Abstract
Objective
Age‐associated decreases in immune functions are precipitated by a variety of mechanisms and affect nearly every immune cell subset. In myeloid cells, aging reduces numbers of phagocytes and impairs their functional abilities, including antigen presentation, phagocytosis, and bacterial clearance. Recently, we described an aging effect on several functions in monocytes, including impaired mitochondrial function and reduced inflammatory cytokine gene expression during stimulation with lipopolysaccharide. We hypothesized that circulating factors altered by the aging process underly these changes. Growth differentiation factor‐15 (GDF‐15) is a distant member of the transforming growth factor‐β superfamily that has known anti‐inflammatory effects in macrophages and has been shown to be highly differentially expressed during aging.
Methods
We used biobanked plasma samples to assay circulating GDF‐15 levels in subjects from our previous studies and examined correlations between GDF‐15 and monocyte function.
Results
Monocyte interleukin‐6 production due to lipopolysaccharide stimulation was negatively correlated to plasma GDF‐15. Additionally, GDF‐15 was positively correlated to circulating CD16 + monocyte proportions and negatively correlated to monocyte mitochondrial respiratory capacity.
Conclusions
These results suggest that GDF‐15 is a potential circulating factor affecting a variety of monocyte functions and promoting monocyte immunosenescence and thus may be an attractive candidate for therapeutic intervention to ameliorate this.
Keywords: immune function, inflammaging, senescence, senescence‐associated secretory phenotype
1. INTRODUCTION
Aging is a multifactorial process leading to disruption of physiological function at subcellular, cellular, tissue, and organismal levels. As such, aging is the largest risk factor for most major chronic diseases. 1 Underlying these changes, at least in part, are the dual concepts of inflammaging and immunosenescence. Inflammaging is a generalized, age‐associated, and chronic low‐grade inflammatory state that is associated with disease progression and is included in the now well‐established Hallmarks of Aging 2 and Pillars of Aging 3 concepts. Immunosenescence is a generalized decline in immune function that leads to increased susceptibility to infection and dysregulated responses to immunological stimuli. 4 Because inflammation is integral to immune responses, these processes have been suggested to share common etiology, 4 but mechanisms linking the two conditions are largely unknown.
In the past several years, our laboratory has examined the effects of the aging process on immunosenescence of the innate immune system, with a focus on dysregulation of immunometabolism in monocytes. 5 , 6 , 7 We were the first to demonstrate impaired mitochondrial function in aged classical monocytes, 5 and we have also found age‐associated reductions in inflammatory cytokine responses to lipopolysaccharide (LPS) 6 and alterations in proportions of circulating monocyte subsets 5 that support previous and subsequent reports. 8 , 9 , 10 , 11 , 12
During the course of these studies, a large proteomics study was published that comprehensively profiled aging‐related alterations in proteins in circulation. 13 Perhaps the most notable finding from this study was the high differential expression of growth differentiation factor‐15 (GDF‐15) between older and younger participants. Being generally unfamiliar with GDF‐15, we performed a literature search and found that the protein, which is a member of the transforming growth factor‐β superfamily, was originally described as macrophage inhibitory cytokine‐1 14 and was shown to be released from macrophages during inflammatory activation (thus potentially linking it to inflammaging) and to subsequently suppress inflammatory activation in macrophages (thus potentially linking it to innate immunosenescence). Moreover, GDF‐15 is a constituent of the senescence‐associated secretory phenotype, 15 , 16 the proteome secreted by senescent cells, 17 which is itself a major promoter of inflammaging. 18
Given these effects, we became interested in determining if GDF‐15 is a link between cellular senescence, inflammaging, and innate immune system immunosenescence. During the course of our previous studies, we had biobanked blood plasma from older and younger adult participants, and therefore we assayed GDF‐15 levels in these samples and conducted a small secondary analysis of our existing datasets to test the hypothesis that GDF‐15 is correlated to indications of monocyte immunosenescence. Although the results of these analyses are associative, they support a potential link between GDF‐15 and age‐related monocyte dysfunction. Given the great interest in GDF‐15 resulting from recent proteomics studies, and the paucity of data demonstrating age‐associated biological effects of the protein, the outcomes reported here are an important preliminary step in the establishment of GDF‐15 as a link between cellular senescence and immune system dysfunction resulting from the aging process.
