Abstract
Objective:
Characterize the types and doses of commonly administered perioperative drugs in inguinal hernia (IH) repair for premature infants.
Study Design:
Single-center, retrospective cohort study
Results:
In total, 112 premature infants underwent IH repair between 2010–2015. Twenty-one drugs were used during IH repair, with each infant receiving a median 7 drugs. Acetaminophen (88%), bupivacaine (84%), cisatracurium (74%), sevoflurane (72%), and propofol (71%) were the most commonly used agents. Thirty-two infants underwent additional procedures with IH repair. Additional procedures were not associated with a higher number of perioperative drugs, however infants with additional procedures were exposed to higher cumulative doses of cisatracurium (p<0.001) and fentanyl (p=0.002).
Conclusion:
There is wide variability in the drugs and doses used for a common surgical procedure in this population, even within a single center. Future research should focus on the safety and efficacy of the most commonly used perioperative drugs described in this study.
Introduction
Among premature infants, early surgery is associated with more brain injury and adverse neurodevelopmental outcomes compared to infants who do not have surgery.1, 2, 3 However, infants undergoing early surgery may be more acutely ill and have additional risk factors for neurodevelopmental impairment than infants not undergoing surgery, thereby confounding the relationship between surgery and later development. Studies have also shown that inadequately treated pain in premature infants can substantially increase stress responses, impair brain development, and negatively affect future pain response.4, 5 As such, the risk of potential neurodevelopmental impairment associated with surgery and exposure to anesthetic drugs must be weighed against evidence that inadequately treated pain may harm neurodevelopment.
In order to better assess the risks of anesthesia drug exposure among premature infants, more data evaluating this population are needed. Premature infants are a particularly vulnerable population at higher baseline risk of neurodevelopmental impairment compared to infants born at term. They also demonstrate a unique physiology that limits extrapolation of pharmacokinetic and pharmacodynamic data from older populations.6 Therefore, studies are needed to assess the safety of anesthesia drugs in premature infants, as well as the use of prolonged anesthesia and variability in drug protocols and doses. These studies should prioritize the most commonly used anesthetic drugs for surgeries in premature infants. The goal of this study is to characterize the types and doses of commonly administered perioperative drugs in inguinal hernia (IH) repair for premature infants at our center.
Materials / Subjects and Methods
After obtaining approval from the University of North Carolina at Chapel Hill (UNC) Institutional Review Board, we identified premature infants (gestational age <37 weeks) born between 2010 and 2015 who underwent IH repair prior to 1 year of age during their initial hospitalization in the 58-bed level IV neonatal intensive care unit (NICU) at UNC Children’s Hospital in Chapel Hill, NC. The NICU is part of a large academic medical center and serves as a regional referral center for the state. We chose IH repair in order to get a more homogenous sample in which we expected use of perioperative agents to be relatively similar across patients.
We performed a chart review of all infants meeting inclusion criteria and recorded demographics, intraoperative characteristics at time of IH repair, and characteristics at time of death or discharge. We identified the frequency and cumulative doses of perioperative drugs administered during the surgical procedure and 24 hours following the end of the repair. Pre-specified drugs of interest included general anesthetics, local anesthetics, inhaled anesthetics, neuromuscular blocking agents, opioid agents, anticholinergic agents, caffeine, dexamethasone, acetaminophen, and clonidine. We did not record exposures to antibiotics. For inhaled anesthetics, we recorded the duration of intraoperative use.
In addition to perioperative drug frequencies and doses, we recorded total anesthesia time and additional procedures performed along with the IH repair. Total anesthesia time was extracted from the operative anesthesia report and calculated from the time of induction to the recorded end anesthesia time. We did not consider circumcision to be an additional procedure due to its relative brevity and minimal impact on total anesthesia time.
Statistical Analysis
We used standard summary statistics, including medians, with interquartile values (IQV), and counts (percentages), to describe categorical study variables. We used Wilcoxon rank-sum test to compare the number of perioperative drugs received in infants who underwent IH repair alone to those receiving additional procedures at the time of IH repair. We also compared doses of the most commonly used drugs in infants who underwent IH repair compared to additional procedures using Wilcoxon rank-sum test. We manually abstracted data from the medical chart using REDCap electronic data capture tools hosted at UNC and reviewed outlying data points for accuracy through a second review of patients’ charts.7, 8 We used Stata (version 15.1 StataCorp, College Station, Texas) to perform all analyses and considered p values <0.05 as significant.
