-cartilage mesenchyme (48 hpf) and the paired trabeculae, ethmoid plate, and Meckel’s cartilage at 5 days post-fertilization (dpf) (Fig. 1B). We also detected rspo3 expression within ethmoid plate chondrocytes at 5 dpf (Fig. 1B). rspo2 expression generally overlapped with rspo3 at both developmental timepoints.
Figure 1.
High resolution gene expression analysis detected dynamic spatiotemporal localization of rspo2 and rspo3 transcripts in zebrafish cranial mesenchyme. (A) Whole-mount RNA in situ hybridization detected rspo2 and rspo3 transcripts in the midbrain (mb), forebrain (f), otic vesicle (o), and Meckel’s cartilage (arrowhead) at 24 and 48 hpf in lateral and dorsal views. Transcript of rspo3 was additionally detected at 24 and 48 hpf in the forebrain (f) and at 48 hpf in the ethmoid plate (open arrowhead). Scale bar: 100 um. (B) Maximum projections of z-stacks of coronal sections of zebrafish embryos, section in plane with eyes. Labeled schematic at left. At 48 hpf, rspo2 and rspo3 transcripts were highly co-localized in the mesenchyme surrounding the condensing trabeculae cartilage mesenchyme, marked by runx2a expression. rspo2 is also detected within the condensing mesenchyme at this timepoint. At 5 dpf rspo2 and rspo3 continue to be co-expressed in mesenchyme and perichondrium surrounding cartilage elements, specifically the ethmoid plate and Meckel’s cartilage. Rspo3 expression is particularly high in runx2a expressing osteogenic precursor cells associated with Meckel’s cartilage, as well as where the palatoquadrate meets the ethmoid plate (white arrowhead). Abbreviations: e: eye, o: olfactory organ, s: stomodeum, t: trabeculae. Scale bar: 100 μm.
Expression of rspo3 is similar in mouse and zebrafish, however rspo2 expression is distinct
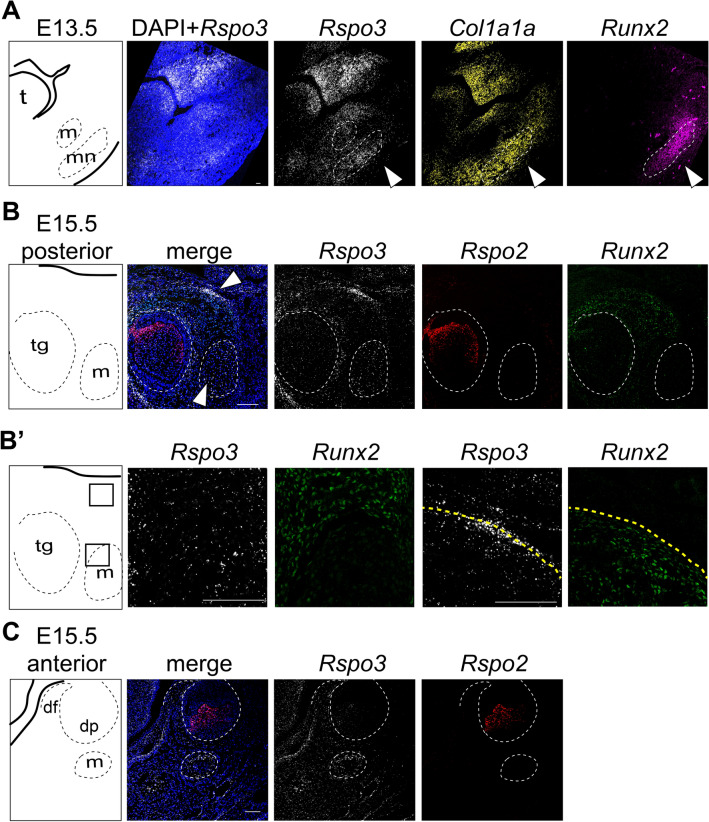
To test the conservation of Rspo2 and Rspo3 expression between vertebrates, we analyzed expression in mouse embryos with RNAscope in situ hybridization and immunofluorescence. At E13.5 we detected Rspo3 expression in regions consistent with osteogenesis, including the developing mandible. We found cellular co-localization of Rspo3, Col1a1 mRNA and Runx2 protein, indicating a potential role in osteogenesis (Fig. 2A). In the E15.5 mouse embryo, Rspo3 transcripts were detected widely throughout the mesenchyme as well as within Meckel’s cartilage (Fig. 2B). Rspo3 expression was also detected within Runx2 positive, presumptive osteoprogenitor cells (Fig. 2B). In contrast to gene expression results in zebrafish, we did not detect Rspo2 expression in the mesenchyme of mouse embryos or associated with cartilage elements. Instead, within the developing mandible, Rspo2 expression was restricted to developing teeth (Fig. 2C). Unlike in zebrafish where rspo2 and rspo3 expression largely overlap, in the mouse transcripts of Rspo2 and Rspo3 appear to be anatomically distinct (Fig. 2).
Figure 2.
RNAscope gene expression analysis of Rspo2 and Rspo3 in mouse embryos. Coronal sections of wild-type mouse embryos at E13.5 and E15.5. Labeled schematic at left. (A) RNAscope revealed diffuse expression of Rspo3 throughout the cranial mesenchyme at E13.5. Col1a1 in situ hybridization and Runx2 immunofluorescence were used to identify osteogenic precursor cells and Rspo3 expression was detected within these cells. Of particular note is Rspo3 expression within Meckel’s cartilage (m) and the developing mandible (arrow; mn). (B) At E15.5, Rspo3 expression is detected diffusely thorough the mesenchyme, including in Meckel’s cartilage (m) and within osteogenic precursor cells (co-expressed with Runx2). Rspo2 expression is isolated to discrete cells within the developing tooth germ (tg). (B’) Higher magnification images (boxes depict location) of Rspo3 and Runx2 co-expression. (C) Within the developing molar at E15.5, Rspo2 and Rspo3 transcripts were detected in distinct non-overlapping regions, with Rspo3 expression in Meckel’s cartilage (m) and the dental follicle (df), while Rspo2 is expressed exclusively within the dental pulp (dp). Scale bar: 100 μm.
rspo2 and rspo3 are differentially expressed within zebrafish dental structures
Given the expression of Rspo2 and Rspo3 in developing mouse teeth, we examined the gene expression of rspo2 and rspo3 within and surrounding the tooth structure in zebrafish. rspo3 gene expression was detected at low levels diffusely throughout the dental pulp and the surrounding mesenchyme (Fig. 3). In contrast, high levels of rspo2 gene expression were detected in the enamel epithelium (Fig. 3). Furthermore, rspo3 gene expression was highest within odontoblasts of regenerating teeth (Fig.3).