2. METHODS
2.1. Subjects
Younger (18‐35 years) and older (60‐80 years) adults were recruited from the greater Memphis area for testing. Subject characteristics and inclusion/exclusion criteria have been previously described. 6 Subject characteristics are reported in Table 1, which was adapted from Cohort 1 data from the corresponding table in our previous paper. 6 All subjects completed informed consent documents prior to enrollment and were free to withdraw at any time. This research complied with the US Federal Policy for the Protection of Human Subjects, and all protocols were approved in advance by the Institutional Review Board at the University of Memphis (protocol #4361). Groups did not differ in any measured demographic or anthropometric characteristic, with the exception of age (P < 0.001), as designed.
Table 1.
Demographic and anthropometric characteristics of subjects
| Aged (n = 9) | Young (n = 9) | Probability | |
|---|---|---|---|
| Age, y (range) | 65.0 ± 1.2 (61‐71) | 25.7 ± 1.9 (18‐33) | P < 0.001 |
| Height, cm (range) | 176.7 ± 3.0 (164‐192) | 169.2 ± 4.1 (157‐189) | P = 0.166 |
| Weight, kg (range) | 79.6 ± 3.6 (57‐93) | 77.3 ± 8.6 (48‐129) | P = 0.814 |
| BMI, kg/m2 (range) | 25.5 ± 1.1 (20‐32) | 26.4 ± 1.9 (19‐36) | P = 0.688 |
| Female, n (%) | 4 (44) | 5 (56) | P = 0.637 |
| White, n (%) | 5 (56) | 6 (67) | |
| Black, n (%) | 4 (44) | 2 (22) | Race: P = 0.415 |
| Hispanic, n (%) | 0 (0) | 1 (11) |
Abbreviation: BMI, body mass index
2.2. Monocyte functional assays
Detailed methods for functional assays for isolated human monocytes are reported in their respective papers 5 , 6 and are briefly summarized here. Monocytes were isolated by immunomagnetic negative selection with CD16 depletion using a commercially available kit, yielding an untouched population of classical (CD14 + CD16‐) monocytes at > 90% purity. Isolated monocytes were immediately used in downstream assays and were not frozen. For analysis of mitochondrial function, a Seahorse Cell Mito Stress Test assay was performed on 1.5 × 10 5 monocytes per well in duplicate as described previously. 5 For determination of monocyte cytokine responses, isolated monocytes were stimulated for 24 hours with 1 ng·ml‐1 LPS, then lysed with TRIzol as previously described. 6 Isolated mRNA was purified using the manufacturer’s instructions and reverse‐transcribed to cDNA using a commercial kit, and cytokine fold expression was quantitated by qPCR using 4 ng cDNA assayed in duplicate against B2M as a housekeeping gene. For monocyte phenotype determination, 100 μl whole blood was stained with anti‐CD14‐PE and anti‐CD16‐Brilliant Violet 421 antibodies and analyzed on an Attune NxT flow cytometer as previously described. 5 Monocytes were gated based on forward‐ and side‐scatter and partitioned into classical (CD14+CD16‐), intermediate (CD14+CD16+), and non‐classical (CD14lowCD16+) subtypes based on cell surface expression of CD14 and CD16.