Results
A total of 112 premature infants underwent IH repair between 2010 and 2015 at our center. The majority (n=81, 72%) were male and the median gestational age at birth was 27 weeks (IQV 25–30) (Table 1). Median age at time of IH repair was 81 days (IQV 61–107) and median anesthesia time was 131 minutes (IQV 102–165). Twenty-seven premature infants underwent circumcision along with IH repair and 32 (29%) underwent one or more additional procedures along with the repair. The median age at discharge was 90 days (IQV 68–122) and 2 infants (2%) died prior to discharge to causes unrelated to IH repair.
Table 1.
Premature infant demographics and perioperative characteristics (N=112).
| n (%) | |
|---|---|
| Demographics | |
| Male | 81 (72) |
| Gestational age in weeks, median (IQV) | 27 (25–30) |
| Birth weight in grams, median (IQV) | 855 (695–1218) |
| Maternal race/ethnicity | |
| White | 62 (55) |
| African-American | 34 (30) |
| Hispanic | 8 (7) |
| Other | 9 (8) |
| Characteristics at time of IH repair | |
| Age in days, median (IQV) | 81 (61–107) |
| Weight in grams, median (IQV) | 2632 (2125–3225) |
| Anesthesia time in minutes, median (IQV) | 131 (102–165) |
| Number of drugs of interest received, median (IQV) | 7 (5–8) |
| Additional procedures: | |
| Abdominal surgery (gastrostomy tube, laparotomy, or peritoneal drain) | 18 |
| Orchiopexy | 6 |
| Abdominal/umbilical hernia repair | 6 |
| Bronchoscopy/laryngoscopy (airway evaluation) | 4 |
| Esophageal sounding | 2 |
| Orchiectomy | 2 |
| Hydrocelectomy/hydrocele drainage | 2 |
| Pyloromyotomy | 1 |
| Ileostomy takedown | 1 |
| Central venous catheter placement | 1 |
| Tracheostomy | 1 |
| Discharge Characteristics | |
| Age in days at discharge, median (IQV) | 90 (68–122) |
| Days from IH repair to discharge, median (IQV) | 6 (4–11) |
| Mortality within 48 hours of IH repair | 0 |
| Hospital mortality > 48 hours after IH repair | 2 (2) |
IQV, interquartile values; IH, inguinal hernia
A total of 21 drugs from the pre-specified drug classes of interest were used in the perioperative period for the IH repair, and the median number administered to each infant was 7 (IQV 2–12). The most commonly used drugs were acetaminophen (88%), bupivacaine (84%), cisatracurium (74%), sevoflurane (72%), and propofol (71%) (Table 2). For local/regional anesthetics, a variety of anesthetic techniques were used, including intrathecal or epidural administration of bupivacaine in 72 infants, intravenous administration of lidocaine in 4 infants, and infiltration of bupivacaine in 22 patients, lidocaine in 1 patient, and ropivacaine in 1 patient.
Table 2.
Frequency and doses of perioperative drugs in 112 premature infants undergoing IH repair.