Figure 3.
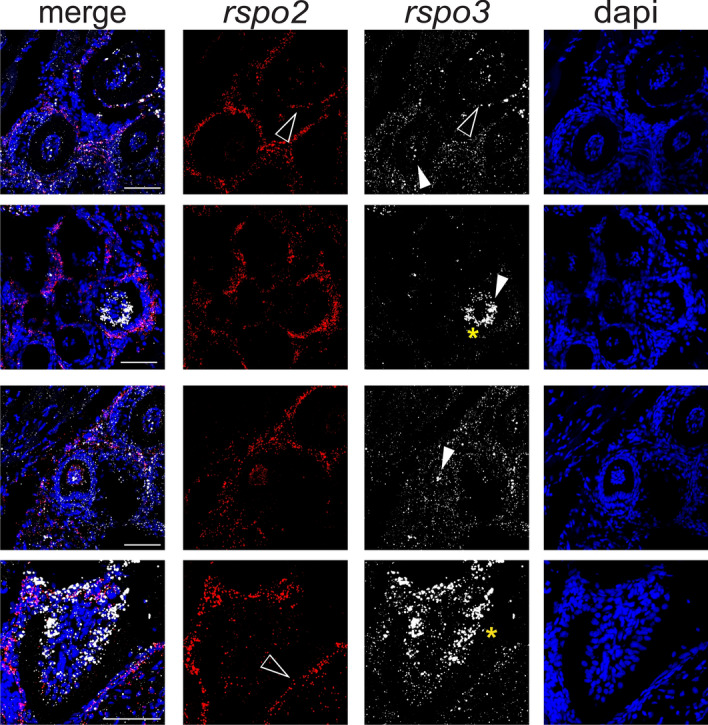
rspo2 and rspo3 are differentially expressed in zebrafish pharyngeal teeth. Representative images of maximum projections of z-stacks of sagittal sections of 180 dpf zebrafish. rspo2 (red) and rspo3 (white) transcripts were detected by RNAscope in situ hybridization. rspo3 is diffusely expressed throughout the supporting mesenchyme and highly expressed in enamel epithelium (open arrow) and some tooth pulp (closed arrow). Often within the tooth pulp, rspo3 expression is restricted to the outermost odontoblasts (*). Meanwhile, rspo2 expression is highest in enamel epithelium (open arrow) and very low within the tooth pulp. Scale bar: 100 μm.
Combined disruption of rspo2 and rspo3 resulted in cartilage dysmorphogenesis
Given the specific expression of rspo3 in early palate and in Meckel’s cartilage development, we used CRISPR/Cas9-mediated genome editing to generate rspo3 mutant alleles. Guide RNAs targeting the rspo3 gene in exon2 were used to create a rspo3 mutant germline allele (Fig. S1A). A frame shift mutation was generated by introducing a − 20 bp gene deletion, as detected by microsatellite genotyping and confirmed by Sanger sequencing (Fig. S1B). The efficiency of this rspo3–20 bp deletion allele (hereafter called rspo3−/−) was assessed by qRT-PCR at 6 hpf, where we observed that rspo3 mRNA was significantly reduced by sixfold in the mutant compared as compared to wild-type clutch-mates (p < 0.05; Fig. S1C).
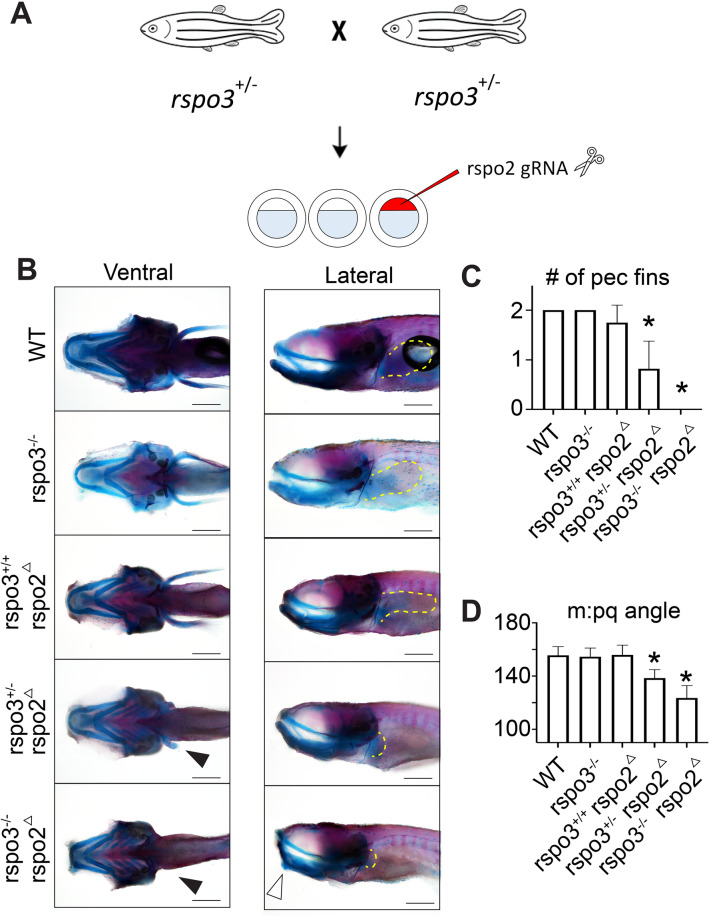
To characterize requirements for rspo3 during early craniofacial morphogenesis, Alcian blue cartilage staining was performed at 5 dpf. The effects of rspo3 disruption on larval cartilage skeleton were found to be subtle. As rspo2 is known to also function in regulating Wnt signaling and has overlapping expression in the zebrafish, we hypothesized that rspo2 action may be compensating for rspo3 germline disruption. Therefore, to determine the combined requirement of rspo2 and rspo3, we targeted rspo2 by injection of multiple gRNA into rspo3 homozygous embryos (Fig. 4A), commonly referred to as a crispant and denoted here as rspo2Δ29. Embryos generated from rspo3+/− in-crossed zebrafish were either raised for analysis of the single mutant or were injected at the 1-cell stage with gRNAs targeting rspo2 (rspo2Δ). The resulting larvae were stained at 9 dpf with Alcian blue and Alizarin Red S. Following imaging and phenotyping, individual larvae were genotyped. We identified a subset of zebrafish with disrupted pectoral fin development where either the fin was partially formed or was absent (Fig. 4B). We found that rspo2 was required for pectoral fin development, and that haploinsufficiency of rspo3 exacerbated the loss of pectoral fin formation (Fig. 4B,C).