2.3. GDF‐15 analysis
For analysis of plasma cytokine levels, whole blood was collected into EDTA vacutainer tubes by venipuncture, and plasma was separated from erythrocytes by centrifugation at 1500 g for 15 min at 4°C. Isolated plasma was aliquoted and frozen at −80°C until analysis. Circulating GDF‐15 levels in plasma were determined by commercial ELISA (DY957, R&D Systems) according to the manufacturer’s instructions. Plasma samples were diluted 10× in reagent diluent and assayed in duplicate against a standard curve. Intra‐assay coefficient of variation was 7.7%. All samples were assayed on a single plate.
2.4. Data analysis
All analyses were conducted in R V. 3.5.1 (R Foundation for Statistical Computing). Demographic data with categorical outcomes (race, sex) were analyzed by Pearson’s chi‐square test. Continuous demographic and anthropometric data, as well as monocyte functional data, were measured by independent samples t‐test with one exception. Welch’s correction was applied to the t‐test for IL6 gene expression, as the data did not meet the criteria for homoscedasticity by Levene’s test. For determination of the associations between circulating GDF‐15 levels and monocyte functional responses, bivariate Pearson’s correlations were performed. The significance level for all tests was set a priori at P ≤ 0.05. Reported results are mean ± SEM.
3. RESULTS
All data used for analyses are available in a dedicated FigShare repository. 19
3.1. Monocyte functional data
Monocyte functional data in Figure 1 are adapted from our previous reports 5 , 6 and demonstrate that monocytes from older adults have reduced mitochondrial respiratory capacity (Figure 1A), impaired cytokine responses to LPS (Figure 1B), and altered proportions of CD16+ (sum of intermediate and non‐classical) subtypes (Figure 1C) compared to monocytes from younger adults. These data are reprinted here to aid in interpretation of subsequent correlational analyses.
Figure 1.
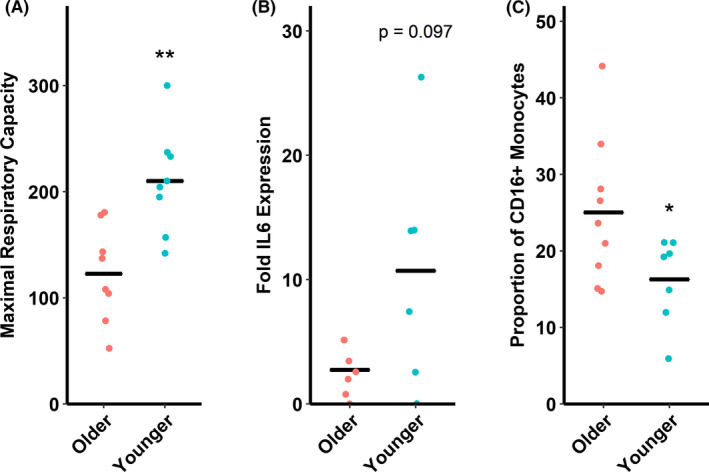
Indications of monocyte dysfunction in older versus younger adults. (A) Older adults (60‐80 y) have reduced mitochondrial respiratory capacity compared to younger adults (18‐35 y). Maximal respiratory capacity is in pmol O2·min−1·(105 monocytes)−1 as measured by a Seahorse XFp analyzer. (B) Older adults have a trend toward reduced IL6 gene expression following lipopolysaccharide stimulation (by 2−ΔΔCt method against B2M) compared to younger adults. (C) Older adults have increased circulating proportions of CD16+ monocytes (as percent total circulating monocytes) compared to younger adults. This subpopulation encompasses the sum total of proportions of intermediate and non‐classical monocytes. *P ≤ 0.05 compared to older adults. **P < 0.01 compared to older adults. n = 6‐9/group depending on assay
3.2. Plasma GDF‐15
It was previously reported that GDF‐15 is one of the most significantly upregulated circulating proteins during the aging process. 13 As expected, circulating GDF‐15 levels in plasma from older adults were elevated nearly threefold in older compared to younger adults in the present study (t 11 = 5.3321, P < 0.001, Figure 2).
Figure 2.