| Infants exposed: n (%) | Cumulative dose in mg/kg: Median (IQV) | |
|---|---|---|
| General anesthetics | ||
| Propofol | 79 (71) | 3.6 (2.3–5.3) |
| Ketamine | 1 (1) | 1.8 |
| Local/Regional anesthetics | ||
| Bupivacaine | 94 (84) | 1 (0.9–1.1) |
| Lidocaine | 5 (4) | 1 (0.5–1.7) |
| Ropivacaine | 1 (1) | 0.8 |
| Inhaled anesthetics | ||
| Sevoflurane* | 81 (72) | 105 (62–149) minutes |
| Desflurane | 26 (23) | 105 (74–131) minutes |
| Isoflurane | 9 (8) | 78 (23–180) minutes |
| Neuromuscular blocking agents | ||
| Cisatracurium* | 83 (74) | 0.21 (0.15–0.34) |
| Vecuronium* | 4 (4) | 0.47 (0.27–1.38) |
| Rocuronium* | 2 (2) | 1 (0.97–1.05) |
| Succinylcholine* | 1 (1) | 2 |
| Opioid agents | ||
| Fentanyl | 42 (38) | 2.7 (1.2–12.2) mcg/kg |
| Morphine | 3 (3) | 0.1 (0.08–0.18) |
| Remifentanil* | 1 (1) | 8.2 mcg/kg |
| Respiratory stimulant | ||
| Caffeine* | 56 (50) | 19 (16–20) |
| Airway anti-inflammatory agent | ||
| Dexamethasone | 33 (29) | 0.49 (0.48–0.56) |
| Anti-cholinergic agents | ||
| Neostigmine | 66 (59) | 0.06 (0.05–0.07) |
| Glycopyrrolate | 56 (50) | 0.01 (0.01–0.01) |
| Other medications | ||