Figure 4.
Synergistic effect of rspo2 and rspo3 ablation on zebrafish limb development and craniofacial morphology. (A) Schematic illustrating experimental design. Targeted mutagenesis of rspo3−/− in zebrafish was carried out using CRISPR/Cas9 gene editing. A – 20 bp deletion was bred to homozygosity. Intercross or rspo3+/− were injected with 4 gRNAs against rspo2 and the resulting larvae were genotyped and analyzed for phenotype. (B) Whole mount ventral and lateral images of Alcian blue/Alizarin red S stained 9 dpf larvae. rspo3−/− embryos that were rspo2 gRNA/Cas9 injected (rspo2Δ) larvae were similar to wild-type except that rspo2Δ larvae exhibited disrupted development of the pectoral fin. Impaired fin development was exacerbated with decreasing genetic dosage of rspo3 (black arrows, dotted yellow lines delineate fins). While craniofacial development in rspo3−/− and rspo2Δ larvae were largely normal, rspo3−/−; rspo2Δ double mutants exhibited a dysmorphic lower jaw (white arrow). Scale bar: 100 μm. (C) Quantification of pectoral fin developmental disruption. rspo2Δ larvae tended to have disrupted development of a single pectoral fin. This effect was significantly exacerbated with decreasing genetic dosage of rspo3, as rspo3−/−; rspo2Δ double mutant larvae failed to develop pectoral fins altogether. (D) Quantification of angle measurements between Meckel’s cartilage (m) and palatoquadrate (pq). While rspo3−/− and rspo2Δ mutants had normal lower jaw morphology, rspo3+/−; rspo2Δ and rspo3−/−; rspo2Δ mutants displayed a significantly decreased angle at the Meckel’s/palatoquadrate joint. N = 10–16. p < 0.01. *Indicates significance relative to wild-type.
In addition to altered fin development, we identified a subset of zebrafish with altered craniofacial morphology affecting the lower jaw. We found that rspo2Δ, rspo3+/− and rspo3−/−; rspo2Δ larvae displayed a significantly reduced angle where the palatoquadrate meets Meckel’s cartilage (Fig. 4B,D).
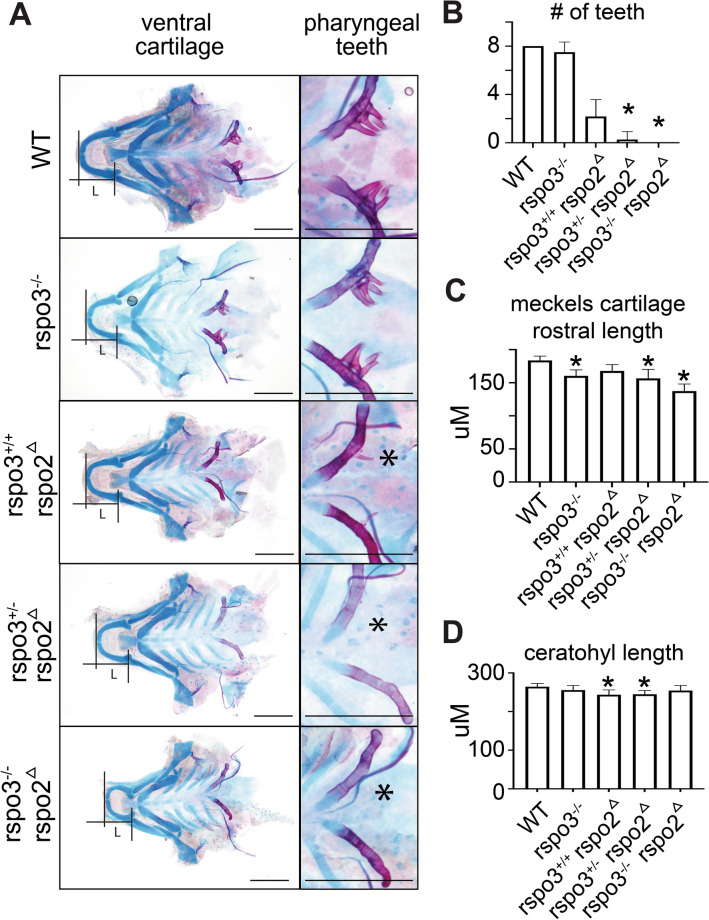
To evaluate craniofacial effects in greater detail and visualize individual cartilage elements, we dissected out the ethmoid plate and ventral cartilages, including the pharyngeal teeth (Fig. 4). Analyses of Alcian blue/Alizarin Red S zebrafish at 9 dpf revealed that disruption of rspo2 caused a decrease in the number of pharyngeal teeth, with an average of 2 total teeth rather than the 8 teeth observed in the control (Fig. 4B). Although rspo3−/− larvae did not exhibit a difference in the number of teeth at 9 dpf, haploinsufficiency of rspo3 decreased tooth number in the rspo2Δ larvae, with the rspo3−/−; rspo2Δ double mutant having no mineralized teeth at 9 dpf (Fig. 4A,B).
Flat-mount imaging of Alcian blue/Alizarin Red S-stained ventral cartilage revealed a significant decrease in anterior–posterior/rostral length of Meckel’s cartilage in the rspo3−/− larvae while rspo2 disruption alone had no effect (Fig. 5A,C). The requirement for rspo3 on Meckel’s cartilage rostral length was significantly exacerbated by rspo2 disruption (Fig. 5C). The effect of rspo3 on Meckel’s cartilage rostral length is specific, rather than due to a total anterior–posterior shortening, as ceratohyal length anterior–posterior length was not different in these zebrafish (Fig. 5D).
Figure 5.