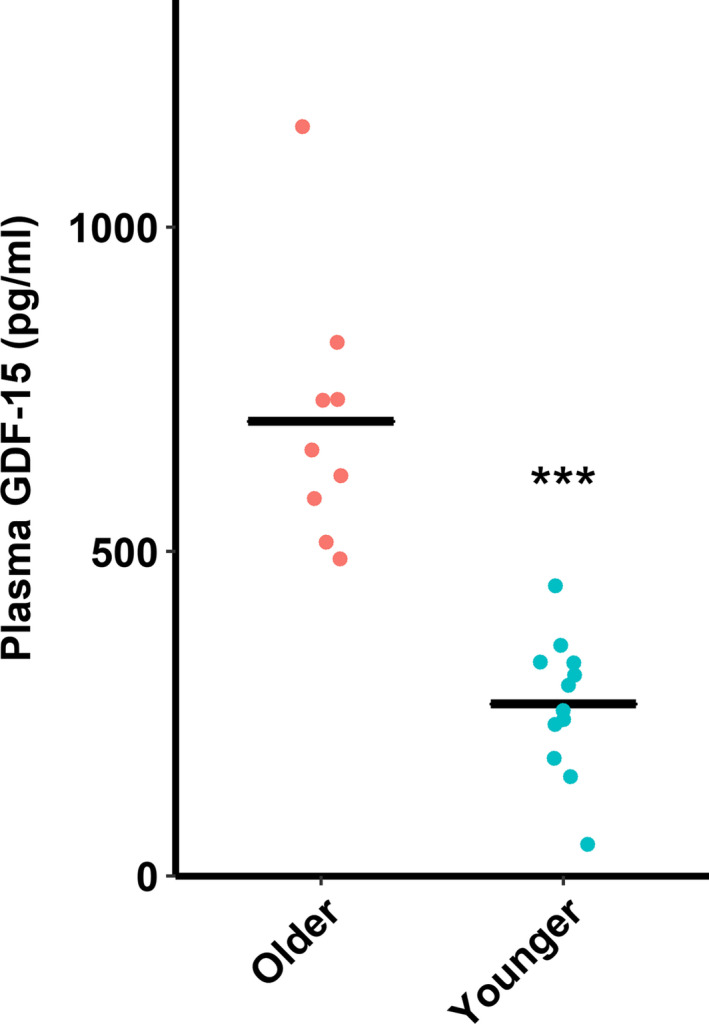
Older (60‐80 y) adults have increased plasma growth differentiation factor‐15 (GDF‐15) levels compared to younger (18‐35 y) adults. ***P < 0.001. n = 9‐12/group
3.3. Correlations
Given the established immunomodulatory properties of GDF‐15 14 and its upregulation with age, we reasoned that circulating levels of GDF‐15 might be associated with immunosenescence. Our ability to conduct secondary analysis using our previous monocyte functional data, coupled with our biobanked human plasma samples, allowed us to conduct correlational analyses between circulating GDF‐15 and indices of monocyte immunosenescence.
Circulating GDF‐15 had strong negative correlation to monocyte maximal respiratory capacity (R = −0.747, P < 0.001, Figure 3A). GDF‐15 had moderate negative correlation to IL6 expression levels after LPS stimulation (R = −0.561, P = 0.046, Figure 3B), and had nonsignificant moderate negative correlation to IL1B and IL10 expression (P > 0.05, data not shown). Finally, GDF‐15 had moderate positive correlation to circulating CD16 + monocyte proportion (sum of intermediate and non‐classical monocyte proportions, R = 0.571, P = 0.021, Figure 3C) and moderate negative correlation to circulating classical (CD14 + CD16‐) monocyte proportion (R = −0.534, P = 0.033, data not shown).
Figure 3.