| Acetaminophen | 99 (88) | 32 (25–48) |
| Clonidine | 2 (2) | 3.2 (1.7–4.7) mcg/kg |
IH, inguinal hernia; IQV, interquartile values; mg, milligrams; kg, kilograms; mcg, micrograms
Indicates US Food and Drug Administration (FDA) approval for use in infants
When comparing infants who underwent IH repair alone to those who underwent additional procedures, there was no significant difference in the number of perioperative drugs received (p=0.55) (Figure 1). However, infants who underwent additional procedures at the time of IH repair were exposed to significantly higher cumulative doses of the following most commonly used drugs: cisatracurium (p<0.001) and fentanyl (p=0.002) (Figure 2).
Figure 1:
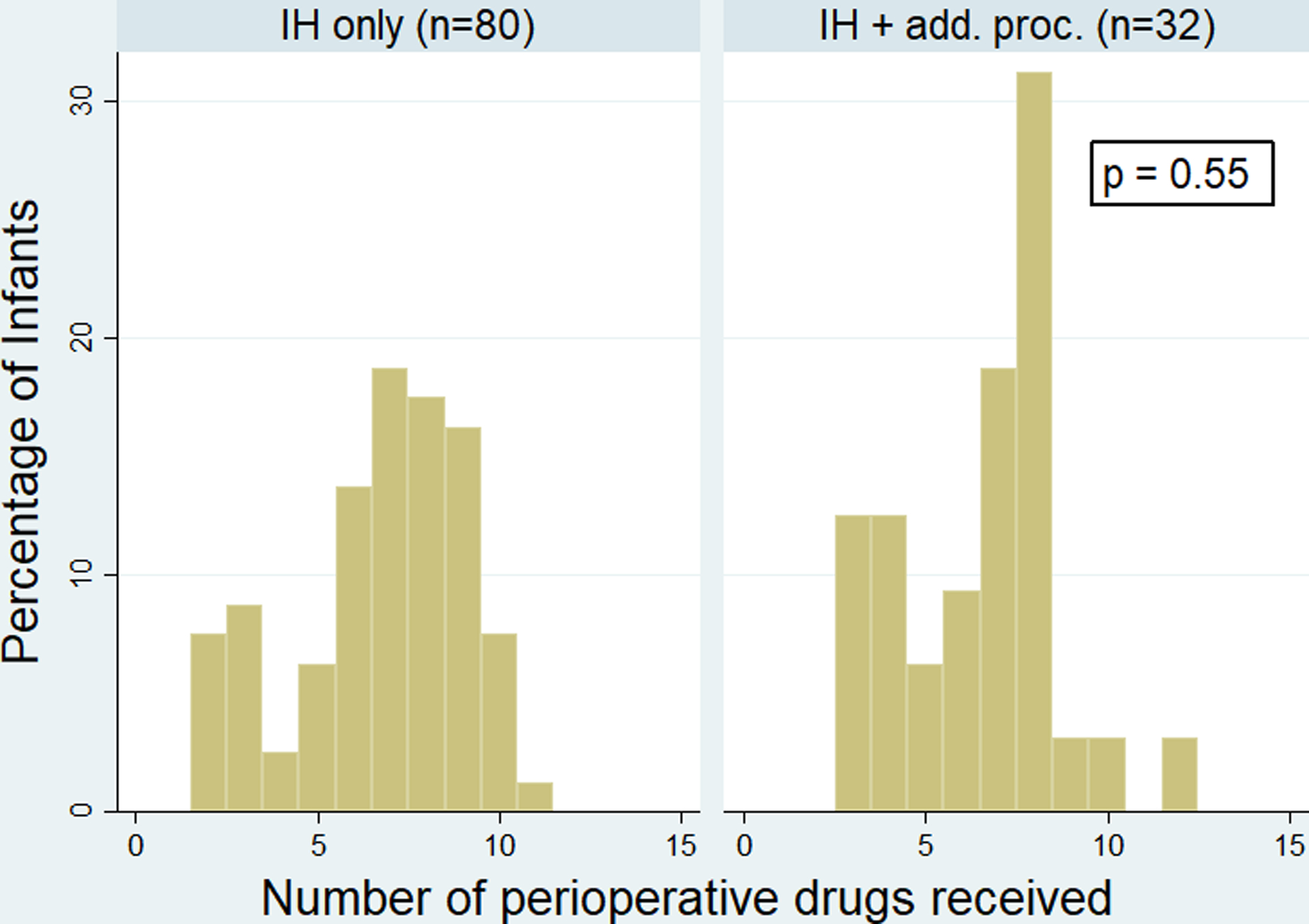
Number of perioperative drugs received by premature infants undergoing inguinal hernia (IH) repair alone compared to infants undergoing IH repair and additional procedure(s).
Figure 2:
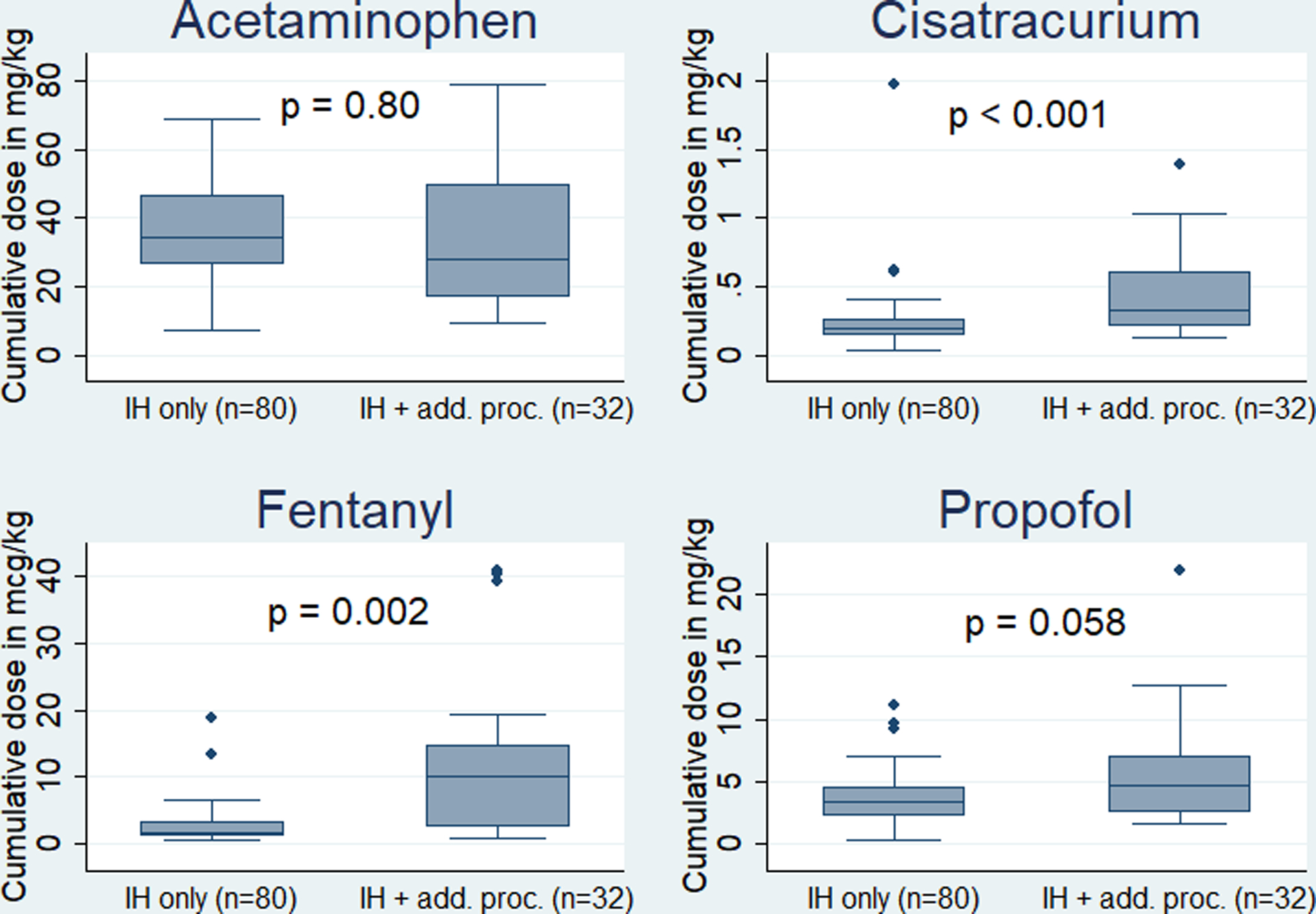
Cumulative doses of acetaminophen, cisatracurium, fentanyl, and propofol in infants undergoing inguinal hernia (IH) repair alone compared to infants undergoing IH repair and additional procedure(s).