Synergistic effect of rspo2 and rspo3 ablation on zebrafish tooth development and Meckel’s cartilage. (A) Flat-mount images of Alcian blue/Alizarin red S stained 9 dpf zebrafish ventral cartilages. Zoom of pharyngeal teeth to right. rspo3−/− larvae displayed anterior shortening of Meckel’s cartilage, which was exacerbated with rspo3−/−; rspo2Δ gRNA disruption (black bars). *indicate absent teeth. Scale bar: 200 μm. (B) Alizarin red S staining of pharyngeal teeth shows that rspo3−/− are generally normal relative to wild-type while rspo2Δ larvae have a reduced number of teeth (average of 2 versus 8). Tooth number in rspo2Δ larvae decreased further with decreasing wild-type alleles of rspo3 (zero teeth detected in rspo3−/−; rspo2Δ mutant). (C) Quantification of the anterior–posterior/rostral length of Meckel’s cartilage shows a primary effect in rspo3−/− larvae, which is exacerbated in rspo3−/−; rspo2Δ mutants. (D) Quantification of the anterior–posterior length of ceratohyal cartilage shows no effect in rspo3−/− larvae, suggesting a cartilage element-specific effect of rspo3 and rspo2. N = 10–16. p < 0.01. *Indicates significance relative to wild-type.
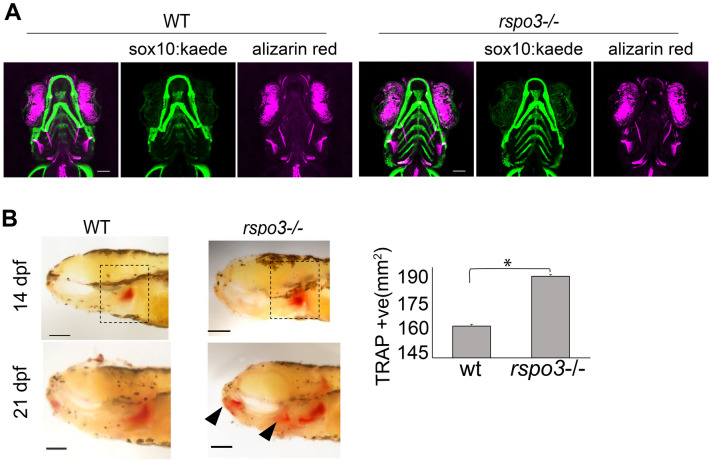
rspo3 influences osteoclast activity during zebrafish development
To assess the role of rspo3 on osteogenesis in developing zebrafish we performed live Alizarin Red S staining on 10 dpf rspo3+/+ and rspo3−/− larvae. The rspo3 mutant allele was also bred onto a sox10:kaede background in order to visualize cartilage elements. Confocal analyses of whole mount embryos revealed no differences in Alizarin Red S intensity (Fig. 6A). No obvious changes in cartilage morphology were observed in the rspo3-/- fish. Interestingly, we did observe increased tartrate-resistant acid phosphatase (TRAP) positive area in rspo3-/- mutants at 14 and 21 dpf, suggesting increased osteoclast number (Fig. 6B). Therefore, these results indicate that rspo3 may regulate aspects of bone homeostasis after larval development as the animals mature during adult life.
Figure 6.
rspo3 ablation does not impact initial bone mineralization but increases osteoclast area of activity. (A) Representative images of maximum intensity projections of confocal z-stack of whole-mount 10 dpf zebrafish. Zebrafish express sox10:kaede transgene allowing fluorescent imaging of cartilage elements. Zebrafish were live-stained with Alizarin red S to visualize mineralized structures. No differences in intensity of Alizarin staining, elements stained, or cartilage architecture were noted in the rspo3−/− fish. Scale bar: 100 μm. (B) Lateral view of 14 dpf showing increased osteoclast activity (red stain in the black dotted box) in rspo3 mutant as compared to wild-type. At 21 dpf, more areas of osteoclast activity in the dentary, hyomandibular, pharyngeal teeth and jaws (solid arrow) were observed in rspo3−/− compared to wild-type. Quantification of total area of red staining. P < 0.05. N = 5. Scale bar: 100 μm.
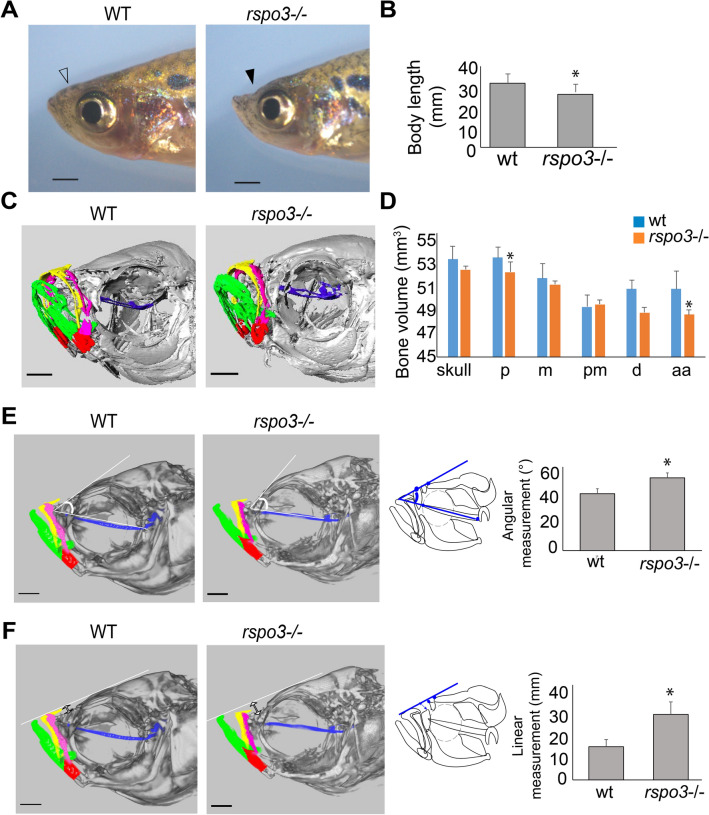
Adult rspo3 zebrafish mutants have decreased body length and exhibit a midface deficiency
As rspo3−/− mutant zebrafish larvae matured to adult fish, we observed midface hypoplasia compared to wild-type clutch-mates (Fig. 7A). Statistically significant differences in body length (measured from tip of mouth opening to the base of the tail, STL) were observed in rspo3−/− mutant as compared to wild-type clutch-mates (Fig. 7B). In addition, rspo3−/− mutant exhibited significantly decreased parasphenoid and anguloarticular bone volume compared to wild-type clutch-mates (Fig. 7C, D). The altered morphology of individual bony elements in rspo3−/− zebrafish also resulted in altered relationships between the bony elements. Cephalometric analysis revealed significant frontal bossing in rspo3−/− mutant adults, with increased parasphenoid-frontal angle (Fig. 7E). Furthermore, we observed midface hypoplasia in adult rspo3−/− zebrafish as compared to wild-type, with significant increased distance between nasal bone and a line drawn between dentary and frontal bone landmarks (Fig. 7F and Supplemental Videos S1, S2).