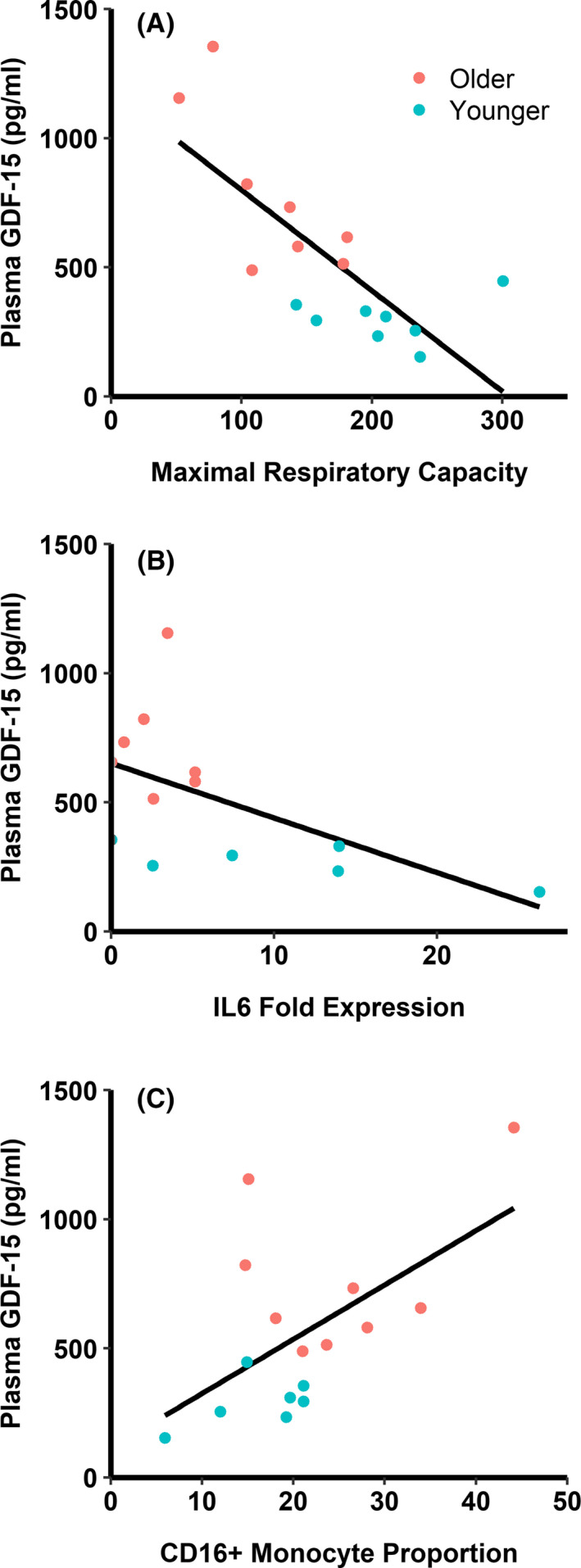
Associations between circulating growth differentiation factor‐15 (GDF‐15) and indices of monocyte dysfunction. (A) GDF‐15 has a strong negative correlation with maximal mitochondrial respiratory capacity (R = −0.747, P < 0.001). Maximal respiratory capacity is in pmol O2·min−1·(105 monocytes) −1 as measured by a Seahorse XFp analyzer. (B) GDF‐15 has a moderate negative correlation with IL6 fold expression following lipopolysaccharide stimulation (by 2−ΔΔCt method against B2M, R = −0.561, P = 0.046). (C) GDF‐15 has moderate positive correlation with CD16+ monocyte proportions (R = 0.571, P = 0.021). n = 6‐9/group depending on assay
4. DISCUSSION
Impaired immune responses are a normal consequence of aging, and increasing evidence suggests a key role of monocytes in these processes. Monocyte (and macrophage) dysfunction may therefore be important mediators of cellular senescence, 20 increased susceptibility to infections, 21 and other hallmarks of innate immunosenescence. Our lab has reported aging‐induced mitochondrial dysfunction in monocytes, altered monocyte population distributions, and attenuated inflammatory responses following LPS stimulation. 5 , 6 , 7 A subset of these previously reported data was used for the analyses reported here.