Discussion
In December 2016, the United States Food and Drug Administration (FDA) released a statement warning that the prolonged or repeated use of sedative and general anesthetic drugs may adversely affect brain development in children <3 years of age.9 This announcement was based on animal studies demonstrating that exposure to anesthetic and sedative drugs for >3 hours caused neuronal apoptosis and alterations in neurogenesis, resulting in long-term neurocognitive deficits in behavior and learning.9, 10, 11, 12, 13 While animal studies on anesthetic agents inform the potential impact on children, evidence is limited on neurodevelopmental outcomes associated with anesthesia drugs in infants. Two cohort studies, the Mayo Anesthesia Safety in Kids (MASK) study and the Pediatric Anesthesia Neurodevelopment Assessment (PANDA) study, found that short exposure to general anesthesia did not result in adverse neurodevelopmental outcomes in healthy children born at term.14, 15 Similarly, the General Anesthesia compared to Spinal (GAS) Trial determined that use of sevoflurane for < 1 hour in children younger than 6 months is unlikely to have an impact on neurocognitive development at 5 years of age when compared to awake-regional anesthesia.16
In this study, we describe the perioperative drugs and doses administered to premature infants undergoing IH repair between 2010 and 2015 at a single center. We determined that there is wide variability in the number of drugs and doses administered to premature infants. We found that infants received a median of 7 perioperative drugs during the procedure and 24 hours following and that there was no significant difference in the number of agents used when an infant underwent IH repair alone versus IH repair with additional procedure(s). However, we did determine that additional procedures were associated with higher cumulative doses of two of the most common agents. To our knowledge, our study is the first to characterize the most common perioperative drugs and doses used in IH repair in premature infants. This has important implications for future research, highlighting the need to not only investigate the anesthetic drugs’ effects on neurodevelopmental outcomes in the preterm infant population, but to assess whether a dose-dependent effect exists.
Propofol was the most commonly used general anesthetic drug in our cohort. Propofol is frequently used for intubations, induction and maintenance of anesthesia, and procedural and prolonged sedations.17, 18 It has been associated with many adverse events in children, including hypotension, pain on injection, Propofol-Related Infusion Syndrome, apneic events, and oxygen desaturations, among others.17 Studies have also shown that induction with IV propofol is less likely to cause adverse respiratory events than inhaled sevoflurane in high-risk children and that the risk of adverse events in healthy children is similar between propofol and other anesthetic agents.19, 20 However, there is a paucity of data on the safety of propofol in premature infants.21, 22
Additionally, bupivacaine, sevoflurane, and cisatracurium were the most commonly used local anesthetic, inhaled anesthetic, and neuromuscular blocking agents. However, data for the use of these drugs in premature infants are similarly lacking.22 Moreover, while sevoflurane and cisatracurium are FDA-approved in this population, the remainder of the most commonly used drugs identified in this study are used off-label, highlighting the need for further research.