Figure 7.
rspo3 mutants exhibited midface deficiency, frontal bossing and decreased body length. Reduced body length, midface deficiency and frontal bossing were observed in adult rspo3−/− (180 dpf). (A) Lateral image of adult zebrafish showing midface depression in rspo3−/− (solid arrow) compared to wild-type (open arrow). (B) Body length was significantly decreased in rspo3−/− mutants relative to wild-type. (C) Oblique micro-CT image of rspo3−/− and wild-type fish at 180 dpf. Individual bone elements are color coded (blue: parashenoid, pink: maxilla, yellow: premaxilla, green: dentary and red: anguloarticular). Scale bar: 10 μm. (D) Bone volume of the skull and of specific bones in rspo3−/− and wild-type individuals demonstrates element-specific differences in volume. Parasphenoid and anguloarticular bone volume were significantly reduced in rspo3−/− compared to wild-type fish. Abbreviations: aa: anguloarticular, d: dentary, m: maxilla, p: parasphenoid, pm: premaxilla. (E) 2D cephalometric analysis obtained from micro-CT of rspo3−/− and wild-type fish. The angle formed by parasphenoid line and a line tangent to frontal bone identified frontal bossing, with increased angle in rspo3−/− compared to wild-type. Diagram of lateral view of adult zebrafish showing the angular measurement. Bar chart showing statistical differences in the angular measurement between rspo3−/− and wild-type. (F) 2D cephalometric analysis of rspo3−/− and wild-type fish. The distance between nasal bone and a line drawn between dentary and frontal bone landmarks were measured. Diagram of lateral view of adult zebrafish showing the linear measurement from nasal bone to a line tangent to the frontal bone and dentary. The linear measurement value was significantly greater in rspo3−/− mutants than in wild-type indicating the presence of midface hypoplasia. *p ≤ 0.05. Scale bar: 100 μm.
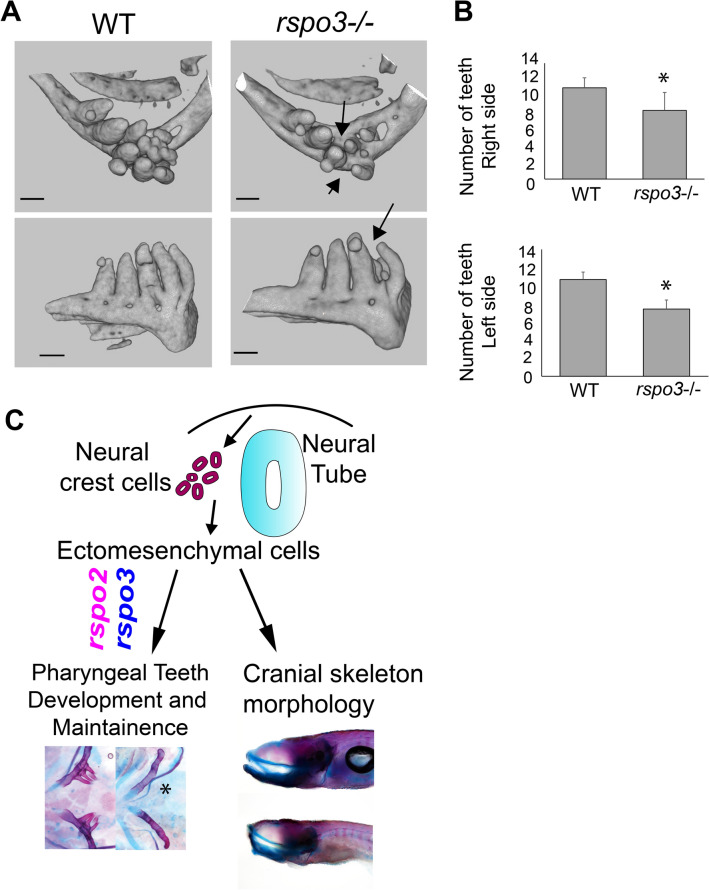
rspo3 is required for normal tooth maintenance
Analysis of pharyngeal tooth morphology in adult zebrafish using micro-CT illustrated decreased tooth number in rspo3−/− mutant zebrafish, as compared to wild-type clutch-mates (Fig. 8A,B). On average, rspo3−/− adult zebrafish had two fewer teeth on both the right and left sides of the jaw (Fig. 8B). As we found no difference in the number of teeth during the larval stage in rspo3−/− animals (Fig. 5), we suggest that rspo3 functions in the maintenance of teeth, rather than tooth development, either by regulating tooth integrity or regulating tooth regeneration.
Figure 8.
Adult rspo3 mutant zebrafish have reduced teeth number. (A) Micro-CT of 180 dpf zebrafish reveals that the adult rspo3−/− animals exhibit decreased tooth number with several sockets missing teeth that are present in the wild-type. (B) rspo3−/− have significantly fewer teeth than wild-type on both right and left sides. N = 9.7. *p < 0.05. (C) Summary diagram illustrating that rspo2 and rspo3 both regulate pharyngeal tooth development as well as have roles in morphogenesis of the craniofacial skeleton.
Discussion
This study reports Rspo2 and Rspo3 gene expressions and functions in craniofacial and dental morphogenesis, using zebrafish and mouse models. Rspo3 is diffusely expressed through the craniofacial mesenchyme whereas Rspo2 is expressed in distinct domains. In zebrafish, there is overlap in rspo2 and rspo3 gene expressions, whereas in the mouse embryo the expression domains of these paralogs are distinct. We showed that Rspo3 is expressed in perichondral cells, and Runx2 positive osteoprogenitors in embryonic palate and Meckel’s cartilage in zebrafish, as well as in mouse osteoprogenitors. In zebrafish teeth, rspo3 is expressed in newly formed replacement teeth, where it is broadly expressed in dental pulp, odontoblasts, and crypt epithelium. Analysis of adult rspo3−/− zebrafish suggest that rspo3 is required for adult teeth maintenance. Loss of rspo3 did not affect larval osteogenesis but did result in increased area of TRAP staining, midface hypoplasia, and reduced numbers of attached teeth in adult zebrafish. Importantly, rspo2 and rspo3 genetically interact, where haploinsufficiency of rspo3 exacerbates defects in tooth formation and pectoral fin bud extension. We show that zebrafish rspo2 and rspo3 are required for limb development, analogous to the mouse function of Rspo2/3 that was previously reported20. Together, these gene expression studies and genetic analyses are consistent with functions for rspo3 in progenitor cell populations contributing to the craniofacial skeleton and teeth, and in the maintenance of craniofacial bones and teeth in zebrafish (Fig. 8C).