GDF‐15 is a cytokine member of the transforming growth factor‐β superfamily and is known to dampen or attenuate innate immune cell responses to a variety of stimuli. GDF‐15 is released from human‐derived macrophages following TNF‐α and IL‐1β stimulation and in turn inhibits LPS‐stimulated TNF‐α production in macrophages. 14 GDF‐15 was recently observed to increase lipid accumulation and impair autophagy in oxLDL‐laden macrophages. 22 Furthermore, tumor‐derived GDF‐15 inhibits the synthesis of nitric oxide and TNF‐α through TGF‐activated kinase‐1 in macrophages. 23 Thus, it appears GDF‐15 blunts macrophage responses from diverse stimuli, but the common underlying mechanism by which GDF‐15 attenuates innate immune cell response is still unknown.
Through secondary analyses of existing samples, we found upregulated circulating GDF‐15 in older adults whose monocytes were tested for functional responses in our previous studies. GDF‐15 has recently been identified as a core member of the senescence‐associated secretory phenotype 24 and has been shown to increase in peripheral circulation with age. 13 Serum GDF‐15 was reported as a strong predictor of mortality in a Swedish male cohort, 25 as well as of all‐cause mortality in community‐dwelling older adults. 26 The approximately threefold increase in serum concentrations of GDF‐15 in older subjects in our study is consistent with these previous reports demonstrating elevated secretions of GDF‐15 with aging.
While the exact role of GDF‐15 in the aging process is unclear, elevated GDF‐15 has been observed in other chronic inflammatory diseases, including cardiovascular disease, 27 obesity, 28 type 2 diabetes, 29 and cancer. 30 Recently, GDF‐15 was found to mediate aging‐related anti‐inflammatory effects in both humans and mice, lending some credence to the idea that the cytokine is a potential mediator of innate immune dysfunction in aged individuals. 31 Together these data highlight a potential role for GDF‐15 in chronic inflammatory diseases and mortality. These conditions are commonly associated with immune and mitochondrial dysfunction, but additional research is necessary to uncover the precise mechanisms by which GDF‐15 participates in the pathogenesis of these diseases, and to establish whether or not it is elevated as a protective agent in pathology or is actively contributing to it.
In the present study, we report, for the first time, associations between GDF‐15 and indices of age‐related monocyte dysfunction. Using a secondary analysis of existing monocyte functional data paired with newly acquired circulating GDF‐15 protein data, we found significant negative correlations between GDF‐15 levels and monocyte maximal respiratory capacity and LPS‐stimulated IL6 gene expression. We also demonstrated a significant positive correlation between GDF‐15 and CD16+ monocytes proportions in peripheral circulation. This study is limited both by the small sample size and the associative nature of the analyses; therefore, we cannot demonstrate a causal link between GDF‐15 and these monocyte functions. However, while associative data must be interpreted cautiously, these data do suggest a possible link between aging, GDF‐15, and monocyte (dys)function.
Mitochondrial integrity and function are critical for monocyte and macrophage homeostasis. In this study, we observed a negative association between plasma GDF‐15 levels and maximal mitochondrial respiratory capacity in monocytes. Respiratory capacity is the ability to maximally generate ATP through mitochondrial respiration. 32 The reduced respiratory capacity we demonstrated in our previous study 5 may render monocytes unable to generate sufficient ATP under aerobic conditions, leading to cellular dysfunction, although currently this is speculative. Because GDF‐15 is thought to be a biomarker for mitochondrial dysfunction 33 , 34 and indeed promotes mitochondrial function in macrophages, 35 it is likely that GDF‐15 is not causally related to mitochondrial dysfunction in monocytes, but circulating levels of the protein may serve as a useful proxy measure for mitochondrial dysfunction. Additionally, monocytes with dysfunctional mitochondria may contribute to increased circulating GDF‐15, although this has not been investigated.