There are several limitations to this study. First, this study was limited to 112 patients from one center and may not be generalizable to other centers. As management guidelines often change from center to center, it is possible that drug exposures described in our study do not reflect practices at other hospitals. In addition, our study included charts up to 2015 and does not include more recent data. Moreover, the majority of our study population was male. However, this is consistent with previous studies showing a higher incidence of IH repair among male preterm infants and is likely representative of the population undergoing IH repair.23 Another limitation is that our perioperative period included 24 after surgery. As such, our analysis likely included drugs that infants were receiving per clinical care, independent of surgery. Another limitation is that we did not differentiate between open and laparoscopic IH repair, which may have influenced length of surgery and post-operative pain control. Finally, our study was subject to similar limitations as other retrospective studies including information bias and errors in data entry.
In conclusion, we determined that there is wide variability in the number and doses of perioperative drugs used in IH repair in preterm infants at our center. This variability likely reflects a lack of standard protocols for perioperative management in premature infants. Our study informs the selection of perioperative drugs for future studies in this population and highlights the need for additional prospective studies exploring their efficacy and safety, specifically the effects on neurodevelopment, in this vulnerable population.
Funding Information:
NHLBI K24 HL143283
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr 2012, 160(3): 409–414. [DOI] [PubMed] [Google Scholar]
- 2.Stolwijk LJ, Keunen K, de Vries LS, Groenendaal F, van der Zee DC, van Herwaarden MYA, et al. Neonatal Surgery for Noncardiac Congenital Anomalies: Neonates at Risk of Brain Injury. J Pediatr 2017, 182: 335–341 e331. [DOI] [PubMed] [Google Scholar]
- 3.Hunt RW, Hickey LM, Burnett AC, Anderson PJ, Cheong JLY, Doyle LW, et al. Early surgery and neurodevelopmental outcomes of children born extremely preterm. Arch Dis Child Fetal Neonatal Ed 2018, 103(3): F227–F232. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet 1987, 1(8527): 243–248. [DOI] [PubMed] [Google Scholar]
- 5.Hatfield LA, Meyers MA, Messing TM. A systematic review of the effects of repeated painful procedures in infants: Is there a potential to mitigate future pain responsivity? J Nurs Educ Pract 2013, 3(8): 99–112. [Google Scholar]
- 6.Hillier SC, Krishna G, Brasoveanu E. Neonatal anesthesia. Semin Pediatr Surg 2004, 13(3): 142–151. [DOI] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009, 42(2): 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019, 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Drug Safety and Availability - FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016. [cited 2020 February 25]Available from: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm
- 10.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology 2011, 115(2): 282–293. [DOI] [PubMed] [Google Scholar]
- 11.Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol 2012, 72(4): 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology 2014, 120(3): 626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward TJ, Timic Stamenic T, Todorovic SM. Neonatal general anesthesia causes lasting alterations in excitatory and inhibitory synaptic transmission in the ventrobasal thalamus of adolescent female rats. Neurobiol Dis 2019, 127: 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. Jama 2016, 315(21): 2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 2018, 129(1): 89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 2019, 393(10172): 664–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs 2015, 29(7): 543–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filho EM, Riechelmann MB. Propofol use in newborns and children: is it safe? A systematic review. J Pediatr (Rio J) 2020, 96(3): 289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramgolam A, Hall GL, Zhang G, Hegarty M, von Ungern-Sternberg BS. Inhalational versus Intravenous Induction of Anesthesia in Children with a High Risk of Perioperative Respiratory Adverse Events: A Randomized Controlled Trial. Anesthesiology 2018, 128(6): 1065–1074. [DOI] [PubMed] [Google Scholar]
- 20.Hong H, Hahn S, Choi Y, Jang MJ, Kim S, Lee JH, et al. Evaluation of Propofol in Comparison with Other General Anesthetics for Surgery in Children Younger than 3 Years: a Systematic Review and Meta-Analysis. J Korean Med Sci 2019, 34(15): e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah PS, Shah VS. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database Syst Rev 2011(3): Cd007248. [DOI] [PubMed] [Google Scholar]
- 22.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg 2008, 106(6): 1681–1707. [DOI] [PubMed] [Google Scholar]
- 23.Kumar VH, Clive J, Rosenkrantz TS, Bourque MD, Hussain N. Inguinal hernia in preterm infants (< or = 32-week gestation). Pediatr Surg Int 2002, 18(2–3): 147–152. [DOI] [PubMed] [Google Scholar]