We showed that high resolution gene expression analysis using RNAscope on sectioned specimens provided greater resolution as compared to whole mount (ISH) approaches (compare Fig. 1A,B). Using RNAscope, we were able to determine that rspo2 and rspo3 transcripts were detected in a cell layer that surrounds the chondrogenic elements in the zebrafish anterior neurocranium, trabeculae and in Meckel’s cartilage. Moreover, Rspo3 is co-expressed with Runx2 (osteoprogenitor marker) in zebrafish and mouse (Figs. 1, 2). In support of our findings, a human genetic study recently reported the involvement of RSPO3 in bone mineral density and bone fractures30. Furthermore, RSPO3 was reported to regulate osteoblastic differentiation31. In addition, human RSPO3 was identified as a candidate gene that contributes to cleft lip/palate and dental anomalies, consistent with its role in skeletal development and in human adipose-derived stem cells19,31. Taken together, this and other studies corroborate that Rspo3 has conserved functions in the development of craniofacial bone and tooth structures across vertebrates.
This study also identified a key requirement for rspo3 in regulating tooth development. Zebrafish teeth are continuously replaced through its life, where the regenerative process is analogous to human adult tooth replacement of a deciduous, baby tooth and to mouse continuously growing incisors28,32,33. We described rspo3 gene expression in dental pulp, odontoblasts and crypt and dental epithelium in zebrafish and mouse, suggesting possible roles in the regulation of tooth development, odontogenesis and ameloblast differentiation. Importantly, rspo3 is highly detected in zebrafish replacement teeth as compared to mature teeth, indicating potential roles in dental progenitor cell populations as compared to more differentiated dental cell types. Moreover, adult rspo3−/− zebrafish exhibit reduced attached tooth numbers as compared to age-matched wild-type fish. The normal tooth formation in rspo3−/− mutant zebrafish at 9 dpf while having reduced tooth number at 180 dpf suggests a role for rspo3 in the maintenance of adult teeth rather than in their initial development. Differences in Rspo2/3 spatiotemporal gene expression in mouse and zebrafish may reflect differences in the regenerative odontogenic potential of zebrafish. Wnt/β- catenin signaling is important for tooth morphogenesis, and consistent with the observation that rspo3 disruption resulted in inhibited dental tissue development25.
Adult rspo3−/− zebrafish exhibited midface hypoplasia, frontal bossing and reduced tooth number as compared to aged-matched wild-type clutch-mates. Our data showed co-expression of rspo3 and col1a1a during embryogenesis, suggesting that these two genes could be functionally associated. Consistent with this result, previous studies reported that patients with osteogenesis imperfecta have mutations in COL1A1A which is characterized by frontal bossing, midface hypoplasia and dentinogenesis imperfecta35–37. Future studies are recommended to investigate the molecular mechanisms regulated by rspo3, including its interactions with Wnt signaling pathway genes in regulating dental and bone development.
Methods
Experimental animals
All animal experiments were approved by the Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee (IACUC) and in compliance with ARRIVE guidelines. Zebrafish embryos and adults were cared for and maintained in this study as previously described38. Wild-type mice were ordered obtained from Jackson Laboratory (C57BL/6J, Bar Harbor, ME, USA) and Rspo3 mutant mice were kindly provided to Dr. Baron by Dr. Christof Nierhs (German Cancer Research Center, Heidelberg, Germany). All methods were carried out in accordance with relevant guidelines and regulations.
Zebrafish CRISPR mutant line, F0 CRISPR disruption and reporter lines
We used targeted genome editing via CRISPR-cas9 mutagenesis in zebrafish to perform functional analysis of rspo3. A rspo3 mutant zebrafish line was created using the cas9 RNA CCTGGCAGCCCTGGGAGCTC, which resulted in a 20 bp deletion (Supplemental Fig. S1). Genotyping primers for the rspo3 mutant line are 5′-AAGCAGCAAAAATAAGTTCCCA-3′ and 5′-CCACTCCCCATTGCTTTATTAC-3′ with FAM modification on the reverse primer for microsatellite analysis. The mutant peak was observed at 337 bp and wild-type peak observed at 357 bp.
CRISPR gRNA were designed using CRISPOR (http://crispor.tefor.net/) to target rspo2 translational start sites as previously described29. Due to the presence of two rspo2 transcript variants with unique translational start sites (TSS), specific pairs of gRNAs (4 total) were designed to flank each TSS. Guides ordered from Synthego were the following: AGCTCATATACGGACCCTGAAGG, AGACGCAGCAGTCCCACCGCTGG, ATGTCTTTGTACCAAACGATTGG, TCCTCTCCCTCCTCAGGAACAGG.
All four gRNAs were co-injected into rspo3+/− in-crossed single cell zebrafish embryos. Each guide was prepared at a final concentration of 1.25 µM and 2 nL were injected into each embryo. Injected embryos were raised to 9 days post fertilization, where they were subsequently fixed and stained for detailed phenotypic analysis. Stained fish were imaged using a Nikon Eclipse 80i compound microscope with a Nikon DS Ri1 camera. Measurements were taken in ImageJ. Transgenic line Tg(sox10:kaede)39 was also used in this study.
Whole mount in situ hybridization analyses
The primers used to generate the rspo3 RNA probe were the forward primer 5′-AACCTGTGGCTTCAAATGG-3′ and reverse primer 5′-TTGTTGTCGCTCATCCAGTA-3′40.
The T7 promotor (gaaattaatacgactcactatagg) was added to all reverse primers. The RNA products were confirmed by gel-electrophoresis. WISH in zebrafish was performed as previously described41.
Skeletal staining
Double Alcian blue/Alizarin red S staining on fixed zebrafish was performed as previously described42. The sample size (n) is 5 embryos per each group. The zebrafish palate and lower jaw were dissected and mounted in 4% methyl cellulose prior to imaging. Tartrate- resistant acid phosphatase (TRAP) staining for osteoclast activity was performed (n = 5 wild-type and 5 rspo3−/−) as adapted from previous study43. Imaging was performed using Nikon Eclipse 80i microscope (Melville, NY, USA) and NIS-Elements Br imaging software version 4.40 (2015). Measurements were taken in ImageJ. In vivo Alizarin red S staining of 9 dpf zebrafish was performed as previously described44. Alizarin red S and sox10:kaede fluorescence was imaged using a Leica SP8 inverted confocal laser scanning microscope. Maximum intensity projections of z-stacks were generated using ImageJ version 2.0.