Finally, non‐classical CD16+ monocytes are typically characterized as anti‐inflammatory and necessary for vascular maintenance. 36 However, non‐classical monocytes have been observed to adopt a pro‐inflammatory phenotype basally and to display features akin to cellular senescence in other cell types. 37 GDF‐15 has been shown to promote cellular senescence in endothelial cells, 38 and thus may play some role in the transition of monocytes to the non‐classical phenotype, which is increased with age. 5 , 8 , 9 , 10 , 11
5. CONCLUSIONS
Here we report associative data suggesting a potential role for GDF‐15 in aging‐induced monocyte dysfunction. Circulating GDF‐15 levels were significantly correlated to impaired mitochondrial function, reduced LPS‐stimulated inflammatory cytokine gene expression, and increased proportion of CD16+ circulating monocytes. While these correlational data are based on a small sample size and are insufficient to demonstrate a direct effect of GDF‐15 on monocyte immunosenescence, our findings highlight a potential role for this protein in regulating age‐associated alterations in immune function.
CONFLICT OF INTERESTS
Nothing to disclose.
AUTHOR CONTRIBUTIONS
B.D.P. conceived and designed the study. B.D.P. and J.R.Y. collected the data. B.D.P. analyzed the data. B.D.P. and R.S.E. drafted the manuscript. B.D.P., R.S.E., and J.R.Y. revised the manuscript drafts. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge the participants in this study. The study was supported by American Heart Association grants 18AIREA33960189 and 19TPA34910232 to B.D.P.
Pence BD, Yarbro JR, Emmons RS. Growth differentiation factor‐15 is associated with age‐related monocyte dysfunction. Aging Med. 2021;4:47–52. 10.1002/agm2.12128
REFERENCES
- 1. Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22(17):R741‐R752. [DOI] [PubMed] [Google Scholar]
- 2. Lopez‐Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm‐aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pence BD, Yarbro JR. Aging impairs mitochondrial respiratory capacity in classical monocytes. Exp Gerontol. 2018;108:112‐117. [DOI] [PubMed] [Google Scholar]
- 6. Pence BD, Yarbro JR. Classical monocytes maintain ex vivo glycolytic metabolism and early but not later inflammatory responses in older adults. Immun Ageing. 2019;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yarbro JR, Pence BD. Classical monocytes from older adults maintain capacity for metabolic compensation during glucose deprivation and lipopolysaccharide stimulation. Mech Ageing Dev. 2019;183:111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120‐126. [PubMed] [Google Scholar]
- 9. McLachlan JA, Serkin CD, Morrey KM, Bakouche O. Antitumoral properties of aged human monocytes. J Immunol. 1995;154:832‐843. [PubMed] [Google Scholar]
- 10. Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11(5):867‐875. [DOI] [PubMed] [Google Scholar]
- 11. Ault R, Dwivedi V, Koivisto E, et al. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp Gerontol. 2018;111:35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30(6):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka T, Biancotto A, Moaddel R, et al. Plasma proteomic signature of age in healthy humans. Aging Cell. 2018;17(5):e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC‐1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF‐beta superfamily. Proc Natl Acad Sci U S A. 1997;94(21):11514‐11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ha G, De Torres F, Arouche N, et al. GDF15 secreted by senescent endothelial cells improves vascular progenitor cell functions. PLoS One. 2019;14(5):e0216602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y, Ayers JL, Carter KT, et al. Senescence‐associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell. 2019;18(6):e13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coppé J‐P, Desprez P‐Y, Krtolica A, Campisi J. The senescence‐associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis. 2010;5(1):99‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular senescence and inflammaging in age‐related diseases. Mediators Inflamm. 2018;2018:9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pence BD. Growth differentiation factor‐15 is associated with age‐related monocyte immunosenescence. FigShare. 2020. 10.1101/2020.02.05.935643. Accessed February 5, 2020. [DOI] [Google Scholar]