RNAscope in situ hybridization, immunofluorescence and confocal imaging
For sample preparation, 48 hpf and 5 dpf zebrafish embryos were fixed using 4% formaldehyde overnight (ON) at 4 °C. Adult zebrafish (6 months old) were fixed using 4% formaldehyde ON at 4 °C and then decalcified ON using 0.35 M EDTA as previously published45. The E13.5 and E15.5 mouse embryos were fixed with 4% formaldehyde ON. n = 3 zebrafish embryos and n = 3 mouse embryos were analyzed.
Subsequently, all samples were placed in 15% sucrose in PBS until the tissue sank, and then placed in 30% sucrose in PBS ON. Samples were then embedded in OCT (Tissue-Tek) and serially sectioned (10 um) in coronal orientation using a Leica CM1850 cryostat.
RNAscope probes included: Dr-rspo3-C2 (catalog number: 555121-C2), Dr-runx2a-C1 (catalog number: 409521), Dr-rspo2-C3 (catalog number: 899271-C3) Mm-Rspo3-C3 (catalog number: 402011-C3), Mm-rspo2-C2 (catalog number: 402008-C2). All probes were manufactured by Advanced Cell Diagnostics in Newark, NJ, USA. Sample pre-treatment and RNAscope were performed according to the manufacturer’s instructions (Advanced Cell Diagnostics, Newark, NJ, USA). Stained slides were imaged using a Leica SP8 inverted confocal laser scanning microscope and image processing was performed using ImageJ version 2.0 (2018). Immunofluorescence detection of mouse Runx2 (Abcam primary antibody, catalog number: ab192256; Invitrogen Alexa Fluor 488 goat anti rabbit secondary antibody) was performed following RNAscope in situ hybridization as described by Advanced Cell Diagnostics.
Micro-computed tomography
Wild-type and rspo3 mutant adult zebrafish were sacrificed at 6 months of age, n = 9 wild-type and 7 mutant zebrafish. All zebrafish were scanned as previously described46. The voxel size of Micro-CT analysis is 10.5 μm. The examiner (K.W.) was blinded to the genotype of the zebrafish. Images were reconstructed, analyzed and viewed using Amira software version 6.
Measurement of bone volume
The reconstructed bitmap image (BMP) files were converted to NIfTI format for simplification, using Amira software. The threshold tool values were consistent between the samples (32–72 threshold logic unit). Each zebrafish skull was segmented into bone elements (dentary, anguloarticular, premaxilla, maxilla and parasphenoid) using Amira manufacture’s instruction. n = 9 wild type and 7 mutant zebrafish were analyzed at 6 months of age.
Quantitative RT-PCR
Three independent samples of wild-type and rspo3 CRISPR/Cas9 (− 20 base pairs micro-deletion mutants) at 6 hpf were collected and measured in triplicate in order to characterize the rspo3 mutant. We decided to collect embryos at 6 hpf, because it has been reported that rspo3 mRNA is highly expressed in zebrafish embryos at this time point34. In addition, three independent 1-cell stage and 24 hpf wild type embryo samples were collected and measured to define the expression of rspo3 mRNAs. RNA extractions were performed using RNeasy Mini Kit (Qiagen). SuperScript First-Strand Synthesis System IV (Thermo Fisher Scientific) was used to synthesize first-strand cDNA. Quantitative reverse-transcription PCR (qRT-PCR) was performed using rspo3 Taqman assay (Dr03109282_m1) Taqman Fast Advanced master mix (Thermo Fisher Scientific) and normalized to 18S rRNA expression (Hs03003631_g1). qPCR was performed on a StepOnePlus Real-Time PCR system (Applied Biosystems).
Statistical analysis
IBM SPSS statistics version 26 was used for all Student’s t-test statistical analyses. Student’s t-test was used to compare between the two groups. Prism 9 software was used to perform Kruskal–Wallis statistical test with multiple comparisons when more than two groups were compared. Statistical significance was set at p-value ≤ 0.05. Asterisks in the figures indicate p-value ≤ 0.05. Data presented as means ± SEM.
Supplementary Information
Acknowledgements
We are grateful for funding support from Shriners Hospital for Children, and the National Institutes of Health R01DE027983. We thank Ms. Jessica Bethoney for excellent management of our aquatic facility. E.C.L. received support from the Laurie and Mason Tenaglia MGH Research Scholar Award. K.C.W. was supported by NIH F32DE029362. M.P.H. was supported in part by NSF/BSF binational Award 201720.
Author contributions
N.A. performed the experiments, wrote the main manuscript and prepared the figures. S.H.C. performed the experiments, wrote the revised manuscript and prepared the figures. K.K. performed the experiments and prepared figures. K.C.W. microCT scanned, reconstructed, and assessed phenotype of a blinded set of mutant and WT skulls. S.A.H. used CRISPR/Cas9-mediated genome editing to generate rspo3 mutant alleles. E.C.L. supervised the work. All the authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nora Alhazmi and Shannon H. Carroll.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85415-y.
References
- 1.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 2.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 3.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass DA, 2nd, Karsenty G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr. Top. Dev. Biol. 2006;73:43–84. doi: 10.1016/S0070-2153(05)73002-7. [DOI] [PubMed] [Google Scholar]
- 5.Albers J, Keller J, Baranowsky A, et al. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J. Cell Biol. 2013;200(4):537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vendrell V, Summerhurst K, Sharpe J, Davidson D, Murphy P. Gene expression analysis of canonical Wnt pathway transcriptional regulators during early morphogenesis of the facial region in the mouse embryo. Gene Exp. Patterns. 2009;9(5):296–305. doi: 10.1016/j.gep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Brugmann SA, Goodnough LH, Gregorieff A, et al. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134(18):3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- 8.Geetha-Loganathan P, Nimmagadda S, Antoni L, et al. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev. Dyn. 2009;238(5):1150–1165. doi: 10.1002/dvdy.21934. [DOI] [PubMed] [Google Scholar]
- 9.Alexander C, Piloto S, Le Pabic P, Schilling TF. Wnt signaling interacts with bmp and edn1 to regulate dorsal-ventral patterning and growth of the craniofacial skeleton. PLoS Genet. 2014;10(7):e1004479. doi: 10.1371/journal.pgen.1004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima M, Kou I, Ohashi H, Ikegawa S. Identification and functional characterizationof RSPO2 as a susceptibility gene for ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 2016;99(1):202–207. doi: 10.1016/j.ajhg.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012;44(5):491–501. doi: 10.1038/ng.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KA, Zhao J, Andarmani S, et al. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle. 2006;5(1):23–26. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- 13.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb. Perspect. Biol. 2013;5(3):a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao HX, Xie Y, Zhang Y, et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485(7397):195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 15.Lebensohn AM, Rohatgi R. R-spondins can potentiate WNT signaling withoutLGRs. Elife. 2018 doi: 10.7554/eLife.33126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight MN, Hankenson KD. R-spondins: Novel matricellular regulators of the skeleton. Matrix Biol. 2014;37:157–161. doi: 10.1016/j.matbio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev. Biol. 2007;301(1):218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Correa-Rodriguez M, Schmidt Rio-Valle J, Rueda-Medina B. The RSPO3 gene as genetic markers for bone mass assessed by quantitative ultrasound in a population of young adults. Ann. Hum. Genet. 2018;82(3):143–149. doi: 10.1111/ahg.12235. [DOI] [PubMed] [Google Scholar]
- 19.Vieira AR, McHenry TG, Daack-Hirsch S, Murray JC, Marazita ML. Candidate gene/loci studies in cleft lip/palate and dental anomalies finds novel susceptibility genes for clefts. Genet. Med. 2008;10(9):668–674. doi: 10.1097/GIM.0b013e3181833793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neufeld S, Rosin JM, Ambasta A, et al. A conditional allele of Rspo3 reveals redundant function of R-spondins during mouse limb development. Genesis. 2012;50(10):741–749. [Google Scholar]
- 21.Jin YR, Yoon JK. The R-spondin family of proteins: Emerging regulators of WNT signaling. Int. J. Biochem. Cell. Biol. 2012;44(12):2278–2287. doi: 10.1016/j.biocel.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Millar SE. Wnt/beta-catenin signaling in oral tissue development and disease. J. Dent. Res. 2010;89(4):318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huysseune A, Soenens M, Elderweirdt F. Wnt signaling during tooth replacement in zebrafish (Danio rerio): Pitfalls and perspectives. Front. Physiol. 2014;5:386. doi: 10.3389/fphys.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003;116(Pt 9):1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Chu EY, Watt B, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008;313(1):210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuang-Hsien HuJ, Mushegyan V, Klein OD. On the cutting edge of organ renewal: Identification, regulation, and evolution of incisor stem cells. Genesis. 2014;52(2):79–92. doi: 10.1002/dvg.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat. Rev. Genet. 2004;5(7):499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 28.Yelick PC, Schilling TF. Molecular dissection of craniofacial development using zebrafish. Crit. Rev. Oral. Biol. Med. 2002;13(4):308–322. doi: 10.1177/154411130201300402. [DOI] [PubMed] [Google Scholar]
- 29.Hoshijima K, Jurynec MJ, Klatt Shaw D, Jacobi AM, Behlke MA, Grunwald DJ. Highly efficient CRISPR-Cas9-based methods for generating deletion mutations and F0 embryos that lack gene function in zebrafish. Dev. Cell. 2019;51(5):645–657. doi: 10.1016/j.devcel.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baniwal SK, Shah PK, Shi Y, et al. Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporos Int. 2012;23(4):1399–1413. doi: 10.1007/s00198-011-1728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Zhang P, Liu Y, et al. RSPO3-LGR4 regulates osteogenic differentiation of human adipose-derived stem cells via ERK/FGF signalling. Sci. Rep. 2017;7:42841. doi: 10.1038/srep42841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobourne MT, Sharpe PT. Making up the numbers: The molecular control of mammalian dental formula. Semin. Cell. Dev. Biol. 2010;21(3):314–324. doi: 10.1016/j.semcdb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Tummers M, Thesleff I. Root or crown: A developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130(6):1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- 34.Verstraeten B, van Hengel J, Huysseune A. Beta-catenin and plakoglobin expression during zebrafish tooth development and replacement. PLoS ONE. 2016;11(3):e0148114. doi: 10.1371/journal.pone.0148114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Fontoura CS, Miller SF, Wehby GL, et al. Candidate gene analyses of skeletal variation in malocclusion. J. Dent. Res. 2015;94(7):913–920. doi: 10.1177/0022034515581643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallos D, Hart PS, Cortelli JR, et al. Novel COL1A1 mutation (G559C) [correction of G599C] associated with mild osteogenesis imperfecta and dentinogenesis imperfecta. Arch. Oral. Biol. 2001;46(5):459–470. doi: 10.1016/s0003-9969(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 37.Gistelinck C, Kwon RY, Malfait F, et al. Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc. Natl. Acad. Sci. USA. 2018;115(34):E8037–E8046. doi: 10.1073/pnas.1722200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty M, Kamel G, Grimaldi M, et al. Distinct requirements for wnt9a and irf6 in extension and integration mechanisms during zebrafish palate morphogenesis. Development. 2013;140(1):76–81. doi: 10.1242/dev.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rong X, Chen C, Zhou P, et al. R-spondin 3 regulates dorsoventral and anteroposterior patterning by antagonizing Wnt/beta-catenin signaling in zebrafish embryos. PLoS ONE. 2014;9(6):e99514. doi: 10.1371/journal.pone.0099514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling IT, Rochard L, Liao EC. Distinct requirements of wls, wnt9a, wnt5b and gpc4 in regulating chondrocyte maturation and timing of endochondral ossification. Dev. Biol. 2017;421(2):219–232. doi: 10.1016/j.ydbio.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007;82(1):23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- 43.Hammond CL, Schulte-Merker S. Two populations of endochondral osteoblasts with differential sensitivity to Hedgehog signalling. Development. 2009;136(23):3991–4000. doi: 10.1242/dev.042150. [DOI] [PubMed] [Google Scholar]
- 44.Bensimon-Brito A, Cardeira J, Dionisio G, Huysseune A, Cancela ML, Witten PE. Revisiting in vivo staining with alizarin red S: a valuable approach to analyse zebrafish skeletal mineralization during development and regeneration. BMC Dev. Biol. 2016;16:2. doi: 10.1186/s12861-016-0102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Copper JE, Budgeon LR, Foutz CA, et al. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish. Comp. Biochem. Physiol. C. 2018;208:38–46. doi: 10.1016/j.cbpc.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charles JF, Sury M, Tsang K, et al. Utility of quantitative micro-computed tomographic analysis in zebrafish to define gene function during skeletogenesis. Bone. 2017;101:162–171. doi: 10.1016/j.bone.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.