- 20. Yarbro JR, Emmons RS, Pence BD. Macrophage immunometabolism and inflammaging: roles of mitochondrial dysfunction, cellular senescence, CD38, and NAD. Immunometabolism. 2020;2(3):e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pence BD. Severe COVID‐19 and aging: are monocytes the key? GeroScience. 2020;42(4):1051‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackermann K, Bonaterra GA, Kinscherf R, Schwarz A. Growth differentiation factor‐15 regulates oxLDL‐induced lipid homeostasis and autophagy in human macrophages. Atherosclerosis. 2019;281:128‐136. [DOI] [PubMed] [Google Scholar]
- 23. Ratnam NM, Peterson JM, Talbert EE, et al. NF‐κB regulates GDF‐15 to suppress macrophage surveillance during early tumor development. J Clin Invest. 2017;127(10):3796‐3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence‐associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1):e300599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiklund FE, Bennet AM, Magnusson PKE, et al. Macrophage inhibitory cytokine‐1 (MIC‐1/GDF15): a new marker of all‐cause mortality. Aging Cell. 2010;9(6):1057‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett‐Connor E. Growth‐differentiation factor‐15 is a robust, independent predictor of 11‐year mortality risk in community‐dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123(19):2101‐2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lind L, Wallentin L, Kempf T, et al. Growth‐differentiation factor‐15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the prospective investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur Heart J. 2009;30(19):2346‐2353. [DOI] [PubMed] [Google Scholar]
- 28. Dostálová I, Roubíček T, Bártlová M, et al. Increased serum concentrations of macrophage inhibitory cytokine‐1 in patients with obesity and type 2 diabetes mellitus: The influence of very low calorie diet. Eur J Endocrinol. 2009;161(3):397‐404. [DOI] [PubMed] [Google Scholar]
- 29. Carstensen M, Herder C, Brunner EJ, et al. Macrophage inhibitory cytokine‐1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: the Whitehall II Study. Eur J Endocrinol. 2010;162(5):913‐917. [DOI] [PubMed] [Google Scholar]
- 30. Wallentin L, Zethelius B, Berglund L, et al. GDF‐15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS One. 2013;8(12):e78797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moon JS, Goeminne LJE, Kim JT, et al. Growth differentiation factor 15 protects against the aging‐mediated systemic inflammatory response in humans and mice. Aging Cell. 2020;19(8):e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Desler C, Hansen TL, Frederiksen JB, Marcker ML, Singh KK, Juel RL. Is there a link between mitochondrial reserve respiratory capacity and aging? J Aging Res. 2012;2012:192503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M. Secreted growth differentiation factor15 as a potential biomarker for mitochondrial dysfunctions in aging and age‐related disorders. Geriatr Gerontol Int. 2016;16:17‐29. [DOI] [PubMed] [Google Scholar]
- 34. Montero R, Yubero D, Villarroya J, et al. GDF‐15 is elevated in children with mitochondrial diseases and is induced by mitochondrial dysfunction. PLoS One. 2016;11(2):e0155172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jung SB, Choi MJ, Ryu D, et al. Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nat Commun. 2018;9(1):1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC. Nonclassical monocytes in health and disease. Annu Rev Immunol. 2019;37(1):439‐456. [DOI] [PubMed] [Google Scholar]
- 37. Ong SM, Hadadi E, Dang TM, et al. The pro‐inflammatory phenotype of the human non‐classical monocyte subset is attributed to senescence article. Cell Death Dis. 2018;9(3)266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park H, Kim CH, Jeong JH, Park M, Kim KS. GDF15 contributes to radiation‐induced senescence through the ros‐mediated p16 pathway in human endothelial cells. Oncotarget. 2016;7(9):9634‐9644. [DOI] [PMC free article] [PubMed] [Google Scholar]


